Abstract
Background
In many countries intrauterine insemination (IUI) is the treatment of first choice for a subfertile couple when the infertility work up reveals an ovulatory cycle, at least one open Fallopian tube and sufficient spermatozoa. The final goal of this treatment is to achieve a pregnancy and deliver a healthy (singleton) live birth. The probability of conceiving with IUI depends on various factors including age of the couple, type of subfertility, ovarian stimulation and the timing of insemination. IUI should logically be performed around the moment of ovulation. Since spermatozoa and oocytes have only limited survival time correct timing of the insemination is essential. As it is not known which technique of timing for IUI results in the best treatment outcome, we compared different techniques for timing IUI and different time intervals.
Objectives
To evaluate the effectiveness of different synchronisation methods in natural and stimulated cycles for IUI in subfertile couples.
Search methods
We searched for all publications which described randomised controlled trials of the timing of IUI. We searched the Cochrane Menstrual Disorders and Subfertility Group Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL) (1966 to October 2014), EMBASE (1974 to October 2014), MEDLINE (1966 to October 2014) and PsycINFO (inception to October 2014) electronic databases and prospective trial registers. Furthermore, we checked the reference lists of all obtained studies and performed a handsearch of conference abstracts.
Selection criteria
Randomised controlled trials (RCTs) comparing different timing methods for IUI were included. The following interventions were evaluated: detection of luteinising hormone (LH) in urine or blood, single test; human chorionic gonadotropin (hCG) administration; combination of LH detection and hCG administration; basal body temperature chart; ultrasound detection of ovulation; gonadotropin‐releasing hormone (GnRH) agonist administration; or other timing methods.
Data collection and analysis
Two review authors independently selected the trials, extracted the data and assessed study risk of bias. We performed statistical analyses in accordance with the guidelines for statistical analysis developed by The Cochrane Collaboration. The overall quality of the evidence was assessed using GRADE methods.
Main results
Eighteen RCTs were included in the review, of which 14 were included in the meta‐analyses (in total 2279 couples). The evidence was current to October 2013. The quality of the evidence was low or very low for most comparisons . The main limitations in the evidence were failure to describe study methods, serious imprecision and attrition bias.
Ten RCTs compared different methods of timing for IUI. We found no evidence of a difference in live birth rates between hCG injection versus LH surge (odds ratio (OR) 1.0, 95% confidence interval (CI) 0.06 to 18, 1 RCT, 24 women, very low quality evidence), urinary hCG versus recombinant hCG (OR 1.17, 95% CI 0.68 to 2.03, 1 RCT, 284 women, low quality evidence) or hCG versus GnRH agonist (OR 1.04, 95% CI 0.42 to 2.6, 3 RCTS, 104 women, I2 = 0%, low quality evidence).
Two RCTs compared the optimum time interval from hCG injection to IUI, comparing different time frames that ranged from 24 hours to 48 hours. Only one of these studies reported live birth rates, and found no difference between the groups (OR 0.52, 95% CI 0.27 to 1.00, 1 RCT, 204 couples). One study compared early versus late hCG administration and one study compared different dosages of hCG, but neither reported the primary outcome of live birth.
We found no evidence of a difference between any of the groups in rates of pregnancy or adverse events (multiple pregnancy, miscarriage, ovarian hyperstimulation syndrome (OHSS)). However, most of these data were very low quality.
Authors' conclusions
There is insufficient evidence to determine whether there is any difference in safety and effectiveness between different methods of synchronization of ovulation and insemination. More research is needed.
