Fig. 6. Comparison of primary structure and post-translational modification of DCFHP produced in Expi293 and CHO cells as measured by LC MS peptide mapping and N-linked oligosaccharide mapping.
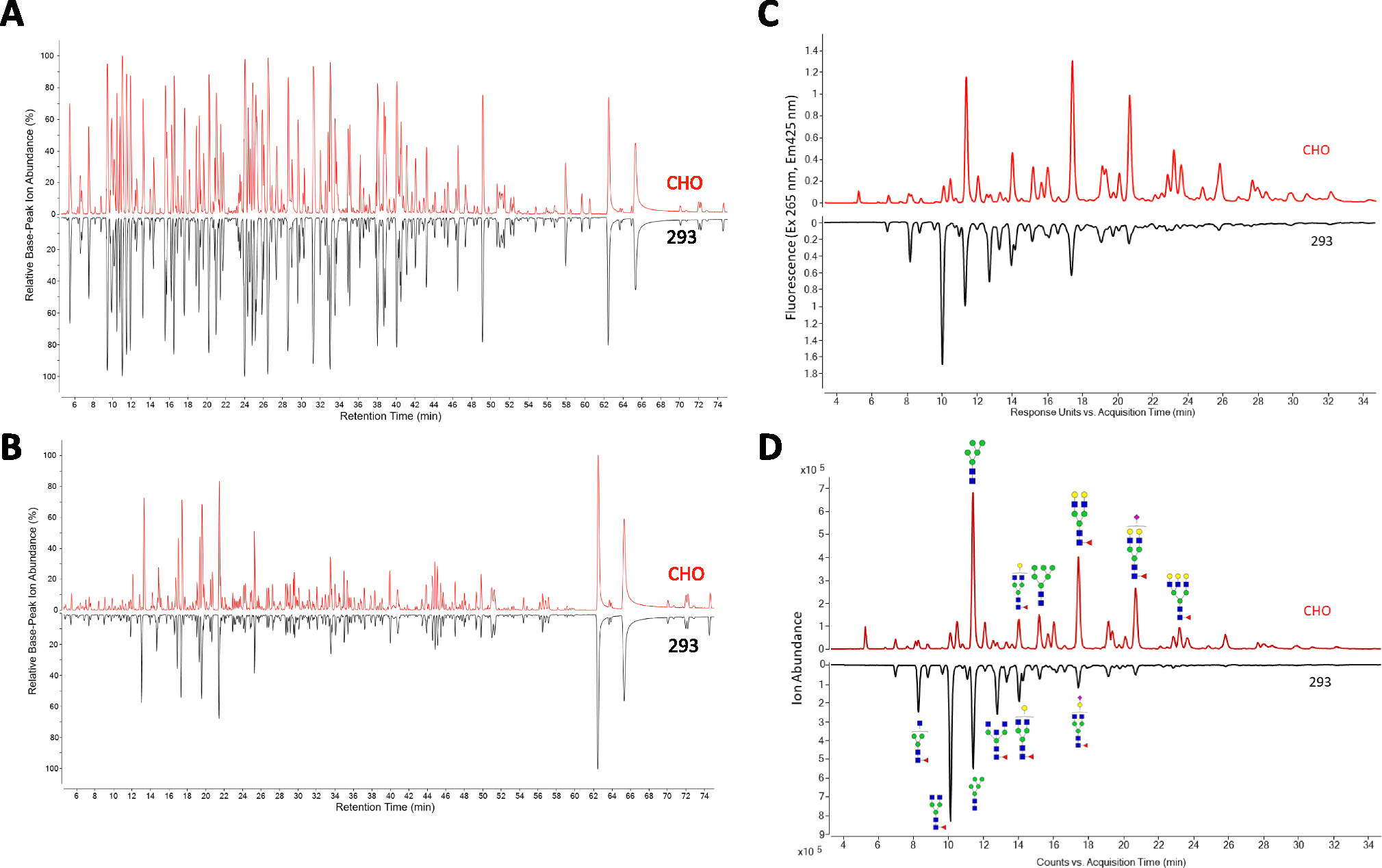
Representative LC-MS peptide mapping chromatograms of DCFHP (pre-treated with PNGaseF) and digested with either A) Trypsin or B) Chymotrypsin. (C, D) Representative oligosaccharide mapping results of labelled N-glycans (removed by PNGaseF treatment) from DCFHP produced in Expi293 and CHO cells. C) fluorescence detection chromatograms or D) total ion count MS detection chromatograms. Data is representative from triplicate experiments.
