Abstract
BACKGROUND
Population-level data are limited regarding contemporary practice and outcomes of isolated tricuspid operations. We evaluated this using The Society of Thoracic Surgeons Adult Cardiac Surgery Database.
METHODS
We identified 14,704 isolated tricuspid operations from The Society of Thoracic Surgeons Adult Cardiac Surgery Database from July 1, 2011 to June 30, 2020. After excluding patients with endocarditis, tricuspid stenosis, emergent/emergent salvage status, previous heart transplants, and missing tricuspid operation type, 6507 patients remained. End-points were operative mortality and composite major comorbidities (permanent stroke, renal failure, prolonged ventilation > 24 hours, deep sternal wound infection, cardiac reoperations, and new permanent pacemaker implantation).
RESULTS
Isolated tricuspid operations increased from 2012 (983 cases) to 2019 (2155 cases, P < .001). Median annual center volume was 2 cases (range, 1-81). In the final cohort (n = 6507; median age, 65 years; 38.5% men), 40% had New York Heart Association class III/IV heart failure and 24% had nonelective operations. The operative mortality was 7.3% (1.7% in patients without these risk factors), and new permanent pacemaker implant rate was 10.8%. In the multivariable analysis, factors associated with operative mortality included New York Heart Association class III/IV heart failure (odds ratio [OR], 1.57), nonelective operations (OR, 1.91), tricuspid replacement (OR, 1.56), annual center volume ≤ 5 cases (OR, 1.37), and higher model for end-stage liver disease scores (all P < .05). Beating heart operation was associated with a lower adjusted risk of pacemaker implant (OR, 0.69), renal failure (OR, 0.75), and blood transfusions (OR, 0.8) compared with full cardioplegic arrest (all P < .05).
CONCLUSIONS
Isolated tricuspid repair was associated with lower adjusted mortality and morbidities than replacement. Beating heart operation was associated with lower adjusted major morbidities. The preoperative model for end-stage liver disease scores may identify high-risk patients, and early referral to higher volume centers may help improve outcomes.
Tricuspid valve operation is historically associated with higher morbidity and mortality than mitral and aortic valve operations. Despite the increased procedural volume, outcomes have not significantly changed over the last decade.1,2 Most tricuspid operations are performed with concomitant left-sided valve operations, and isolated tricuspid operations make up only 14% to 20%.2,3 Patients with isolated tricuspid disease represent a heterogeneous population, and perioperative mortality for those undergoing operations has ranged from 3% to 11%.4-6
Existing guidelines for isolated tricuspid operations are generally conservative,7,8 based on expert opinion and limited data, and reflect the historically poor outcomes in the context of late referral, advanced heart failure symptoms, and right ventricular dysfunction. Single-center series of isolated tricuspid operations are limited by small sample sizes, often combining decades of operative practices, and therefore do not reflect contemporary practice.9-11 In addition insufficient clinical granularity limits analyses of large administrative claims databases.4,5,12 Therefore, we examined The Society of Thoracic Surgeons Adult Cardiac Surgery Database (STS-ACSD) to evaluate contemporary patient characteristics, operative approaches, and clinical outcomes of isolated tricuspid operations, with a focus on patients with nonendocarditis-related tricuspid regurgitation (TR).
PATIENTS AND METHODS
DATA SOURCE.
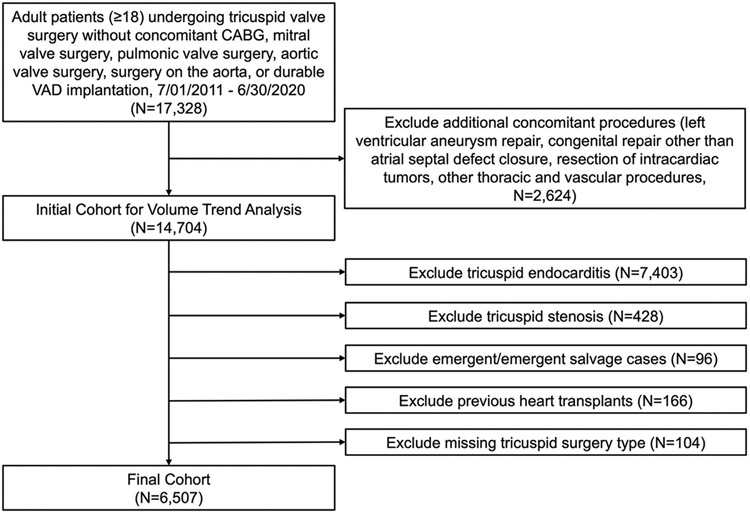
Adult patients undergoing tricuspid operations from July 1, 2011 to June 30, 2020 without concomitant coronary artery bypass grafting, other heart valve operations, aortic operations, or durable ventricular-assist device implantation were identified from the STS-ACSD, a repository for more than 7 million records encompassing data from 1030 participant groups.13 Exclusion criteria included additional concomitant operations (Figure 1). This created an initial cohort of 14,704 isolated tricuspid operations to analyze overall volume trends. Next, patients with endocarditis (identified as having active endocarditis, endocarditis as the etiology for tricuspid disease, or tricuspid valvectomy), tricuspid stenosis, emergent/emergent salvage status, history of previous heart transplants, and missing tricuspid operation type were excluded (Figure 1), creating a final cohort of 6507 cases. Although endocarditis is a major indication for isolated tricuspid operation, we excluded patients with endocarditis from the final cohort because a prior STS-ACSD analysis evaluated this population.14 For completeness, baseline patient characteristics and unadjusted outcomes are included in Supplemental Tables 1 and 2.
