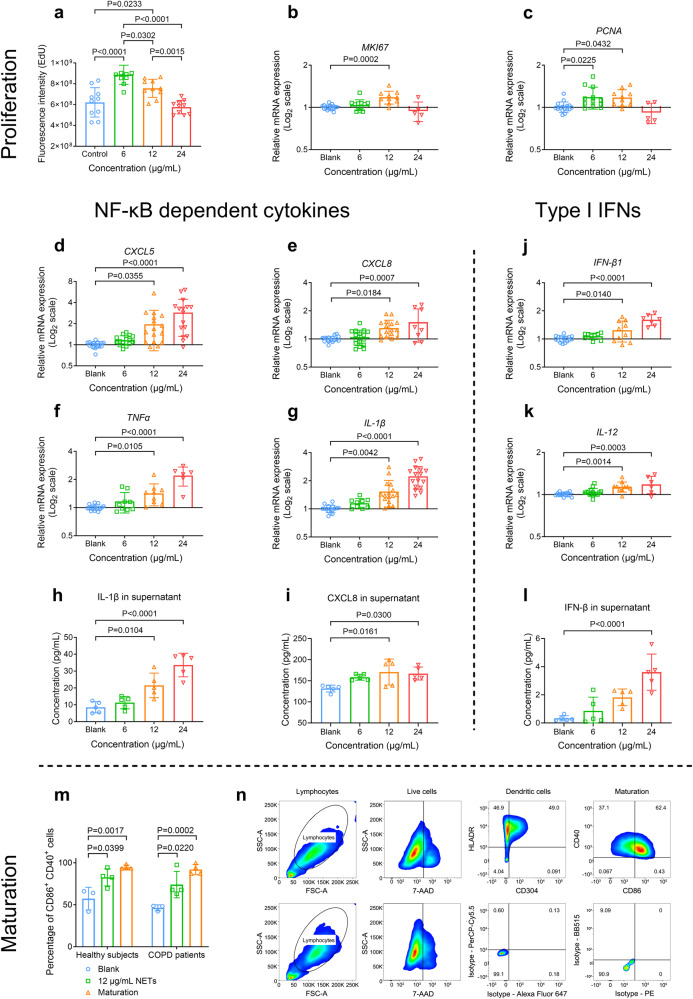
Fig. 3.
Neutrophil extracellular traps (NETs) induced by cigarette smoke extract (CSE-NETs) dose-dependently promote the proliferation and the gene expressions of both nuclear factor kappa B (NF-κB)-dependent inflammatory cytokines and type-I interferons (IFNs) in the human airway epithelial cells (hAECs); CSE-NETs promote the maturation of human dendritic cells (DCs). Statistical analysis: n = 3–16 for each bar in (a–m) from at least three independent experiments, data were presented as the mean ± standard deviation; Differences are assessed by the (a–l) one-way or (m) two-way ANOVA analysis of variance, followed Tukey’s honest significant test; P < 0.05 represents a significant difference, the scattered samples and the p values are displayed in figures. a–c Human peripheral neutrophils were stimulated by 5% CSE for 18 h to induce NETs, which were subsequently collected and quantified for DNA concentrations. Effects of 6, 12, and 24 μg/mL NETs (incubated for 48 h) on the proliferation of hAECs, as assessed by the a EdU proliferation assay (supplementary Method 11) and mRNA expressions of b MKI67 and c PCNA (both are markers of proliferation, supplementary Method 13). d–l Effects of 6, 12, and 24 μg/mL NETs on the mRNA expressions and soluble levels of NF-κB-dependent inflammatory cytokines and type-I IFNs of hAECs (supplementary Method 13, 26): d mRNA expression of CXCL5 (C-X-C motif chemokine ligand 5), e mRNA expression of CXCL8, f mRNA expression of TNFα (tumour necrosis factor-alpha), g mRNA expression of IL-1β (interleukin 1β), h soluble levels of IL-1β in cell-culture supernatants, i soluble levels of CXCL8 in cell-culture supernatants, j mRNA expression of IFN-β1, k mRNA expression of IL-12 and l soluble levels of IFN-β in cell-culture supernatants. m, n 12 μg/mL of NETs promote the maturation of dendritic cells (differentiated from peripheral blood monocytes) from both healthy participants and patients with COPD (supplementary Method 15); the maturation is evaluated by m the percentage of CD86+ CD40+ cells, as assessed by flow cytometry with a gating strategy illustrated in (n) (Supplementary Method 17)