Abstract
Background
Dysphagia after stroke is common and can impact morbidity and death. The purpose of this population‐based study was to determine specific epidemiological and health risk factors that impact development of dysphagia after acute stroke.
Methods and Results
Ischemic and hemorrhagic stroke cases from 2010 and 2015 were identified via chart review from the GCNKSS (Greater Cincinnati Northern Kentucky Stroke Study), a representative sample of ≈1.3 million adults from southwestern Ohio and northern Kentucky. Dysphagia status was determined on the basis of clinical assessments and necessity for alternative access to nutrition via nasogastric or percutaneous endoscopic gastrostomy tube placement. Comparisons between patients with and without dysphagia were made to determine differences in baseline characteristics and premorbid conditions. Multivariable logistic regression determined factors associated with increased risk of dysphagia. Dysphagia status was ascertained from 4139 cases (1709 with dysphagia). Logistic regression showed that increased age, Black race, higher National Institutes of Health Stroke Scale score at admission, having a hemorrhagic stroke (versus infarct), and right hemispheric stroke increased the risk of developing dysphagia after stroke. Factors associated with reduced risk included history of high cholesterol, lower prestroke modified Rankin Scale score, and white matter disease.
Conclusions
This study replicated previous findings of variables associated with dysphagia (older age, worse stroke, right‐sided hemorrhagic lesions), whereas other variables identified were without clear biological rationale (eg, Black race, history of high cholesterol, and presence of white matter disease) and should be investigated in future studies to determine biological relevance and potential influence in stroke recovery.
Keywords: dysphagia, feeding, stroke, swallowing
Subject Categories: Big Data and Data Standards, Clinical Studies, Epidemiology, Risk Factors, Complications
Clinical Perspective.
What Is New?
Patients who are older, Black, or have a right‐sided or hemorrhagic stroke are more likely to develop poststroke dysphagia.
Presence of white matter disease and a history of high cholesterol were associated with a decreased risk of developing dysphagia after stroke.
What Are the Clinical Implications?
Well‐designed prospective trials are needed to investigate the biological rationale for the association between dysphagia and Black race, as well as high cholesterol or presence of white matter disease being protective factors for developing poststroke dysphagia.
Nonstandard Abbreviations and Acronyms
- EMR
electronic medical record
- GCNKSS
Greater Cincinnati Northern Kentucky Stroke Study
- mRS
modified Rankin Scale
- NIHSS
National Institutes of Health Stroke Scale
- WMD
white matter disease
The development of swallowing impairments (dysphagia) has been shown to have an impact on hospitalization timeline and comorbidities associated with acute stroke recovery. 1 Specifically, patients with dysphagia have poor nutrition and hydration, 1 both of which are critical for neural recovery after stroke. 1 , 2 Dysphagia is also associated with higher risk of pneumonia, a serious and deadly complication of swallowing impairment. 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10
Prior investigations of dysphagia after ischemic or hemorrhagic stroke have focused primarily on characterizing specific impairments in swallow physiology on the basis of lesion location and size 11 , 12 , 13 or reporting on specific outcomes of these impairments in this population. 3 , 14 These investigations are highly relevant for assessing and treating the disorder and understanding disposition of these patients. However, patient‐specific factors related to increased risk of developing dysphagia after stroke and patient characteristics linked to severity of dysphagia are not well understood. These patient‐specific factors include both premorbid health conditions (eg, heart disease) and patient demographics (eg, sex, age, race), which in part derive social determinants of health (eg, economic stability and health literacy/education) 15 and are known to impact health outcomes at multiple levels. 16 Gaining an understanding of underlying conditions, biological, and socioeconomic risk factors that may predispose patients to developing swallowing disorders after stroke would provide opportunities for preventative care. To date, there have been no study populations large enough to address these gaps.
The goal of this work is to investigate incidence of dysphagia after stroke in a large retrospective, population‐based data set to determine what patient‐specific factors may influence dysphagia status or severity of impairment. These data will guide future investigations to better understand how and why certain individuals develop swallowing impairments after stroke and what can be done in acute clinical management of stroke to help prevent development of swallowing disorders.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Institutional review board approval was obtained; consent requirement was waived for the nature of this retrospective chart review study.
The GCNKSS (Greater Cincinnati Northern Kentucky Stroke Study) has been previously described in detail 17 , 18 , 19 , 20 , 21 ; in brief, this is a population‐based study using retrospective ascertainment of physician‐adjudicated stroke cases in a sample of ≈1.3 million people across 5 counties in southwestern Ohio and northern Kentucky. All hospitalized adult (aged ≥18 years) cases of ischemic and hemorrhagic stroke from 2010 and 2015 were identified using International Classification of Diseases, Ninth (ICD‐9) and Tenth Revision (ICD‐10) stroke codes (ICD‐9, 430–436; ICD‐10, I60‐I69) at 15 area hospitals.
Case Definition: Dysphagia Versus No Dysphagia
Dysphagia status was identified through electronic medical record (EMR) review by a study team of registered nurses. Individuals with documentation in the EMR of swallowing impairment were included in the dysphagia group. Given the known variability in swallowing assessment practices both within and across institutions (eg, timing of initial screening, formal evaluation by a speech–language pathologist versus bedside screen by nursing staff), 22 , 23 documentation of dysphagia varied depending on the case, the treating institution, and clinical practices at the time of stroke. Assessment of dysphagia status was also documented in the EMR. This could include a formal swallow assessment (bedside with or without instrumental by a speech–language pathologist) or a nursing bedside screen. Only individuals who were determined not to have dysphagia after ≥1 of these assessments were included in the no‐dysphagia group. Within the identified dysphagia group, individuals were compared on the basis of their need for access to alternative nutrition during the acute hospital stay. Individuals were categorized as 1 of the following (dysphagia by mouth subgroups): (1) dysphagia not requiring percutaneous endoscopic gastrostomy (PEG)/nasogastric tube (ie, dysphagia management required only a modified texture with or without modified liquids ), (2) dysphagia requiring nasogastric tube, or (3) dysphagia requiring PEG. Oral status was determined and confirmed through same chart review procedures previously described. For cases in which dysphagia status changed (ie, progressed) throughout inpatient stay, the most invasive intervention required was documented (ie, PEG was placed after nasogastric tube).