FIGURE 1.
Cohort identification. (CABG, coronary artery bypass grafting; VAD, ventricular assist device.)
Comparisons were made between patients who underwent repair (n = 3308) vs replacement (n = 3199) and those who underwent beating heart (n = 2435) vs full cardioplegic arrest (n = 3901) operations. Data access for this study was approved using the STS Participant User File research program (PUF-ACSD-2021-011). The Institutional Review Board at Cedars-Sinai Medical Center approved the study protocol, with a waiver of informed consent (STUDY00001188, approved on February 19, 2021). All patient characteristics and study endpoints were defined according to STS-ACSD definitions.
STUDY ENDPOINTS.
The primary endpoint was operative mortality (death during the same hospitalization as operation or after discharge but within 30 days of operation). Secondary endpoints were composite major complications (defined as permanent stroke, renal failure, prolonged ventilation > 24 hours, deep sternal wound infection, cardiac reoperations, or postoperative new permanent pacemaker implantation) and their individual components.
STATISTICAL ANALYSIS.
Baseline characteristics are reported as either mean with SD deviation or median with interquartile range (IQR) for continuous variables and proportions for categorical variables. Between-group comparisons were performed using the Student’s t test or Wilcoxon rank-sum test for continuous variables and Pearson’s χ2 test for categorical variables. The annual hospital volume of isolated tricuspid operation was calculated as the number of operations performed by a hospital in a calendar year. Because only 6 months of data were available in 2011 and 2020, volume data from these 2 years were normalized to 12 months. For missing data, single imputation was used for variables with <5% missingness, similar to methods described and validated in previous STS risk prediction models (Supplemental Methods).15 Included variables with missing data and the percentage of missingness are reported in Supplemental Table 3.
To determine factors associated with operative mortality, we fitted a multivariable generalized estimating equation model with a logit link function and binomial distribution adjusting for clustering at the center level. Variables included in the final model were selected a priori based on data availability and clinical significance (Supplemental Methods). For the secondary endpoint of composite major complications, another model was created including the same covariates as the operative mortality model. Additional models were similarly constructed to provide adjusted comparisons of tricuspid operation type (repair vs replacement) and operative strategy (beating heart vs full cardioplegic arrest) regarding postoperative complications. Additionally because the model for end-stage liver disease (MELD) score is an important risk-stratification tool, we created 2 additional models using 4960 of 6507 patients with available MELD scores to explore the association between MELD and operative mortality and composite major complications, respectively.
All tests were 2-tailed with an alpha level of 0.05. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
STUDY POPULATION.
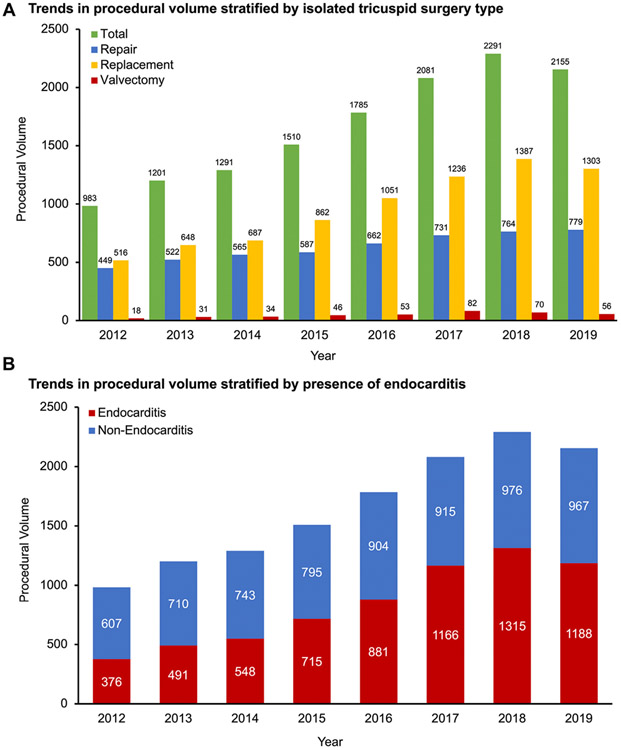
During the study period 14,704 isolated tricuspid operations were performed by 967 hospitals. Annual procedural volume increased from 983 in 2012 to 2155 in 2019 (P < .001) (Figure 2A). A median of 2 cases (range, 1-81) was performed annually per hospital, and 93% of hospitals performed 5 cases or less per year on average. The proportion of patients with endocarditis increased from 38.3% in 2012 to 55.1% in 2019 (P < .001) (Figure 2B).
