Figure 2. STM2457 treatment decreases neuroblastoma cell proliferation and global levels of m6A in vitro and impairs the growth of neuroblastoma xenografts in vivo.
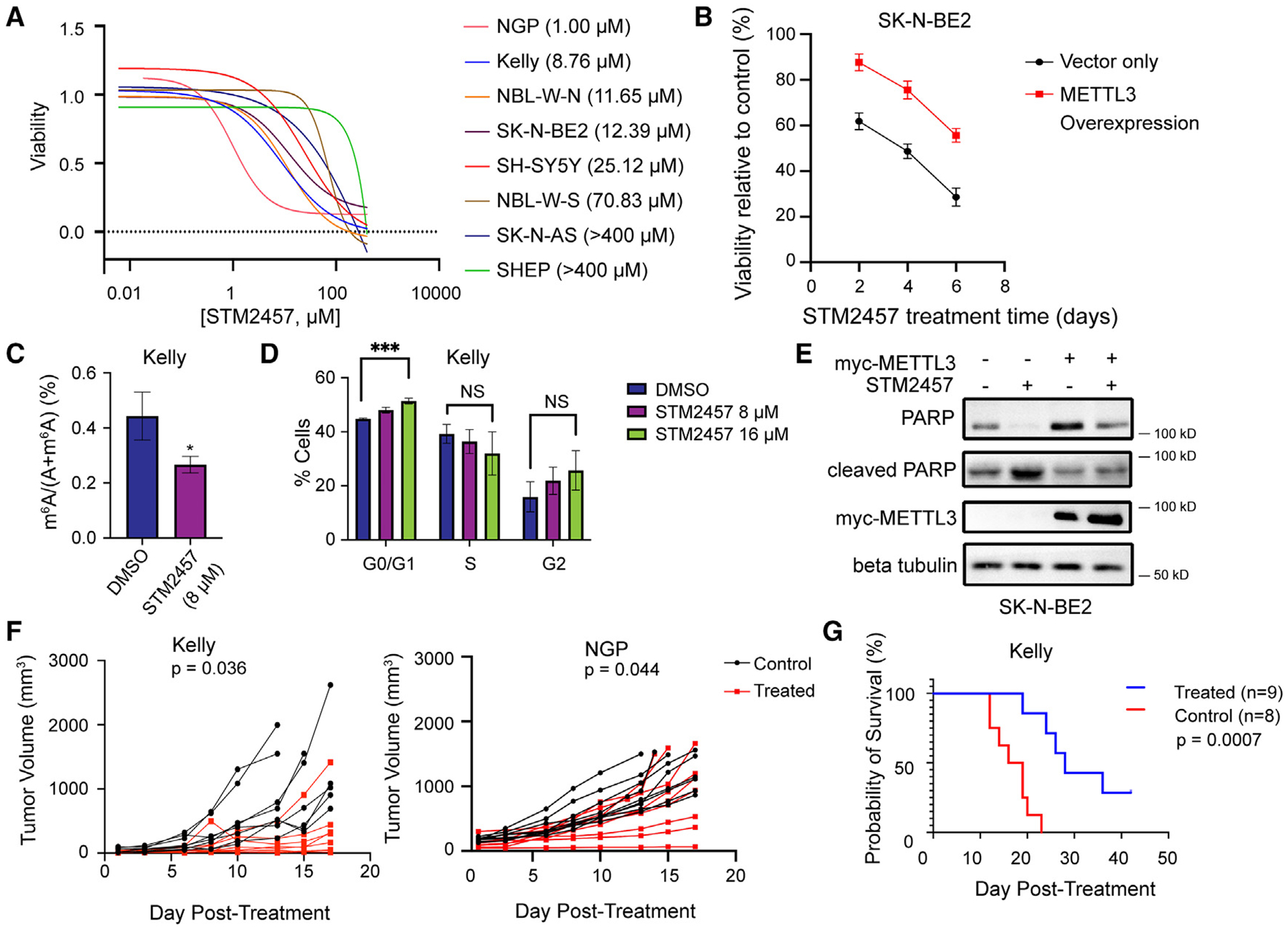
(A) STM2457 dose-response curves for 8 neuroblastoma cell lines (mean curve of 3 biological replicates).
(B) The viability of SK-N-BE2 cells with overexpression of METTL3 treated with 16 μM STM2457 is higher relative to control cells (n = 3, p < 0.05, unpaired t test).
(C) Quantitative m6A analysis by LC-MS QQQ shows that global m6A levels are significantly decreased in Kelly cells treated with 8 μM STM2457 compared to cells treated with DMSO control (n = 3, p < 0.05, unpaired t test).
(D) Cell cycle analysis shows that treatment with 16 μM STM2457 increases the distribution of Kelly cells in G0/G1 compared to DMSO (n = 3, p < 0.001, unpaired t test).
(E) Western blot analysis shows that cleaved PARP expression is decreased in SK-N-BE2 cells with METTL3 overexpression treated with 16 μM STM2457 compared to control cells treated with STM2457.
(F) STM2457 treatment (50 mg/kg/day × 14 days) suppresses the growth of subcutaneous neuroblastoma xenografts (mm3) comprised of Kelly cells (p = 0.036, unpaired t test) or NGP cells (p = 0.044) compared to vehicle control.
(G) STM2457 treatment improves overall survival for mice compared to vehicle control in neuroblastoma xenografts comprised of Kelly cells (p = 0.0007, log-rank test).
Bar graphs show mean and SD of data from 3 independent experiments.