Patient Characteristics: Demographics, Clinical Presentation, Premorbid Conditions
Patients included in the analyses were further characterized and compared (dysphagia versus no‐dysphagia, and dysphagia by mouth subgroups). The following patient characteristics were available for analysis, abstracted during the registered nurse chart review process: (1) patient demographic and social determinants of health data: age, sex, race, ethnicity, and estimation of a neighborhood deprivation index score 24 ; (2) clinical presentation at time of acute injury: National Institutes of Health Stroke Scale (NIHSS), prestroke modified Rankin Scale (mRS), and Glasgow Coma Scale scores; (3) stroke/lesion characteristics: type of case (ischemic/hemorrhagic), lesion location (hemisphere), ischemic stroke subtype (small vessel, cardioembolic, large vessel, other, or undetermined); (4) comorbidities (premorbid conditions): history of hypertension, diabetes, elevated cholesterol, coronary artery disease, myocardial infarction, congestive heart failure, prior stroke, prior transient ischemic attack, dementia, brain injury or tumor, history of smoking, current smoker (within the past 6 months), alcohol use, heavy alcohol use, and any white matter disease (WMD; new or old). All data regarding race and ethnicity categorization were abstracted directly from the EMR; as such, we are unable to definitively determine the source of the EMR reporting of race and ethnicity, whether it was self‐identification or assigned by the admitting medical professional.
Statistical Analysis
Study variables were summarized using descriptive statistics (mean and SD or median and interquartile range or range for continuous variables, and frequencies and percentages for categorical variables) by study groups (dysphagia status: yes versus tested and no; dysphagia subgroups: dysphagia not requiring PEG/nasogastric tube versus dysphagia requiring nasogastric tube versus dysphagia requiring PEG; assessment: assessed versus not assessed). Distribution was examined for all continuous variables. Associations between categorical variables and study groups were tested using either χ2 or Fisher's exact test. Associations between continuous variables and study groups were tested using either 2‐sample t‐test, ANOVA, Wilcoxon rank‐sum test, or Kruskal–Wallis test. Comparisons among the 3 dysphagia subgroups were adjusted for multiple comparisons using Bonferroni correction for categorical variables, NIHSS, and Glasgow Coma Scale and by applying Dunnett's test, following ANOVA, for age and deprivation index. Multivariable logistic regression analysis was carried out for dysphagia status (yes versus assessed and no) and dichotomized dysphagia subgroups (dysphagia requiring PEG or nasogastric tube versus dysphagia not requiring PEG or nasogastric tube). P values ≤0.05 were declared as statistically significant. All analyses were carried out in SAS 9.4 (SAS Institute, Cary, NC).
Results
A total of 5592 (2010, N=2644; 2015, N=2948) physician‐adjudicated and confirmed hospitalized strokes from 15 area hospitals in the Greater Cincinnati and northern Kentucky region were reviewed.
Dysphagia Status and Dysphagia Subgroup
Dysphagia status could be ascertained from only 4139 cases, which made up the final sample for the analyses (Figure 1). Of 4139 cases, 1709 were determined to have dysphagia. Of these cases, 944 did not require alternative access to nutrition (ie, PEG/nasogastric), 437 required nasogastric, and 328 required PEG placement (Table 1). In comparing the subgroups by year, there were no differences between 2015 and 2010 in terms of proportion of individuals per dysphagia subgroup.
Figure 1. Number of strokes included from the Greater Cincinnati Northern Kentucky Stroke Study in 2010 and 2015.
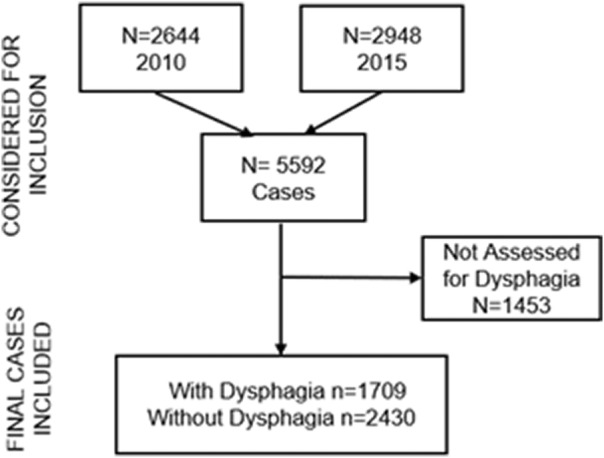
Table 1.
Dysphagia Subgroup Status by Year
| Dysphagia subgroup (n=709) | Dysphagia not requiring PEG/nasogastric tube (n=944) | Dysphagia requiring nasogastric tube (n=437) | Dysphagia requiring PEG (n=328) |
|---|---|---|---|
| Year, n (%) | |||
| 2010 (n=782) | 406 (51.9) | 223 (28.5) | 153 (19.6) |
| 2015 (n=927) | 538 (58.0) | 214 (23.1) | 175 (18.9) |
| P value (2010 vs 2015) | 0.03 | 0.03 | 1.0 |
PEG indicates percutaneous endoscopic gastrostomy.
Patient Characteristics: Demographics and Clinical Presentation
Patients with dysphagia were significantly older (mean, 72 years versus 67.2 years; P<0.0001); a larger proportion were women (57% versus 52.2%; P=0.002), had higher median NIHSS scores (8 versus 2; P<0.0001), had lower median Glasgow Coma Scale scores (14 versus 15; P<0.0001), had higher median prestroke mRS scores (2 versus 1; P<0.0001), and scored slightly higher on the mean deprivation index (P=0.01; Table 2).
Table 2.