FIGURE 2.
Procedural trends of all isolated tricuspid operations (2012-2019). (A) Trends in procedural volume stratified by isolated tricuspid surgery type; (B) trends in procedural volume stratified by presence of endocarditis. All 14,704 patients undergoing isolated tricuspid surgery were included except cases from 2011 and 2020 (because only six months of data were available). 104 patients had missing tricuspid operation type.
In the final cohort of 6507 patients with nonendocarditis-related TR (median age, 65 years [IQR, 52-74]; 38.5% men), 40% had New York Heart Association class III or IV heart failure. The median MELD score (available in 4960/6507 patients) was 10.1 (IQR, 7.5-13.7). Other baseline characteristics are outlined in Table 1. Among 3658 patients (56.2%) with available tricuspid disease etiology, 52.1% had functional disease, 12.8% had degenerative disease, 9.8% had pacing wire/catheter-induced dysfunction, 7.1% had congenital disease, and 5.4% had failed previous tricuspid interventions (Supplemental Table 4). Twenty-four percent of cases were nonelective. Minimally invasive approaches were used in 11.3% (Table 2). Full cardioplegic arrest was used in 60.0%, fibrillatory arrest in 2.6%, and on-pump beating heart operation in 37.4%. Tricuspid repair was performed in 3308 patients (50.8%) and replacement in 3199 patients (49.2%). In patients with tricuspid repair, 85.4% received a ring/band annuloplasty ± leaflet reconstruction (median implant size, 30 mm [IQR, 28-32]), 4.8% received leaflet reconstruction alone, and 0.5% received a pericardium annuloplasty ± leaflet reconstruction.
TABLE 1.
Baseline Characteristics Stratified by Tricuspid Repair vs Replacement and Beating Heart vs Full Arrest Operations
| Characteristics | All Patients (N = 6507) |
Repair (n = 3308) |
Replacement (n = 3199) |
P Value | Beating Heart (n = 2435) |
Full Arrest (n = 3901) |
P Value |
|---|---|---|---|---|---|---|---|
| Age, y | 65 (52-74) | 65 (51-74) | 66 (53-74) | .42 | 68 (57-75) | 63 (49-73) | <.001 |
| Male sex | 38.5 (2507) | 39.0 (1290) | 38.4 (1217) | .43 | 39.8 (969) | 37.8 (1475) | .11 |
| Body mass index, kg/m2 | 26.9 (23.4-31.3) | 27.1 (23.7-31.5) | 26.8 (23.3-31.2) | .06 | 26.9 (23.6-31.2) | 27.0 (23.4-31.4) | .92 |
| White race | 77.6 (5047) | 77.5 (2562) | 77.7 (2485) | .20 | 77.9 (1898) | 77.4 (3018) | .16 |
| Diabetes | 23.6 (1537) | 22.2 (733) | 25.1 (804) | .005 | 27.3 (665) | 21.0 (823) | <.001 |
| Dialysis dependency | 4.7 (307) | 4.8 (157) | 4.7 (150) | .91 | 6.2 (149) | 3.9 (152) | <.001 |
| Chronic lung disease | 26.7 (1742) | 25.3 (837) | 28.3 (905) | .007 | 30.2 (736) | 24.7 (962) | <.001 |
| Liver disease | 14.5 (940) | 10.3 (339) | 18.8 (601) | <.001 | 17.8 (434) | 12.5 (486) | <.001 |
| Peripheral vascular disease | 7.3 (476) | 6.9 (229) | 7.7 (247) | .22 | 9.4 (229) | 6.0 (232) | <.001 |
| Cerebrovascular disease | 15.4 (1003) | 15.1 (498) | 15.8 (505) | .41 | 18.9 (460) | 13.3 (518) | <.001 |
| Atrial fibrillation | 47.1 (3062) | 47.0 (1555) | 47.1 (1507) | .93 | 51.3 (1250) | 44.3 (1729) | <.001 |
| Previous myocardial infarction | 10.0 (648) | 10.0 (330) | 9.9 (318) | .96 | 13.5 (329) | 7.7 (299) | <.001 |
| Left ventricular ejection fraction, % | 58 (52-60) | 58 (53-61) | 58 (52-60) | .16 | 56 (50-60) | 58 (53-62) | <.001 |
