Table 2 ∣.
Bispecific antibodies approved or nearing approval by the FDA and EMA
| Target | Antibody | Structure | Format | Major indications | Adverse effects |
|---|---|---|---|---|---|
| T cell engagers | |||||
| CD19xCD3 | Blinatumomab |
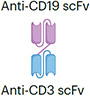
|
Lacks Fc (short half-life ~2 h) ~54 kDa |
B cell precursor ALL | CRS Neurotoxicity Infection Cytopenia TLS |
| CD20xCD3 | Mosunetuzumab |
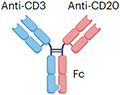
|
IgG1 Fc (half-life extension) N297G mutation (aglycosylation and reduced ADCC) ~146 kDa |
Lymphoma | CRS Neurotoxicity Infection Cytopenia Embryo–fetal toxicity |
| Epcoritamab |
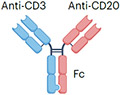
|
IgG1 Fc (half-life extension) L234F, L235E, D265A mutation (reduced ADCC) ~146 kDa |
Lymphoma | CRS Neurotoxicity Infection Cytopenia TLS |
|
| Glofitamab |
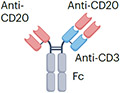
|
Two anti-CD20 scFvs linked with one anti-CD3 scFv IgG1 Fc (half-life extension) P329G, L234A, L235A mutation (reduced ADCC) ~194 kDa |
Lymphoma | CRS Neurotoxicity Infection Cytopenia |
|
| Imvotamab (in clinical trial) |
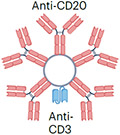
|
IgM pentamer (ten CD20-binding sites) Albumin fusion (half-life extension) ~960 kDa |
Lymphoma | CRS Cytopenia Hypophosphatemia |
|
| BCMAxCD3 | Teclistamab |
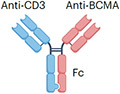
|
IgG4 Fc (half-life extension) S228P (hinge stabilization), and F234A and L235A mutation (reduced FcγR binding) ~143 kDa |
Multiple myeloma | CRS Neurotoxicity Infection Cytopenia Hepatotoxicity Embryo–fetal toxicity |
| Elranatamab |
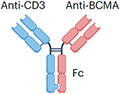
|
IgG2 Fc (half-life extension) ~145 kDa |
Multiple myeloma | CRS Neurotoxicity Cytopenia |
|
| GPRC5DxCD3 | Talquetamab |
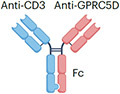
|
IgG4 Fc (half-life extension) S228P (hinge stabilization), and F234A and L235A mutation (reduced FcγR binding) ~147 kDa |
Multiple myeloma | CRS Neurotoxicity Cytopenia Skin rash Hepatotoxicity Embryo–fetal toxicity |
| GP100xCD3 | Tebentafusp |

|
Lacks Fc, MW above renal filtration cut-off (half-life 6–8 h) ~75–77 kDa |
Melanoma | CRS Skin rash Hepatotoxicity Embryo–fetal toxicity |
| Receptor blockers | |||||
| EGFRxcMET | Amivantamab |
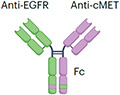
|
IgG1 (half-life extension) Fc afucosylation (enhanced ADCC) K409R, F405L mutation (for Fab exchange) ~146 kDa |
Lung cancer | Skin rash Stomatitis Muscle pain Cytopenia Electrolyte abnormality Embryo–fetal toxicity |
| Her2xHer2 | Zanidatamab (in clinical trial) |
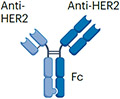
|
Two anti-HER2 scFvs (bind two distinct HER2 epitopes) IgG1 (half-life extension) ~125 kDa |
HER2+ cancers | Diarrhoea Infusion reaction Cardiac failure |
| PD1xCTLA4 | Volrustomig MEDI5752 (in clinical trial) |
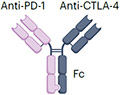
|
IgG1 (half-life extension) L234F, L235E, P331S mutation (reduced FcγR binding) ~145 kDa (estimated) |
Multiple solid tumours | Checkpoint inhibitor-associated irAEs: Diarrhoea Thyroid disorders Skin rash Hepatotoxicity |
ADCC, antibody-dependent cellular cytotoxicity; ALL, acute lymphoblastic leukaemia; CRS, cytokine release syndrome; Ig, immunoglobulin; irAEs, immune-related adverse events; IV, intravenous; kDa, kilodalton; scFv, single-chain variable fragment; TLS, tumour lysis syndrome. Fc-bearing bispecific antibodies also carry Fc mutations that enable pairing of the heterogenous heavy chains (not shown).
