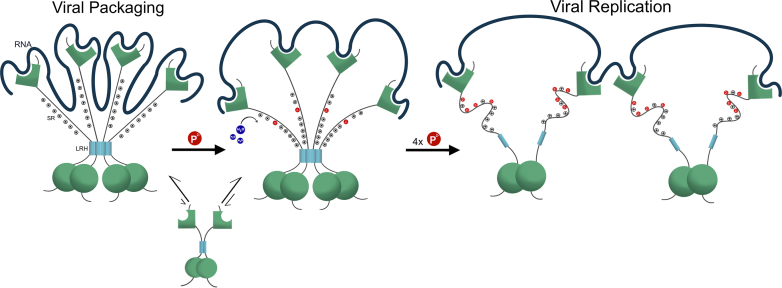
Figure 6.
Proposed model of how phosphorylation acts as a molecular switch for the role of N from viral packaging to viral replication. FL-N is depicted here using similar illustration as in Figure 1C. (Left) Unphosphorylated FL-N is in a dimer-tetramer equilibrium which shifts to tetramer when bound to RNA. The positive charges in the SR-rich region cause elongation due to charge–charge repulsion. RNA intercalates by binding to the SR region of the linker, resulting in a compacted RNA expected to be most populated in LLPS and in viral packaging. (Middle) A single phosphorylation event (red) introduces negative charges in the SR causing some structural change. RNA remains bound to the NTD but does not bind the linker resulting in reduced compaction. (Right) Four phosphorylation events with GSK-3 causing significant structural changes in the SR-region of GSK hyperphosphorylated FL-N and dissociation of the 4-helix bundle tetramers. The resulting structure of FL-N is a dimer with significant flexibility in the linker after dissociation of the LRH causing significant dispersing of the RNA, consistent with the model expected in viral replication. Two N proteins are shown to illustrate multivalent binding. Phosphates are indicated by red circles, and arginines by circled pluses. RNA is depicted by the dark blue line. FL-N, full length N protein; GSK-3, glycogen synthase kinase 3β; NTD, N-terminal domain; SR-rich, serine/arginine-rich.