| New York Heart Association class III/IV heart failure | 40.0 (2599) | 34.7 (1149) | 45.3 (1450) | <.001 | 51.09 (1244) | 33.1 (1290) | <.001 |
| Previous cardiac surgery | 41.3 (2686) | 32.4 (1072) | 50.5 (1614) | <.001 | 59.0 (1436) | 29.0 (1132) | <.001 |
| Previous tricuspid valve surgery | 8.4 (548) | 2.0 (66) | 15.1 (482) | <.001 | 10.3 (250) | 7.1 (278) | <.001 |
| Creatinine (mg/dL) | 1.0 (0.8-1.3) | 1.0 (0.8-1.2) | 1.0 (0.8-1.4) | <.001 | 1.1 (0.9-1.5) | 1.0 (0.8-1.2) | <.001 |
| Total bilirubin (mg/dL) | 0.8 (0.5-1.2) | 0.8 (0.5-1.1) | 0.9 (0.6-1.3) | <.001 | 0.9 (0.6-1.3) | 0.8 (0.5-1.2) | <.001 |
| Model for end-stage liver disease | 10.1 (7.5-13.7) | 9.4 (7.5-12.9) | 10.7 (8.4-14.3) | <.001 | 11.1 (8.4-15.3) | 9.3 (7.5-12.6) | <.001 |
Values are in % (n) or median (interquartile range).
TABLE 2.
Operative Characteristics Stratified by Tricuspid Repair vs Replacement and Beating Heart vs Full Arrest Operations
| Characteristics | All Patients (n = 6507) |
Repair (n = 3308) |
Replacement (n = 3199) |
P Value | Beating Heart (n = 2435) |
Full Arrest (n = 3901) |
P Value |
|---|---|---|---|---|---|---|---|
| Non-elective surgery | 23.9 (1557) | 21.3 (706) | 26.6 (851) | <.001 | 28.3 (690) | 20.9 (816) | <.001 |
| Tricuspid replacement | 3199 (49.2) | - | 100 (3199) | - | 57.3 (1394) | 43.6 (1701) | <.001 |
| Operative approach | |||||||
| Sternotomy | 78.9 (5131) | 78.1 (2583) | 79.7 (2548) | .12 | 63.6 (1548) | 89.8 (3501) | <.001 |
| Full thoracotomy | 7.7 (501) | 6.7 (222) | 8.7 (279) | .002 | 14.3 (349) | 2.7 (107) | <.001 |
| Mini thoracotomy/minimally invasive | 11.3 (737) | 12.8 (423) | 9.8 (314) | <.001 | 18.8 (457) | 6.2 (241) | <.001 |
| CPB method | |||||||
| Full arrest | 60.0 (3901) | 66.5 (2200) | 53.2 (1701) | <.001 | - | 100 (3901) | - |
| Fibrillating arrest | 2.6 (171) | 2.0 (67) | 3.3 (104) | .002 | - | - | - |
| Beating heart | 37.4 (2435) | 31.5 (1041) | 43.6 (1394) | <.001 | 100 (2435) | - | - |
| Total bypass time (minutes) | 92 (69-126) | 87 (64-121) | 97 (75-132) | <.001 | 88 (65-119) | 95 (72-130) | <.001 |
| Post-bypass residual tricuspid regurgitation | <.001 | <.001 | |||||
| None/trace | 57.7 (3754) | 53.7 (1777) | 61.8 (1977) | 55.9 (1361) | 58.6 (2287) | ||
| Mild | 12.8 (831) | 18.6 (616) | 6.7 (215) | 13.1 (318) | 12.7 (495) | ||
| Moderate | 3.6 (232) | 5.6 (186) | 1.4 (46) | 4.0 (98) | 3.3 (130) | ||
| Severe | 2.1 (134) | 2.1 (68) | 2.1 (68) | 1.9 (47) | 2.1 (83) | ||
| Unknown | 23.9 (1556) | 20.0 (661) | 28.0 (895) | 25.1 (611) | 23.2 (906) |
Values are in % (n) or median (interquartile range). CPB, cardiopulmonary bypass.
SHORT-TERM OUTCOMES.
The operative mortality was 7.3%, rate of composite major complications was 32.0%, and 10.8% required new permanent pacemaker implantation (Table 3). Operative mortality was 1.7% in 869 elective patients without heart failure, organ dysfunction, or prior surgery. Operative mortality was higher with increasing MELD score (MELD < 10, 3.2%; MELD ≥ 20, 15.9%; P < .001). Additionally, increasing annual hospital volume was associated with a significant decrease in operative mortality (1-5 cases, 7.9%; 6-10 cases, 6.6%; >10 cases, 5.8%; P = .01) (Supplemental Figure 1).
