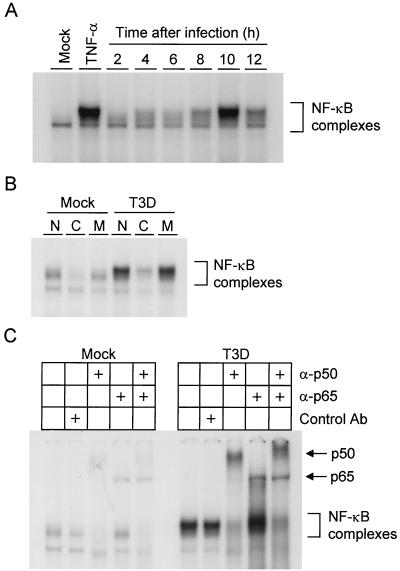
FIG. 2.
(A) Time course of NF-κB gel shift activity in nuclear extracts prepared from reovirus-infected HeLa cells. Cells (5 × 106) were either mock infected or infected with T3D at an MOI of 100 PFU per cell and incubated at 37°C for the times shown. Uninfected cells also were treated with 20 ng of TNF-α per ml for 1 h. Nuclear extracts were prepared and incubated with a 32P-labeled oligonucleotide consisting of the NF-κB consensus binding sequence. Incubation mixtures were resolved by acrylamide gel electrophoresis, dried, and exposed to film. NF-κB-containing complexes are indicated. (B) Specificity of NF-κB gel shift activity. Nuclear extracts were prepared as in panel A 10 h after viral adsorption. Extracts were incubated with 32P-labeled NF-κB consensus oligonucleotide alone (lanes N), a 10-fold excess of unlabeled consensus probe (lanes C), or a 10-fold excess of unlabeled mutant probe consisting of the NF-κB consensus sequence with a point mutation that abolishes NF-κB binding (lanes M). NF-κB-containing complexes are indicated. (C) Identification of NF-κB family members activated by reovirus infection. Nuclear extracts were prepared as in panel A 10 h after viral adsorption. Extracts were incubated with no antibody, a control antibody (Ab), p50-specific antiserum, p65-specific antiserum, or both p50- and p65-specific antisera. NF-κB complexes not shifted by antibody and supershifted complexes containing p50 or p65 are indicated.