Demographics and Clinical Presentation by Dysphagia Status
| Dysphagia | No dysphagia | P value | |
|---|---|---|---|
| No. | 1709 | 2430 | |
| Age, y, mean±SD | 72.0±14.7 | 67.2±14.8 | <0.0001 |
| Race, n (%) | |||
| White + other | 1278 (74.8) | 1877 (77.3) | 0.06 |
| Black | 431 (25.2) | 551 (22.7) | |
| Unknown* | 1 (0.06) | 1 (0.04) | |
| Ethnicity, n (%) | |||
| Hispanic | 6 (0.4) | 14 (0.6) | 0.02 |
| Non‐Hispanic | 1211 (70.9) | 1804 (74.3) | |
| Unknown | 492 (28.8) | 611 (25.2) | |
| Sex, n (%) | |||
| Male | 735 (43.0) | 1161 (47.8) | 0.002 |
| Female | 974 (57.0) | 1268 (52.2) | |
| Baseline NIHSS | |||
| Median (25th–75th percentile) | 8 (3–16) | 2 (1–5) | <0.0001 |
| Minimum–maximum | 0–40 | 0–39 | |
| Glasgow Coma Scale | |||
| Median (25th–75th percentile) | 14 (11–15) | 15 (15–15) | <0.0001 |
| Minimum, maximum | 3–15 | 3–15 | |
| Baseline mRS | |||
| Median (25th–75th percentile) | 2 (0–3) | 1 (0–3) | <0.0001 |
| Minimum, maximum | 0–5 | 0–5 | |
| Baseline mRS score 0–1, n (%) | 610 (35.8) | 1280 (52.7) | <0.0001 |
| Deprivation index, mean±SD | 0.38±0.13 | 0.37±0.13 | 0.01 |
Unknown not included in statistical comparison.
mRS indicates modified Rankin Scale; and NIHSS, National Institutes of Health Stroke Scale.
Stroke lesion characteristics between dysphagia groups differed (Table 3): the dysphagia group had a larger proportion of hemorrhagic strokes as compared with those with the no‐dysphagia group (16.5% versus 11.8%; P<0.0001); the dysphagia group had a greater proportion of any right hemisphere stroke when compared with the no‐dysphagia group (51.3% versus 45.8%; P=0.002); among ischemic stroke cases, the dysphagia group had a smaller proportion of small‐vessel strokes (9.3% versus 20.7%; P<0.0001), a greater proportion of cardioembolic strokes (38.5% versus 21.9%; P<0.0001), and a smaller proportion of strokes for which subtype could not be determined (32% versus 38.2%; P=0.0008).
Table 3.
Lesion Characteristics by Dysphagia Status
| Dysphagia | No dysphagia | P value | |
|---|---|---|---|
| No. | 1709 | 2430 | |
| Type of case, n (%) | <0.0001* | ||
| Infarct | 1426 (83.4) | 2142 (88.1) | |
| Hemorrhagic (ICH/SAH) | 281 (16.5) | 287 (11.8) | |
| Unknown‡ | 2 (0.1) | 1 (0.03) | |
| Lesion location(s), n (%) | <0.0001* | ||
| Left hemisphere only | 681 (39.8) | 1017 (41.8) | |
| Any left hemisphere | 946 (55.4) | 1292 (53.2) | 0.64† |
| Right hemisphere only | 610 (35.7) | 854 (35.1) | |
| Any right hemisphere | 877 (51.3) | 1113 (45.8) | 0.002† |
| Brain stem only | 4 (0.2) | 7 (0.3) | |
| Any brain stem | 153 (9.0) | 187 (7.7) | 0.57† |
| Mixed hemispheric (left + right only) | 171 (10.0) | 165 (6.8) | |
| Mixed (left and/or right + brain stem) | 149 (8.7) | 180 (7.4) | |
| Unknown | 94 (5.5) | 207 (8.5) | |
| Stroke subtype infarct only, n (%) | <0.0001* | ||
| Small vessel | 132 (9.3) | 444 (20.7) | <0.0001† |
| Cardioembolic | 549 (38.5) | 470 (21.9) | <0.0001† |
| Large vessel | 189 (13.3) | 294 (13.7) | 0.97† |
| Other identified | 99 (7.0) | 116 (5.4) | 0.24† |
| Undetermined | 456 (32.0) | 818 (38.2) | 0.0008† |
ICH indicates intracerebral hemorrhage; and SAH, subarachnoid hemorrhage.
Overall significance.
After correcting for multiple testing.
Unknown not included in the analysis.
Patient Characteristics: Premorbid Conditions
A larger proportion of individuals in the dysphagia group as compared with the no‐dysphagia group had a history of hypertension (84.5% versus 81%; P=0.004), a history of coronary artery disease (36.2% versus 32%; P=0.0005), history of congestive heart failure (23.5% versus 15.6%; P<0.0001), history of prior stroke (28.4% versus 25.6%; P=0.04), and history of dementia (16.7% versus 8.4%; P<0.0001; Table 4). There was a larger proportion of individuals in the no‐dysphagia group who had a history of transient ischemic attack (16.6% versus 13%; P=0.002), who were current smokers (28.2% versus 23.8%; P=0.002), who reported alcohol use (at least 1–2 drinks per day; 41.7% versus 34.7%, P<0.0001), and who had WMD (71.6% versus 68.5%; P=0.03).
Table 4.