TABLE 3.
Short-term Outcomes Stratified by Tricuspid Repair vs Replacement and Beating Heart vs Full Arrest Operations
| Characteristics | All Patients (n = 6507) |
Repair (n = 3308) |
Replacement (n = 3199) |
P Value | Beating Heart (n = 2435) |
Full Arrest (n = 3901) |
P Value |
|---|---|---|---|---|---|---|---|
| Operative mortality | 7.3 (474) | 5.2 (173) | 9.4 (301) | <.001 | 9.2 (225) | 6.0 (235) | <.001 |
| Composite major complications | 32.0 (2082) | 23.1 (765) | 41.3 (1320) | <.001 | 34.7 (845) | 30.2 (1179) | <.001 |
| Permanent stroke | 1.5 (100) | 1.2 (38) | 1.9 (62) | .01 | 1.9 (47) | 1.2 (48) | .03 |
| Prolonged ventilation | 20.2 (1315) | 14.8 (490) | 25.8 (825) | <.001 | 24.4 (594) | 17.4 (677) | <.001 |
| Cardiac reoperation | 4.3 (281) | 5.1 (167) | 7.4 (238) | <.001 | 7.4 (179) | 5.6 (217) | .004 |
| New PPM/ICD implantation | 10.8 (702) | 6.1 (200) | 15.7 (502) | <.001 | 9.2 (223) | 11.9 (464) | <.001 |
| Renal failure | 6.8 (444) | 4.5 (148) | 9.3 (296) | <.001 | 8.1 (196) | 6.1 (237) | .003 |
| New dialysis requirement | 5.5 (359) | 3.4 (113) | 7.7 (246) | <.001 | 6.7 (163) | 4.8 (186) | .001 |
| Deep sternal wound infection | 0.4 (28) | 0.3 (9) | 0.6 (19) | .05 | 0.4 (10) | 0.5 (18) | .77 |
| Blood product transfusions | 46.4 (3022) | 37.6 (1242) | 55.6 (1779) | <.001 | 52.7 (1284) | 42.2 (1646) | <.001 |
| Hospital length of stay, d | 8 (6-12) | 7 (5-11) | 9 (6-14) | <.001 | 9 (6-14) | 7 (5-11) | <.001 |
Values are in % (n) or median (interquartile range). ICD, implantable cardioverter-defibrillator; PPM, permanent pacemaker.
In the multivariable analysis, factors significantly associated with operative mortality were age > 50 years, moderate or severe chronic lung disease, New York Heart Association class III or IV congestive heart failure, atrial fibrillation, nonelective operation, concomitant aortic stenosis, tricuspid valve replacement, and annual volume ≤ 5 cases (Supplemental Table 5). Factors associated with composite major complications are presented in Supplemental Table 6. When only patients with available MELD scores were analyzed (n = 4960), increasing MELD score was independently associated with both increased operative mortality (10-14, adjusted odds ratio [aOR] 1.69; 15-19, aOR 2.21; ≥20, aOR 3.13, reference group, <10; all P < .01) and increased composite major complications (Supplemental Table 7).
TRICUSPID REPAIR VS REPLACEMENT.
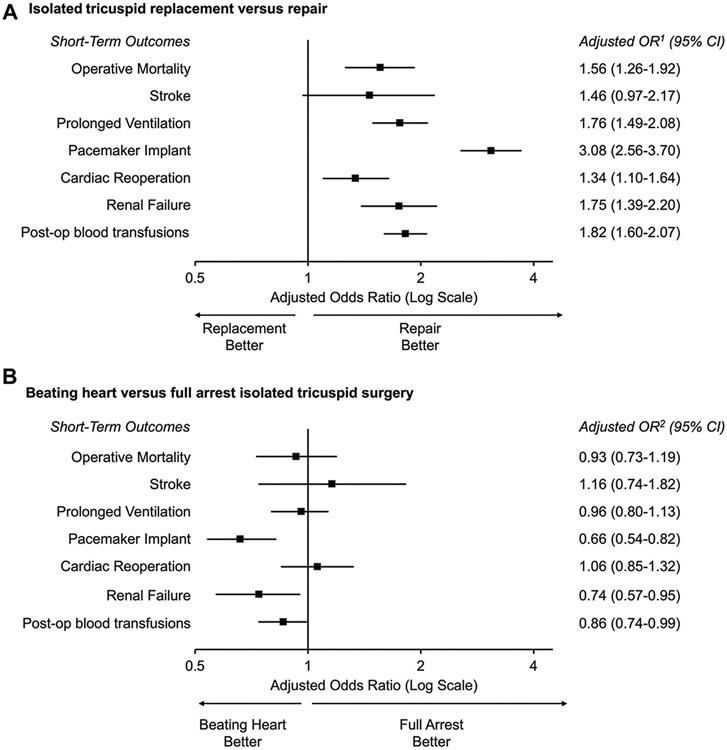
Patients undergoing tricuspid replacement (n = 3199; median valve size, 31 mm [IQR, 29-33]) had more comorbidities (Table 1) and nonelective operations (26.6% vs 21.3%, P < .001) (Table 2) than those undergoing repair (n = 3308). In 2332 patients who underwent replacement with available prosthesis type, 2155 (92.4%) received a bioprosthetic valve, and this proportion remained steady from 2015 (94.8%) to 2019 (93.9%, P = .37). Patients who underwent replacement had less functional disease and more carcinoid disease, rheumatic disease, pacing wire/catheter-induced dysfunction, or failed prior tricuspid interventions (Supplemental Table 4). Replacement was associated with a higher unadjusted operative mortality (9.4% vs 5.2%) and composite major complications (41.3% vs 23.1%, both P < .001) (Table 3). After adjustment, replacement remained associated with increased operative mortality (aOR, 1.56; 95% CI, 1.25-1.94) and composite major comorbidities (aOR, 2.22; 95 % CI, 1.92-2.57; both P < .001), including a higher risk of prolonged mechanical ventilation, permanent pacemaker implant, renal failure, and postoperative blood product transfusions (Figure 3A).
