Fig. 7. Physiochemical characterization of DCFHP produced in Expi293 and CHO cells as measured by biophysical and ACE2 receptor binding studies.
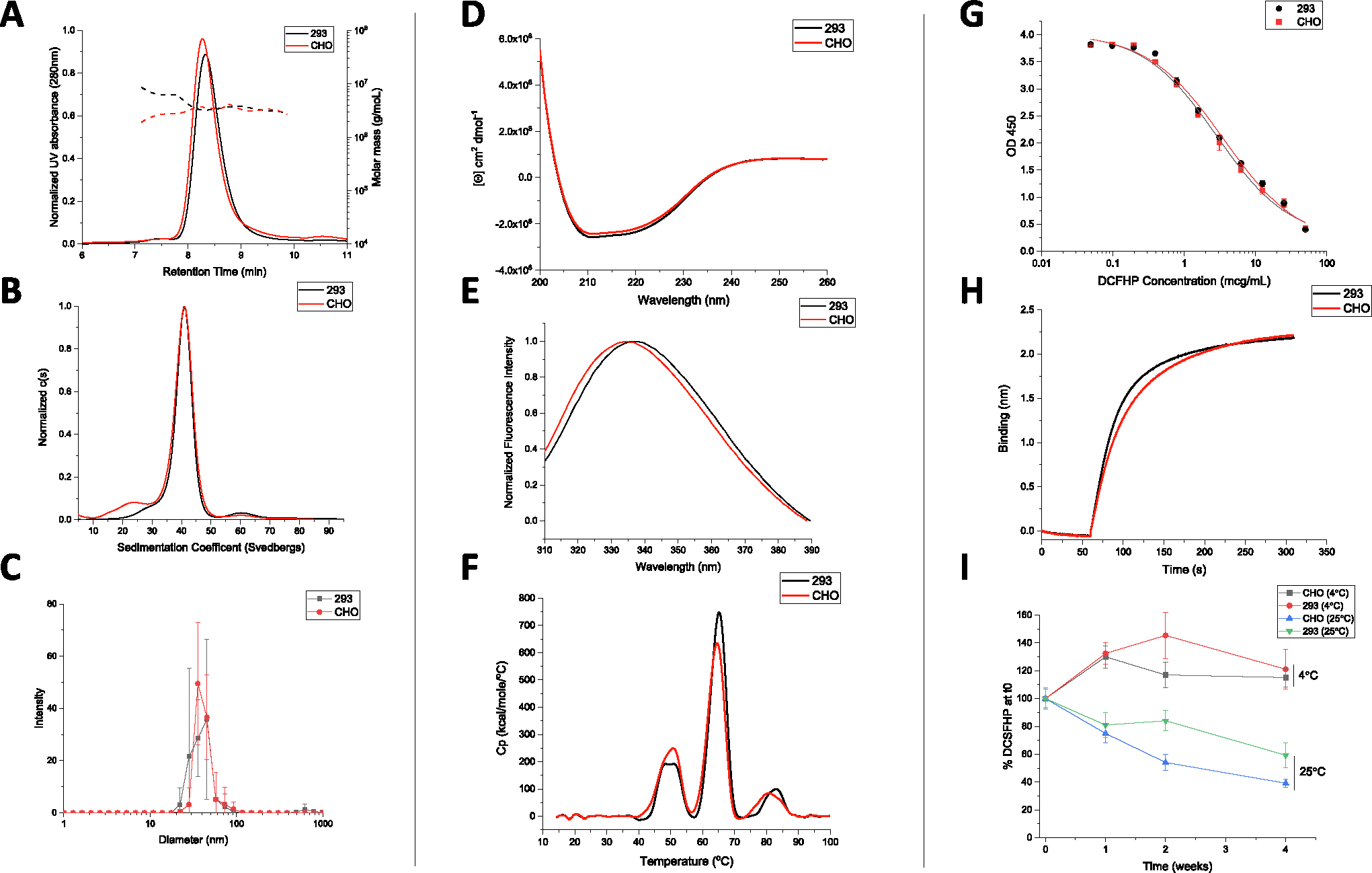
Molecular size characterization in (A) SEC-MALS, (B) SV-AUC and (C) DLS. Overall secondary and tertiary structural integrity analysis at 10°C as measured by (D) circular dichroism, and (E) intrinsic fluorescence spectroscopy, respectively, and overall conformational stability profile as determined by (F) DSC. Representative binding curves of each DCFHP antigen to ACE2 as measured by (G) competitive ELISA, and (H) BLI. (I) Relative ACE2 binding (normalized to time zero) as measured by competitive ELISA in 293 and CHO produced DCFHP samples adsorbed to AH as a function of storage temperature (4 and 25°C) and time (up to 4 weeks). Samples were formulated in 20 mM Tris, 150 mM NaCl, 5% Sucrose, pH 7.5 buffer. Data are the mean of at least three independent measurements with the error bars representing the standard deviation. Representative data are shown in panels A, D, G, and H with the mean data are displayed in panels B, C, E, F, G and I.