Premorbid Conditions by Dysphagia Status
| Dysphagia, n (%) | No dysphagia, n (%) | P value | |
|---|---|---|---|
| No. | 1709 | 2430 | |
| History of hypertension | 1444 (84.5) | 1969 (81.0) | 0.004 |
| History of diabetes | 611 (35.8) | 870 (35.8) | 0.97 |
| History of elevated cholesterol | 944 (55.2) | 1405 (57.8) | 0.10 |
| Coronary heart disease | 794 (46.5) | 938 (38.6) | <0.0001 |
| History of coronary artery disease | 618 (36.2) | 777 (32.0) | 0.005 |
| History of myocardial infarction | 255 (14.9) | 341 (14.0) | 0.42 |
| History of congestive heart failure | 401 (23.5) | 379 (15.6) | <0.0001 |
| Prior stroke | 486 (28.4) | 621 (25.6) | 0.04 |
| Prior transient ischemic attack | 223 (13.0) | 404 (16.6) | 0.002 |
| History of dementia | 286 (16.7) | 204 (8.4) | <0.0001 |
| History of brain injury/tumor | 12 (0.7) | 15 (0.6) | 0.84 |
| History of smoking (cigarettes) | 949 (55.5) | 1400 (57.6) | 0.18 |
| Current smoker (cigarettes) | 407 (23.8) | 686 (28.2) | 0.002 |
| Alcohol use | 593 (34.7) | 1013 (41.7) | <0.0001 |
| Heavy alcohol use | 159 (9.3) | 212 (8.7) | 0.52 |
| White matter disease (any) | 1171 (68.5) | 1739 (71.6) | 0.03 |
Logistic Regression Model for Factors Related to Dysphagia Status
The variables that were considered for the logistic regression can be found in Figure 2. Variables that were included in the final model included age (per year increase), race (Black versus non‐Black), baseline mRS score (0–1 [functional] versus 2–5), NIHSS (per point increase), hemorrhage versus infarct, lesion on the right side, high cholesterol, coronary artery disease, presence of WMD, deprivation index, and year of stroke (2015 versus 2010). The logistic regression model (receiver operating characteristic=0.78; Figure 2) for dysphagia status revealed an increased risk of developing dysphagia after stroke for older individuals (age/1 year odds ratio [OR], 1.022; P<0.0001), people of Black race (OR, 1.225; P=0.04), cases with higher NIHSS score (per point increase: OR, 1.133; P<0.001), cases with a hemorrhagic stroke versus an ischemic infarct (OR, 1.472; P=0.0004), and having the stroke occur in the right hemisphere (OR, 1.269; P=0.0011). Factors showing decreased risk of dysphagia included having lower baseline mRS scores of 0 to 1 (OR, 0.657; P<0.0001), having high cholesterol (OR, 0.84; P=0.02), having WMD (new or old; OR, 0.801; P=0.01), and having been a case in 2015 (OR, 0.82; P=0.007).
Figure 2. Logistic regression by dysphagia status.
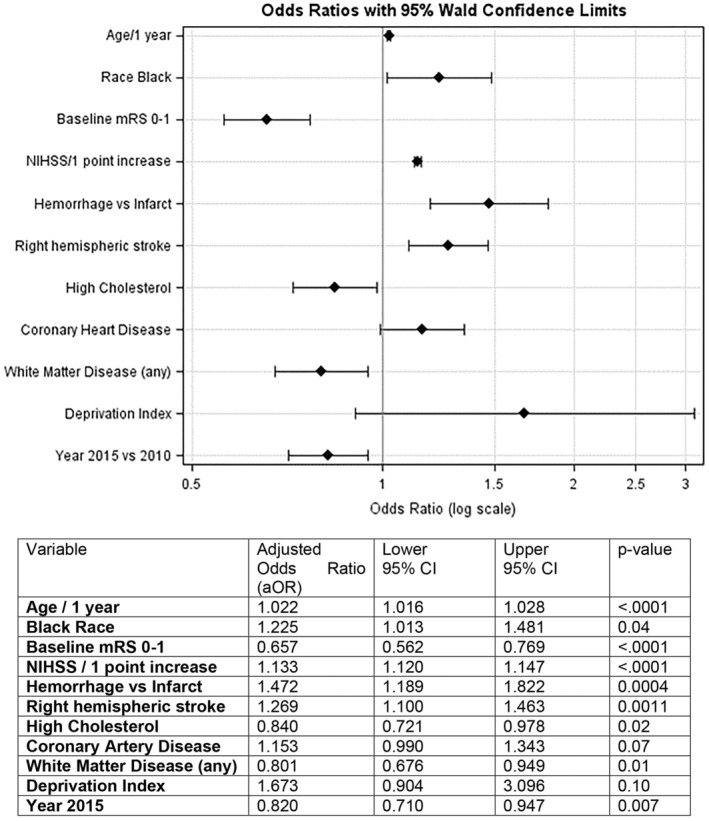
mRS indicates Modified Rankin Score; and NIHSS, National Institutes of Health Stroke Scale.
Discussion
In this large, population‐based study we sought to characterize differences in patient factors underlying dysphagia status for individuals after stroke and to determine which of these factors were associated with an increased risk of developing dysphagia following stroke.
Less than half of our final sample with positive dysphagia status required alternative access to nutrition via PEG or nasogastric tube (Table 1). We found a greater proportion of individuals with dysphagia compared with those with no dysphagia were older, were women, and had higher impairment scores upon stroke admission (ie, NIHSS, Glasgow Coma Scale) and prestroke mRS (Table 2). It has been well established that increased age results in age‐related changes to swallow movements and timing 25 , 26 , 27 , 28 , 29 that may predispose older individuals to developing swallowing impairments after stroke. Regarding our finding associating female sex with swallowing impairments after stroke, a recent study showed that women had more severe outcomes after ischemic stroke as compared with men, which could result in worse swallowing outcomes after stroke. 30 However, another study of 578 patients in Seoul, South Korea, found no relationship between swallowing outcomes after stroke and patient sex. 31 These alternative findings could be due to regional and cultural differences. Published evidence about influence of racial and ethnic background on stroke severity and outcome measures is mixed. 32 However, some literature suggests an ethnicity–age interaction, which could not be examined in these data due to a small number of Hispanic individuals in our study population, could be influential. Other evidence points to some impact of socioeconomic disparities among specific ethnic groups could be a contributing factor, 33 which is also supported by our data. Our data also showed that deprivation index scores were slightly higher in the dysphagia group as compared with the no‐dysphagia group; however, these were not found to be influential in the logistic regression (Figure 2). These discrepancies in the literature regarding the influence of sex and ethnicity in stroke‐related outcomes, in general and specific to swallowing, highlight that more work is needed to investigate biological drivers for these differences.
Interestingly, the model showed that individuals with strokes in the year 2015 were less likely to have dysphagia as compared with 2010. Although no formal data exist regarding specific practice patterns in dysphagia assessment and screening practices regionally or globally, available literature indicates an uptick in more bedside and instrumental evaluation of swallowing disorders in the acute‐stroke population between 2010 and 2015. 34 , 35 However, improved practices of identifying even mild dysphagia at the bedside would result in a larger dysphagia group, which would seemingly influence this model to predict higher risk of dysphagia in 2015. Given this, we could hypothesize that these findings suggest that advances in acute‐stroke care between 2010 and 2015 resulted in fewer severe strokes overall, with fewer individuals with resulting dysphagia in 2015 as compared with 2010. 36 , 37 This idea is supported by the breakdown of dysphagia severity categories across years showing that there was a greater proportion, overall, of individuals in 2015 as compared with 2010 who had dysphagia but did not require alternative access to nutrition (Table 1).