FIGURE 3.
Risk-adjusted comparison of patients undergoing (A) isolated tricuspid replacement vs repair and (B) beating heart operation vs full cardioplegic arrest. Adjusted OR1 = tricuspid repair and adjusted OR2 = full cardioplegic arrest operations. Analyses were performed in 6507 patients in the final cohort with non-endocarditis-related tricuspid regurgitation. (OR, odds ratio.)
FULL CARDIOPLEGIA ARREST VS BEATING HEART OPERATION.
Patients undergoing beating heart operation (n = 2435) were older and had more comorbidities than those who had full cardioplegia arrest (n = 3901) (Table 1). Beating heart operations were more often nonelective (28.3% vs 20.9%) and performed using minimally invasive access (18.8% vs 6.2%, both P < .001) (Table 2). Unadjusted operative mortality and composite major complication rate were both higher after beating heart operations (9.2% vs 6.0% and 34.7% vs 30.2%, respectively, both P < .001) (Table 3). After adjustment, beating heart operation was not significantly associated with operative mortality (aOR, 0.91; 95% CI, 0.71-1.16; P = .44) but was associated with a decreased risk of composite major complications (aOR, 0.85; 95% CI, 0.74-0.98; P = .03), including reduced risk of permanent pacemaker implant, renal failure, and postoperative blood product transfusions (Figure 3B).
COMMENT
This analysis of the STS-ACSD represents the largest contemporary series of patients undergoing isolated tricuspid operation. Detailed clinical and operative data in the STS-ACSD provided additional granularity compared with previous analyses of administrative claims databases. There are several important findings. First, although procedural volume has increased, isolated tricuspid operation remains infrequently performed, and operative mortality for patients with nonendocarditis-related TR remains relatively high at 7.3%. Second, in patients with TR, surrogates of advanced disease were associated with increased operative mortality. Third, after adjustment, valve replacement was associated with increased morbidity and mortality, whereas beating heart operation was associated with decreased morbidity. Finally, annual hospital volume was significantly associated with early outcomes after isolated tricuspid operation.
Current guidelines regarding isolated tricuspid operation for TR are discordant, with no class I indications in the American College of Cardiology/American Heart Association guidelines and only 1 class I indication for severe symptomatic TR in the European Society of Cardiology guidelines.7,8 In this context patients are frequently considered for operation later in the disease course. We observed that markers of advanced diseases, such as New York Heart Association classIII/IV heart failure, nonelective operations, and increased MELD score were all independently associated with increased operative mortality. In patients without these risk factors, operative mortality was 1.7%. In patients with all 3 of these risk factors (including MELD score > 10), operative mortality was nearly 20%. These findings are consistent with prior analyses and may help identify high-risk patients who may be better suited for transcatheter tricuspid interventions. They also suggest a potential role for earlier surgical intervention.16,17 However, because the exact indication and timing of operation in the disease course were unavailable in the STS-ACSD, the current study does not provide sufficient evidence to definitively recommend this practice.