Keywords: Adult; Female; Humans; Male; Young Adult; Body Temperature; Chorionic Gonadotropin; Chorionic Gonadotropin/administration & dosage; Gonadotropin‐Releasing Hormone; Gonadotropin‐Releasing Hormone/agonists; Infertility; Infertility/therapy; Insemination, Artificial; Insemination, Artificial/methods; Luteinizing Hormone; Luteinizing Hormone/blood; Luteinizing Hormone/urine; Ovulation Detection; Ovulation Detection/methods; Randomized Controlled Trials as Topic; Time Factors
Plain language summary
What is the best timing technique for intrauterine insemination in subfertile couples
Review question. Cochrane authors reviewed the evidence about the effectiveness of different timing techniques for intrauterine insemination in subfertile couples.
Background. Couples that have not reached pregnancy after trying for at least a year are defined as subfertile. This affects approximately 10% of couples trying to have a baby. A procedure that may assist couples is intrauterine insemination (IUI). This is an assisted reproduction procedure where sperm are placed directly into the uterus at a specific time in the woman's menstrual cycle (as close to ovulation as possible). It remains unclear which technique of timing for IUI results in the best treatment outcome, a healthy live birth. Timing of IUI is most frequently performed with hormone (luteinising hormone (LH)) detection in urine or blood, or human chorionic gonadotropin (hCG) injection. The usefulness of urinary LH monitoring is hampered by the possibility of false‐negative results which can cause inaccurate timing and significantly reduce pregnancy rates. On the other hand, the ease of performing a test at home, the lower costs and the non‐invasiveness are advantages. Limitations of timing by ultrasound and hCG administration are frequent hospital visits and the occurrence of premature LH surges or the possibility of triggering ovulation in the presence of an immature follicle. The major advantage of this hCG method is the clinical predictability of the ovulation.
Study characteristics. We found 18 randomised controlled trials, all comparing different timing methods in one treatment cycle for IUI, with a total of 2279 couples. The evidence was current to October 2013.
Key results. We found no evidence of a difference in live birth rates between timing methods. We also found no evidence of a difference between any of the groups in rates of pregnancy or adverse events (multiple pregnancy, miscarriage, ovarian hyperstimulation syndrome (OHSS)).
Quality of the evidence. Most of the evidence was of low or very low quality. The main limitations were poor reporting of study methods, imprecision and losses to follow up. More research is needed.
Summary of findings
Summary of findings for the main comparison. hCG compared to LH surge for intrauterine insemination in subfertile couples.
| hCG compared to LH surge for intrauterine insemination in subfertile couples | ||||||
| Population: women undergoing intrauterine insemination Intervention: hCG Comparison: LH surge | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| LH surge | HCG | |||||
| Live birth rate per couple | 83 per 1000 | 83 per 1000 (5 to 621) | OR 1 (0.06 to 18.08) | 24 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| Pregnancy rate per couple | 146 per 1000 | 185 per 1000 (110 to 295) | OR 1.33 (0.72 to 2.45) | 275 (4 studies) | ⊕⊕⊝⊝ low1,3 | |
| Multiple pregnancy rate per pregnancy | 59 per 1000 | 66 per 1000 (11 to 323) | OR 1.12 (0.17 to 7.6) | 42 (2 studies) | ⊕⊝⊝⊝ very low1,2 | |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Methods used for random sequence generation and/or allocation concealment were unclear.
2There was very serious imprecision, with small sample sizes and very few events.
3There was serious imprecision: findings were compatible with substantial benefit in either group, or with no effect.
Summary of findings 2. u‐hCG compared to r‐hCG for intrauterine insemination in subfertile couples.
