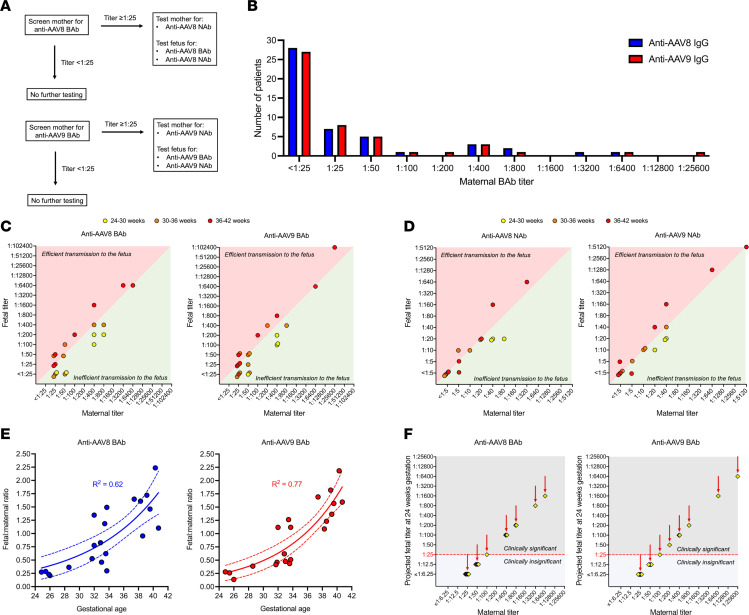
Figure 5. Maternal-fetal anti-AAV antibody transmission efficiency is determined by gestational age in human pregnancy.
(A) Testing strategy. Maternal serum was first tested for anti-AAV8 and anti-AAV9 IgG BAbs. Titers of 1:25 or higher were considered seropositive and prompted additional testing of both mother and fetus. Titers lower than 1:25 were considered seronegative, and no further testing of either mother or fetus was performed. (B) Histogram of maternal BAb titers. Less than half of mothers screened positive for AAV8 and AAV9, with low-level immunity (<1:200) being most common among the seropositive cases. (C) Comparison of maternal and fetal BAb titers. Shown are the fetal titer (y axis) and maternal titer (x axis) for each dyad screening seropositive for anti-AAV IgG. Fetal titers were generally higher than maternal titers at 36–42 weeks gestation, equal to or below maternal titers at 30–36 weeks gestation, and far below maternal titers at 24–30 weeks gestation. (D) Comparison of maternal and fetal NAb titers. NAb followed a pattern similar to BAb. Note that hemolysis interferes with luciferase-based assays, resulting in falsely elevated titers. NAb data extracted from hemolyzed samples were therefore excluded. (E) Nonlinear regression analysis of fetal/maternal IgG BAb ratio by gestational age. Ratio values greater than 1 indicate efficient antibody transmission to the fetus, and values less than 1 indicate inefficient transmission to the fetus. (F) Projected fetal titer at 24 weeks. Using the results of the regression analysis demonstrating a fetal/maternal ratio of 0.25 at 24 weeks, fetal titer at 24 weeks gestation was projected for all seropositive mothers. For 12 of 20 (60%) mothers seropositive for AAV8 and 13 of 21 (62%) mothers seropositive for AAV9, the fetal titer at 24 weeks gestation was projected to be less than 1:25 and therefore clinically insignificant.