Our analysis demonstrated that tricuspid replacement was associated with increased short-term mortality and morbidity compared with repair, similar to prior results from large clinical series and meta-analyses.18-21 We also observed that valve replacement was more frequently performed in sicker patients, possibly due to the concern of reoperation associated with an inadequate repair. Additionally the association between worse outcomes and replacement may reflect the fact that surgeons recognize that repair is less likely to be successful when markers of more advanced disease are present, such as extreme annular dilatation, visible leaflet malcoaptation, massive or torrential TR, leaflet tethering, and failed percutaneous repair. Together our findings may indicate that tricuspid repair is reasonable in less-sick patients. However a low threshold for valve replacement may still be appropriate in patients with high surgical risk or markers of more advanced disease. These findings may also have important implications for transcatheter tricuspid interventions.
Almost 40% of patients in our series underwent beating heart tricuspid operation, highlighting the lack of consensus regarding whether beating heart or arrested heart tricuspid operation is superior. In real-world practice this may be dictated by surgeon preference. Small, single-institution reports comparing these 2 techniques have demonstrated similar results,22,23 whereas a recent multicenter study showed that beating heart operation was associated with a decreased rate of postoperative renal failure in propensity-matched patients.24 We observed that beating heart operation was more frequently performed in higher-risk patients. After adjustment, beating heart operation was independently associated with lower risks of permanent pacemaker implantation, postoperative renal failure, and reduced postoperative blood product transfusions. Therefore beating heart operation may represent an attractive alternative to arrested heart operation for surgeons who are comfortable with this technique.
LIMITATIONS.
The main strength of this study was the ability to evaluate contemporary outcomes of isolated tricuspid operation using a large prospective registry with clinical granularity. However this approach has several limitations. First, because markers of right ventricular dysfunction were largely missing, we could not evaluate this critical prognostic factor. Second, no long-term outcomes were available in the STS-ACSD. Third, some data elements were incomplete and may be subject to inaccurate coding, particularly those related to tricuspid disease etiology. We therefore did not include tricuspid disease etiology in our multivariable modeling. The classification of tricuspid disease etiology within the database also changed after July 2014, limiting the ability to evaluate trends in the underlying etiology. Fourth, we were unable to identify patients who underwent tricuspid replacement after attempted repair or those with prophylactic permanent epicardial pacing lead placement at the time of tricuspid replacement. Finally, selection bias may still exist despite the use of various clinical and operative characteristics to adjust for potential confounding.
CONCLUSION.
This national analysis suggests that the unadjusted operative mortality was relatively high at 7.3% for adult patients undergoing isolated tricuspid operation for nonendocarditis-related TR. In these patients valve repair was associated with lower operative mortality and major morbidities than replacement. Beating heart operation was associated with lower rates of pacemaker implant, renal failure, and postoperative blood transfusions compared with full cardioplegic arrest. Preoperative MELD scores may identify high-risk patients, and early referral to higher volume centers may help improve outcomes.
Supplementary Material
FUNDING SOURCES
Qiudong Chen, Jad Malas, and Amy Roach are supported by grants from the National Institutes of Health for advanced heart disease research (T32HL116273).
Footnotes
The Supplemental Material can be viewed in the online version of this article [https://dx.doi.org/10.1016/j.athoracsur.2022.12.041] on https://www.annalsthoracicsurgery.org.
Presented at the Fifty-ninth Annual Meeting of The Society of Thoracic Surgeons, San Diego, CA, Jan 21-23, 2023. Richard E. Clark Memorial Paper for Adult Cardiac Surgery.
Data for this research was provided by The Society of Thoracic Surgeons National Database Participant User File Research Program. Data analysis was performed at the investigators’ institutions.