Lesion type differed between the dysphagia and no‐dysphagia groups, with a greater proportion of hemorrhagic and right hemisphere strokes in the dysphagia group, which is supported by the literature. 13 , 38 , 39 Similarly, our findings of more dysphagia occurring with increasing stroke severity (NIHSS, Glasgow Coma Scale), initial prestroke status as measured by the mRS, hemorrhagic stroke, and right hemisphere lesions are also consistent with prior literature. 13 , 39 , 40 , 41 , 42 , 43 , 44 There have been some other studies, with smaller samples, 45 , 46 , 47 questioning the influence of laterality in development of dysphagia, but our findings provide more evidence toward hemispheric location of lesion being a potential risk factor for developing swallowing impairments. Our findings for stroke subtype also demonstrated that there was a greater proportion of cardioembolic strokes in the dysphagia group with ischemic stroke. This could be related to the increased likelihood of cardioembolic infarcts resulting in hemorrhagic transformation, 48 and, as noted, hemorrhagic strokes may be more likely to present with dysphagia.
Regarding premorbid factors and development of dysphagia, we found that a greater proportion of individuals with a history of coronary artery disease, congestive heart failure, prior stroke, dementia, and hypertension in the dysphagia group as compared with the no‐dysphagia group. 49 , 50 Alcohol use, current cigarette smoking status, high cholesterol, and prior transient ischemic attack were also significantly different between the groups, with a higher proportion of individuals in the no‐dysphagia group. While prior studies do not directly study effects of alcohol consumption on development of dysphagia, alcohol use is known as risk factor for stroke. 51 , 52 However, studies have also shown that moderate alcohol use may reduce the risk of ischemic stroke 53 and that alcohol use does not affect stroke severity or outcomes after stroke. 54 These mixed results suggest that there may be a more complex relationship between alcohol consumption and stroke‐related recovery, including swallowing, that has yet to be fully discovered.
Prior literature clearly demonstrates that individuals with cardiac conditions and dysphagia have a more difficult recovery and often poorer outcomes. 55 , 56 However, it is difficult to determine whether these cardiac conditions made patients more susceptible to developing dysphagia after stroke simply because these individuals are more medically compromised or if there is a causal relationship between these conditions and swallowing impairments. In fact, our logistic regression results did not indicate increased risk of dysphagia development for patients with a history of coronary artery disease after accounting for other potential risk factors (Table 4), which may indicate the former hypothesis being more explanatory of these findings.
Other factors that were predictive of developing dysphagia after stroke included increased age, Black race, higher NIHSS score at presentation, having a hemorrhagic stroke (versus infarct), and right hemispheric stroke. Factors associated with decreased risk included patients having a history of high cholesterol, lower prestroke mRS, and any WMD detected on clinical imaging. Several findings were either surprising or are still unclear in their meaning. As discussed, literature on ethnic and racial disparities in stroke severity and poststroke care shows very mixed evidence and appears to be influenced by regional location or size of study. Prior published data have indicated that Black individuals have more severe strokes acutely than White individuals. 57 Current evidence provided in our study provides further emphasis of need for better understanding contributions of biological versus socioeconomic disparity to stroke‐related outcomes in Black patients versus patients of other races. 58
The findings regarding hyperlipidemia and any evidence of WMD on clinical stroke imaging being protective for risk of developing dysphagia after stroke were also unexpected. One hypothesis regarding the relationship between hyperlipidemia and lesser risk of dysphagia could be due to use of statin medications in this population. Literature supports the possibility that use of statins before stroke may reduce the severity of stroke. 59 One study specifically looking at effects of statin therapy and development of poststroke pneumonia suggested that patients who were using statins before admission for stroke and were treated with thrombolysis during admission were less likely to develop a pneumonia. 60 This finding should be explored more specifically in future studies of poststroke dysphagia development, as this could be a potential protective treatment for individuals at risk of both stroke and dysphagia. In terms of any evidence of WMD being protective, this could be an incidental finding driven by interdependency among the many factors explored in this study. Given that we were unable to measure new WMD in these patients at the time of clinical imaging (ie, differentiate new WMD from existing WMD before acute admission), it is difficult to tease out the meaning of this particular finding. The design of future studies of WMD and dysphagia could potentially include a cohort of patients with repeat stroke with available prior clinical imaging in an attempt to better understand the relationship between existing and new WMD in poststroke dysphagia.
Limitations
The primary limitations of this study are due to the nature of retrospective analysis of data collected as part of a large data set; therefore, several broad and more nuanced factors were not available for use in our data analysis for both epidemiological factors and patient medical history. Most notably, this included the type of medical professional completing dysphagia evaluation or screening (eg, speech–language pathologist or nursing professional). Additionally, given that this is a multi‐institutional study, practices in poststroke dysphagia screening and assessment may differ. However, to account for this variability we only included patients in the final analysis that had some sort of documented dysphagia testing or evaluation. Another limitation related to the retrospective nature of the data set is that we are unable to verify how patient demographic factors related to race and ethnicity were determined (ie, self‐report versus determination by a medical professional). These factors may have impacted classification of patients within certain groupings in our analysis. Further, the inability to differentiate new versus old WMD limits our ability to fully interpret the role of WMD in development of dysphagia after stroke.
Conclusions
This large, population‐based study confirms several factors that have been frequently demonstrated in the literature to be associated with developing dysphagia after stroke, including older age, dementia status, more severe stroke, and lesion type and location. However, other factors emerged that are not clear in their biological meaning or association, particularly findings related to certain races and ethnicities being significant factors for dysphagia development after stroke. These and other factors indicating a reduced risk (ie, hyperlipidemia and any presence of WMD) should be considered in future prospective work to better understand these relationships and how these conditions may affect acute stroke recovery in the setting of dysphagia.
Sources of Funding
This work was supported by the National Institute of Neurologic Disorders and Stroke (R01NS030678).