DISCLOSURES
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Vassileva CM, Shabosky J, Boley T, Markwell S, Hazelrigg S. Tricuspid valve surgery: the past 10 years from the Nationwide Inpatient Sample (NIS) database. J Thorac Cardiovasc Surg. 2012;143:1043–1049. [DOI] [PubMed] [Google Scholar]
- 2.Kilic A, Saha-Chaudhuri P, Rankin JS, Conte JV. Trends and outcomes of tricuspid valve surgery in North America: an analysis of more than 50,000 patients from The Society of Thoracic Surgeons Database. Ann Thorac Surg. 2013;96:1546–1552. [DOI] [PubMed] [Google Scholar]
- 3.Alqahtani F, Berzingi CO, Aljohani S, Hijazi M, Al-Hallak A, Alkhouli M. Contemporary trends in the use and outcomes of surgical treatment of tricuspid regurgitation. J Am Heart Assoc. 2017;6:e007597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zack CJ, Fender EA, Chandrashekar P, et al. National trends and outcomes in isolated tricuspid valve surgery. J Am Coll Cardiol. 2017;70:2953–2960. [DOI] [PubMed] [Google Scholar]
- 5.Kundi H, Popma JJ, Cohen DJ, et al. Prevalence and outcomes of isolated tricuspid valve surgery among Medicare beneficiaries. Am J Cardiol. 2019;123:132–138. [DOI] [PubMed] [Google Scholar]
- 6.Dreyfus J, Flagiello M, Bazire B, et al. Isolated tricuspid valve surgery: impact of aetiology and clinical presentation on outcomes. Eur Heart J. 2020;41:4304–4317. [DOI] [PubMed] [Google Scholar]
- 7.Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72–e227. [DOI] [PubMed] [Google Scholar]
- 8.Vahanian A, Beyersdorf F, Praz F, et al. 2021 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2022;43:561–632. [DOI] [PubMed] [Google Scholar]
- 9.Hamandi M, Smith RL, Ryan WH, et al. Outcomes of isolated tricuspid valve surgery have improved in the modern era. Ann Thorac Surg. 2019;108:11–15. [DOI] [PubMed] [Google Scholar]
- 10.Sala A, Lorusso R, Bargagna M, et al. Isolated tricuspid valve surgery: first outcomes report according to a novel clinical and functional staging of tricuspid regurgitation. Eur J Cardiothorac Surg. 2021;60:1124–1130. [DOI] [PubMed] [Google Scholar]
- 11.Pfannmueller B, Budde L-M, Etz CD, et al. Postoperative outcome after reoperative isolated tricuspid valve surgery—is there a predictor for survival? Eur J Cardiothorac Surg. 2021;60:867–871. [DOI] [PubMed] [Google Scholar]
- 12.Kawsara A, Alqahtani F, Nkomo VT, et al. Determinants of morbidity and mortality associated with isolated tricuspid valve surgery. J Am Heart Assoc. 2021;10:e018417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowdish ME, D’Agostino RS, Thourani VH, et al. STS Adult Cardiac Surgery Database: 2021 update on outcomes, quality, and research. Ann Thorac Surg. 2021;111:1770–1780. [DOI] [PubMed] [Google Scholar]
- 14.Slaughter MS, Badhwar V, Ising M, et al. Optimum surgical treatment for tricuspid valve infective endocarditis: an analysis of Thoracic Surgeons national database. J Thorac Cardiovasc Surg. 2021;161: 1227–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahian DM, O’Brien SM, Filardo G, et al. The Society of Thoracic Surgeons 2008 cardiac surgery risk models: part 1—coronary artery bypass grafting surgery. Ann Thorac Surg. 2009;88:S2–S22. [DOI] [PubMed] [Google Scholar]
- 16.Färber G, Marx J, Scherag A, et al. Risk stratification for isolated tricuspid valve surgery assisted using the model for end-stage liver disease score. J Thorac Cardiovasc Surg. Published online March 12, 2022. 10.1016/j.jtcvs.2021.11.102 [DOI] [PubMed] [Google Scholar]
- 17.Dreyfus J, Ghalem N, Garbarz E, et al. Timing of referral of patients with severe isolated tricuspid valve regurgitation to surgeons (from a French nationwide database). Am J Cardiol. 2018;122:323–326. [DOI] [PubMed] [Google Scholar]
- 18.Wang TKM, Griffin BP, Miyasaka R, et al. Isolated surgical tricuspid repair versus replacement: meta-analysis of 15 069 patients. Open Heart. 2020;7:e001227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wong W-K, Chen S-W, Chou A-H, et al. Late outcomes of valve repair versus replacement in isolated and concomitant tricuspid valve surgery: a nationwide cohort study. J Am Heart Assoc. 2020;9: e015637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JW, Jang M-J, Kim KH, Hwang HY. Repair versus replacement for the surgical correction of tricuspid regurgitation: a meta-analysis. Eur J Cardiothorac Surg. 2018;53:748–755. [DOI] [PubMed] [Google Scholar]
- 21.Russo M, Di Mauro M, Saitto G, et al. Outcome of patients undergoing isolated tricuspid repair or replacement surgery. Eur J Cardiothorac Surg. 2022;62:ezac230. [DOI] [PubMed] [Google Scholar]
- 22.Pfannmüller B, Davierwala P, Misfeld M, Borger MA, Garbade J, Mohr FW. Postoperative outcome of isolated tricuspid valve operation using arrested-heart or beating-heart technique. Ann Thorac Surg. 2012;94:1218–1222. [DOI] [PubMed] [Google Scholar]
- 23.Baraki H, Saito S, Al Ahmad A, Fleischer B, Haverich A, Kutschka I. Beating heart versus arrested heart isolated tricuspid valve surgery. Int Heart J. 2015;56:400–407. [DOI] [PubMed] [Google Scholar]
- 24.Russo M, Di Mauro M, Saitto G, et al. Beating versus arrested heart isolated tricuspid valve surgery: long-term outcomes. Ann Thorac Surg. 2022;113:585–592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.