Disclosures
Dr Krekeler reports research support from the National Institutes of Health(NIH)/National Center for Medical Rehabilitation Research/Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr Khoury reports research support from the NIH/National Institute of Neurological Disorders and Stroke (NINDS). M. Haverbusch reports research support from the NIH/NINDS. Dr Adeoye reports research support from the NIH/NINDS and is an equity holder and cofounder of Sense Diagnostics, Inc. Dr Mackey reports research support from the NIH/NINDS. Dr Woo reports research support from the NIH/NINDS. Dr Flaherty reports the following: speaker's bureau for CSL Behring and Alexion; cofounder, employee, and equity holder of Sense Diagnostics, Inc.; and clinical trial end point adjudication committee member for Boehringer Ingelheim and LG Chem. Dr Walsh reports the following: AstraZeneca Speaker's Bureau and grant funding/research support from the American Heart Association, NIH, Sense Diagnostics LLC, and Jan Medical Inc. Dr Mistry reports research support from the NIH/NINDS. Dr Kissela reports research support from the NIH/NINDS. The remaining authors have no disclosures to report.
This manuscript was sent to Kori S. Zachrison, MD, MSc, Associate Editor, for review by expert referees, editorial decision, and final disposition.
Preprint posted on MedRxiv August 31, 2023. doi: https://doi.org/10.1101/2023.08.29.23294807.
For Sources of Funding and Disclosures, see page 9.
References
- 1. Crary MA, Humphrey JL, Carnaby‐Mann G, Sambandam R, Miller L, Silliman S. Dysphagia, nutrition, and hydration in ischemic stroke patients at admission and discharge from acute care. Dysphagia. 2013;28:69–76. doi: 10.1007/s00455-012-9414-0 [DOI] [PubMed] [Google Scholar]
- 2. Ojo O, Brooke J. The use of enteral nutrition in the management of stroke. Nutrients. 2016;8:827. doi: 10.3390/nu8120827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martino R, Foley N, Bhogal S, Diamant N, Speechley M, Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36:2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb [DOI] [PubMed] [Google Scholar]
- 4. Perry L, Love CP. Screening for dysphagia and aspiration in acute stroke: a systematic review. Dysphagia. 2001;16:7–18. doi: 10.1007/PL00021290 [DOI] [PubMed] [Google Scholar]
- 5. Eslick GD, Talley N. Dysphagia: epidemiology, risk factors and impact on quality of life–a population‐based study. Aliment Pharmacol Ther. 2008;27:971–979. doi: 10.1111/j.1365-2036.2008.03664.x [DOI] [PubMed] [Google Scholar]
- 6. Humbert IA, Robbins J. Dysphagia in the elderly. Phys Med Rehabil Clin N Am. 2008;19:853–866. doi: 10.1016/j.pmr.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Altman KW, Yu G‐P, Schaefer SD. Consequence of dysphagia in the hospitalized patient: impact on prognosis and hospital resources. Arch Otolaryngol Head Neck Surg. 2010;136:784–789. doi: 10.1001/archoto.2010.129 [DOI] [PubMed] [Google Scholar]
- 8. Cabre M, Serra‐Prat M, Palomera E, Almirall J, Pallares R, Clavé P. Prevalence and prognostic implications of dysphagia in elderly patients with pneumonia. Age Ageing. 2010;39:39–45. doi: 10.1093/ageing/afp100 [DOI] [PubMed] [Google Scholar]
- 9. Cichero JA, Altman KW. Definition, prevalence and burden of oropharyngeal dysphagia: a serious problem among older adults worldwide and the impact on prognosis and hospital resources. Stepping Stones to Living Well with Dysphagia. Karger Publishers; 2012:1–11. doi: 10.1159/000339974 [DOI] [PubMed] [Google Scholar]
- 10. Cohen DL, Roffe C, Beavan J, Blackett B, Fairfield CA, Hamdy S, Havard D, McFarlane M, McLauglin C, Randall M. Post‐stroke dysphagia: a review and design considerations for future trials. Int J Stroke. 2016;11:399–411. doi: 10.1177/1747493016639057 [DOI] [PubMed] [Google Scholar]
- 11. Wilmskoetter J, Bonilha L, Martin‐Harris B, Elm JJ, Horn J, Bonilha HS. Mapping acute lesion locations to physiological swallow impairments after stroke. Neuroimage Clin. 2019;22:101685. doi: 10.1016/j.nicl.2019.101685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilmskoetter J, Martin‐Harris B, Pearson WG Jr, Bonilha L, Elm JJ, Horn J, Bonilha HS. Differences in swallow physiology in patients with left and right hemispheric strokes. Physiol Behav. 2018;194:144–152. doi: 10.1016/j.physbeh.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Daniels SK, Pathak S, Mukhi SV, Stach CB, Morgan RO, Anderson JA. The relationship between lesion localization and dysphagia in acute stroke. Dysphagia. 2017;32:777–784. doi: 10.1007/s00455-017-9824-0 [DOI] [PubMed] [Google Scholar]
- 14. Mann G, Hankey GJ, Cameron D. Swallowing function after stroke: prognosis and prognostic factors at 6 months. Stroke. 1999;30:744–748. doi: 10.1161/01.STR.30.4.744 [DOI] [PubMed] [Google Scholar]
- 15. Kunitz SJ. Sex, race and social role—history and the social determinants of health. Int J Epidemiol. 2006;36:3–10. doi: 10.1093/ije/dyl296 [DOI] [PubMed] [Google Scholar]
- 16. Office of Disease Prevention and Health Promotion . (n.d.). Social determinants of health. Healthy People 2030. U.S. Department of Health and Human Services. Accessed [December, 2022]. https://health.gov/healthypeople/objectives‐and‐data/social‐determinants‐health
- 17. Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, Gebel J, Mills D, Minneci L, Shukla R. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first‐ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.STR.29.2.415 [DOI] [PubMed] [Google Scholar]
- 18. Zakaria T, Lindsell CJ, Kleindorfer D, Alwell K, Moomaw CJ, Woo D, Szaflarski JP, Khoury J, Miller R, Broderick JP. Age accounts for racial differences in ischemic stroke volume in a population‐based study. Cerebrovasc Dis. 2008;26:376–380. doi: 10.1159/000151641 [DOI] [PubMed] [Google Scholar]
- 19. Kissela B, Broderick J, Woo D, Kothari R, Miller R, Khoury J, Brott T, Pancioli A, Jauch E, Gebel J. Greater Cincinnati/Northern Kentucky Stroke Study: volume of first‐ever ischemic stroke among blacks in a population‐based study. Stroke. 2001;32:1285–1290. doi: 10.1161/01.STR.32.6.1285 [DOI] [PubMed] [Google Scholar]
- 20. Madsen TE, Khoury JC, Leppert M, Alwell K, Moomaw CJ, Sucharew H, Woo D, Ferioli S, Martini S, Adeoye O. Temporal trends in stroke incidence over time by sex and age in the GCNKSS. Stroke. 2020;51:1070–1076. doi: 10.1161/STROKEAHA.120.028910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Madsen TE, Cummings OW, De Los Rios La Rosa F, Khoury JC, Alwell K, Woo D, Ferioli S, Martini S, Adeoye O, Khatri P. Substance use and performance of toxicology screens in the Greater Cincinnati Northern Kentucky Stroke Study. Stroke. 2022;53:3082–3090. doi: 10.1161/STROKEAHA.121.038311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen BJ, Suolang D, Frost N, Faigle R. Practice patterns and attitudes among speech–language pathologists treating stroke patients with dysphagia: a nationwide survey. Dysphagia. 2022;37:1715–1722. doi: 10.1007/s00455-022-10432-6 [DOI] [PubMed] [Google Scholar]
- 23. Fairfield CAG, Smithard D. Assessment and management of dysphagia in acute stroke: an initial service review of international practice. Geriatrics. 2020;5:4. doi: 10.3390/geriatrics5010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brokamp C, Beck AF, Goyal NK, Ryan P, Greenberg JM, Hall ES. Material community deprivation and hospital utilization during the first year of life: an urban population–based cohort study. Ann Epidemiol. 2019;30:37–43. doi: 10.1016/j.annepidem.2018.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Robbins J, Humpal NS, Banaszynski K, Hind J, Rogus‐Pulia N. Age‐related differences in pressures generated during isometric presses and swallows by healthy adults. Dysphagia. 2016;31:90–96. doi: 10.1007/s00455-015-9662-x [DOI] [PubMed] [Google Scholar]
- 26. Azzolino D, Damanti S, Bertagnoli L, Lucchi T, Cesari M. Sarcopenia and swallowing disorders in older people. Aging Clin Exp Res. 2019;31:799–805. doi: 10.1007/s40520-019-01128-3 [DOI] [PubMed] [Google Scholar]
- 27. Wakabayashi H. Presbyphagia and sarcopenic dysphagia: association between aging, sarcopenia, and deglutition disorders. J Frailty Aging. 2014;3:97–103. doi: 10.14283/jfa.2014.8 [DOI] [PubMed] [Google Scholar]
- 28. Robbins J, Levine R, Wood J, Roecker EB, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. J Gerontol A Biol Sci Med Sci. 1995;50:M257–M262. doi: 10.1093/gerona/50A.5.M257 [DOI] [PubMed] [Google Scholar]
- 29. Robbins JA. Normal Swallowing and Aging. Seminars in Neurology. 1996; 16(4):309–317. [DOI] [PubMed] [Google Scholar]
- 30. Phan HT, Reeves MJ, Blizzard CL, Thrift AG, Cadilhac DA, Sturm J, Otahal P, Rothwell P, Bejot Y, Cabral NL. Sex differences in severity of stroke in the INSTRUCT study: a meta‐analysis of individual participant data. J Am Heart Assoc. 2019;8:e010235. doi: 10.1161/JAHA.118.010235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim J, Oh B‐M, Lee GJ, Lee SA, Chun SW, Han TR. Clinical factors associated with severity of post‐stroke dysphagia. Brain Neurorehabil. 2011;4:116–120. doi: 10.12786/bn.2011.4.2.116 [DOI] [Google Scholar]
- 32. Ellis C, Hyacinth HI, Beckett J, Feng W, Chimowitz M, Ovbiagele B, Lackland D, Adams R. Racial/ethnic differences in poststroke rehabilitation outcomes. Stroke Res Treat. 2014;2014:950746. doi: 10.1155/2014/950746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36:374–386. doi: 10.1161/01.STR.0000153065.39325.fd [DOI] [PubMed] [Google Scholar]
- 34. O'Horo JC, Rogus‐Pulia N, Garcia‐Arguello L, Robbins J, Safdar N. Bedside diagnosis of dysphagia: a systematic review. J Hosp Med. 2015;10:256–265. doi: 10.1002/jhm.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kertscher B, Speyer R, Palmieri M, Plant C. Bedside screening to detect oropharyngeal dysphagia in patients with neurological disorders: an updated systematic review. Dysphagia. 2014;29:204–212. doi: 10.1007/s00455-013-9490-9 [DOI] [PubMed] [Google Scholar]
- 36. Chang P, Prabhakaran S. Recent advances in the management of acute ischemic stroke. F1000Res. 2017;6:F1000 Faculty Rev‐484. doi: 10.12688/f1000research.9191.1 [DOI] [Google Scholar]
- 37. Robbins BT, Howington GT, Swafford K, Zummer J, Woolum JA. Advancements in the management of acute ischemic stroke: a narrative review. J Am Coll Emerg Physicians Open. 2023;4:e12896. doi: 10.1002/emp2.12896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paciaroni M, Mazzotta G, Corea F, Caso V, Venti M, Milia P, Silvestrelli G, Palmerini F, Parnetti L, Gallai V. Dysphagia following stroke. Eur Neurol. 2004;51:162–167. doi: 10.1159/000077663 [DOI] [PubMed] [Google Scholar]
- 39. Suntrup S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, Heindel W, Wiendl H, Dziewas R. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 1: dysphagia incidence, severity and aspiration. Eur J Neurol. 2015;22:832–838. doi: 10.1111/ene.12670 [DOI] [PubMed] [Google Scholar]
- 40. Henke C, Foerch C, Lapa S. Early screening parameters for dysphagia in acute ischemic stroke. Cerebrovasc Dis. 2017;44:285–290. doi: 10.1159/000480123 [DOI] [PubMed] [Google Scholar]
- 41. Jeyaseelan RD, Vargo MM, Chae J. National Institutes of Health Stroke Scale (NIHSS) as an early predictor of poststroke dysphagia. PM R. 2015;7:593–598. doi: 10.1016/j.pmrj.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 42. Crary MA, Carnaby‐Mann GD, Miller L, Antonios N, Silliman S. Dysphagia and nutritional status at the time of hospital admission for ischemic stroke. J Stroke Cerebrovasc Dis. 2006;15:164–171. doi: 10.1016/j.jstrokecerebrovasdis.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 43. Khedr EM, Abbass MA, Soliman RK, Zaki AF, Gamea A. Post‐stroke dysphagia: frequency, risk factors, and topographic representation: hospital‐based study. Egypt J Neurol Psychiatr Neurosurg. 2021;57:1–8. doi: 10.1186/s41983-021-00281-9 [DOI] [Google Scholar]
- 44. Robbins J, Levine RL, Maser A, Rosenbek JC, Kempster GB. Swallowing after unilateral stroke of the cerebral cortex. Arch Phys Med Rehabil. 1993;74:1295–1300. doi: 10.1016/0003-9993(93)90082-L [DOI] [PubMed] [Google Scholar]
- 45. Fernández‐Pombo A, Seijo‐Raposo IM, López‐Osorio N, Cantón‐Blanco A, González‐Rodríguez M, Arias‐Rivas S, Rodríguez‐Yáñez M, Santamaría‐Nieto A, Díaz‐Ortega C, Gómez‐Vázquez E, et al. Lesion location and other predictive factors of dysphagia and its complications in acute stroke. Clin Nutr ESPEN. 2019;33:178–182. doi: 10.1016/j.clnesp.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 46. Suntrup‐Krueger S, Kemmling A, Warnecke T, Hamacher C, Oelenberg S, Niederstadt T, Heindel W, Wiendl H, Dziewas R. The impact of lesion location on dysphagia incidence, pattern and complications in acute stroke. Part 2: oropharyngeal residue, swallow and cough response, and pneumonia. Eur J Neurol. 2017;24:867–874. doi: 10.1111/ene.13307 [DOI] [PubMed] [Google Scholar]
- 47. Sharma J, Fletcher S, Vassallo M, Ross I. Prognostic value of CT scan features in acute ischaemic stroke and relationship with clinical stroke syndromes. Int J Clin Pract. 2000;54:514–518. doi: 10.1111/j.1742-1241.2000.tb10914.x [DOI] [PubMed] [Google Scholar]
- 48. Hornig CR, Bauer T, Simon C, Trittmacher S, Dorndorf W. Hemorrhagic transformation in cardioembolic cerebral infarction. Stroke. 1993;24:465–468. doi: 10.1161/01.STR.24.3.465 [DOI] [PubMed] [Google Scholar]
- 49. Easterling CS, Robbins E. Dementia and dysphagia. Geriatr Nurs. 2008;29:275–285. doi: 10.1016/j.gerinurse.2007.10.015 [DOI] [PubMed] [Google Scholar]
- 50. Smithard DG, O'Neill PA, England RE, Park CL, Wyatt R, Martin DF, Morris J. The natural history of dysphagia following a stroke. Dysphagia. 1997;12:188–193. doi: 10.1007/PL00009535 [DOI] [PubMed] [Google Scholar]
- 51. Shiotsuki H, Saijo Y, Ogushi Y, Kobayashi S. Relationship between alcohol intake and stroke severity in Japanese patients: a sex‐and subtype‐stratified analysis. J Stroke Cerebrovasc Dis. 2022;31:106513. doi: 10.1016/j.jstrokecerebrovasdis.2022.106513 [DOI] [PubMed] [Google Scholar]
- 52. O'Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, Rao‐Melacini P, Rangarajan S, Islam S, Pais P, McQueen MJ, et al; INTERSTROKE investigators. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case‐control study. Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3 [DOI] [PubMed] [Google Scholar]
- 53. Elkind MS, Sciacca R, Boden‐Albala B, Rundek T, Paik MC, Sacco RL. Moderate alcohol consumption reduces risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2006;37:13–19. doi: 10.1161/01.STR.0000195048.86810.5b [DOI] [PubMed] [Google Scholar]
- 54. Fekete K, Szatmári S, Szőcs I, Szekeres C, Szász J, Mihálka L, Smolanka V, Kardos L, Csiba L, Bereczki D. Prestroke alcohol consumption and smoking are not associated with stroke severity, disability at discharge, and case fatality. J Stroke Cerebrovasc Dis. 2014;23:e31–e37. doi: 10.1016/j.jstrokecerebrovasdis.2013.08.006 [DOI] [PubMed] [Google Scholar]
- 55. Matsuo H, Yoshimura Y, Fujita S, Maeno Y. Dysphagia is associated with poor physical function in patients with acute heart failure: a prospective cohort study. Aging Clin Exp Res. 2020;32:1093–1099. doi: 10.1007/s40520-019-01287-3 [DOI] [PubMed] [Google Scholar]
- 56. Matsuo H, Yoshimura Y, Fujita S, Maeno Y. Incidence of dysphagia and its association with functional recovery and 1‐year mortality in hospitalized older patients with heart failure: a prospective cohort study. J Parenter Enteral Nutr. 2021;45:372–380. doi: 10.1002/jpen.1845 [DOI] [PubMed] [Google Scholar]
- 57. Jones MR, Horner RD, Edwards LJ, Hoff J, Armstrong SB, Smith‐Hammond CA, Matchar DB, Oddone EZ. Racial variation in initial stroke severity. Stroke. 2000;31:563–567. doi: 10.1161/01.STR.31.3.563 [DOI] [PubMed] [Google Scholar]
- 58. Howard VJ. Reasons underlying racial differences in stroke incidence and mortality. Stroke. 2013;44:S126–S128. doi: 10.1161/STROKEAHA.111.000691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hong K‐S, Lee JS. Statins in acute ischemic stroke: a systematic review. J Stroke. 2015;17:282–301. doi: 10.5853/jos.2015.17.3.282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Scheitz JF, Endres M, Heuschmann PU, Audebert HJ, Nolte CH. Reduced risk of poststroke pneumonia in thrombolyzed stroke patients with continued statin treatment. Int J Stroke. 2015;10:61–66. doi: 10.1111/j.1747-4949.2012.00864.x [DOI] [PubMed] [Google Scholar]