| u‐hCG compared to r‐hCG for intrauterine insemination in subfertile couples | ||||||
| Population: women undergoing intrauterine insemination Intervention: u‐hCG Comparison: r‐hCG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| R‐hCG | U‐hCG | |||||
| Live birth rate per couple | 221 per 1000 | 249 per 1000 (162 to 365) | OR 1.17 (0.68 to 2.03) | 284 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Pregnancy rate per couple | 261 per 1000 | 265 per 1000 (187 to 357) | OR 1.02 (0.65 to 1.57) | 409 (2 studies) | ⊕⊕⊝⊝ low2,3 | |
| Multiple pregnancy rate per pregnancy | 184 per 1000 | 182 per 1000 (83 to 358) | OR 0.99 (0.4 to 2.47) | 109 (2 studies) | ⊕⊕⊝⊝ low2,3 | |
| Miscarriage rate per pregnancy | 84 per 1000 | 50 per 1000 (12 to 185) | OR 0.57 (0.13 to 2.47) | 109 (2 studies) | ⊕⊝⊝⊝ very low2,3,4 | |
| OHSS rate per cycle | See comment | See comment | Not estimable | 468 (2 studies) | ⊕⊕⊕⊝ moderate3 | There were no events in either study |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Methods used for random sequence generation and/or allocation concealment were unclear.
2There was serious imprecision: findings were compatible with substantial benefit in either group, or with no effect.
3One study did not report the method of allocation concealment used.
4There was very serious imprecision, with very few events and wide confidence intervals.
Summary of findings 3. Short interval compared to long interval for intrauterine insemination in subfertile couples.
| Short interval compared to long interval for intrauterine insemination in subfertile couples | ||||||
| Population: women undergoing intrauterine insemination Intervention: short interval Comparison: long interval | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long interval | Short interval | |||||
| Live birth rate per couple ‐ 24 hours versus 34 to 36 hours | 298 per 1000 | 181 per 1000 (103 to 298) | OR 0.52 (0.27 to 1) | 204 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Pregnancy rate per couple ‐ 24 hours versus 34 to 36 hours | 397 per 1000 | 266 per 1000 (170 to 392) | OR 0.55 (0.31 to 0.98) | 234 (2 studies) | ⊕⊕⊝⊝ low1,2 | |
| Pregnancy rate per couple ‐ 24 hours versus 48 hours | 600 per 1000 | 398 per 1000 (130 to 742) | OR 0.44 (0.1 to 1.92) | 30 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Pregnancy rate per couple ‐ 34 to 36 hours versus 48 hours | 600 per 1000 | 465 per 1000 (174 to 788) | OR 0.58 (0.14 to 2.48) | 30 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Miscarriage rate per pregnancy ‐ 24 hours versus 34 to 36 hours | 116 per 1000 | 172 per 1000 (44 to 484) | OR 1.58 (0.35 to 7.16) | 67 (2 studies) | ⊕⊝⊝⊝ very low1,3 | |
| Miscarriage rate per pregnancy ‐ 24 hours versus 48 hours | 111 per 1000 | 333 per 1000 (33 to 880) | OR 4 (0.27 to 58.56) | 15 (1 study) | ⊕⊝⊝⊝ very low1,3 | |
| Miscarriage rate per pregnancy ‐ 34 to 36 hours versus 48 hours | 111 per 1000 | 142 per 1000 (9 to 764) | OR 1.33 (0.07 to 25.91) | 16 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Methods used for random sequence generation or allocation concealment were unclear.
2There was serious imprecision: findings were compatible with substantial benefit in the long interval group, or with no effect. (See comment) 3There was very serious imprecision, with very few events and wide confidence intervals
Summary of findings 4. hCG compared to GnRH‐a for intrauterine insemination in subfertile couples.
| hCG compared to GnRH‐a for intrauterine insemination in subfertile couples | ||||||
| Population: women undergoing intrauterine insemination Intervention: hCG Comparison: GnRH‐a | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| GnRH‐a | HCG | |||||
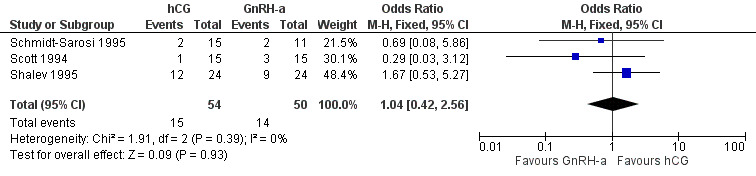
| Live birth rate per couple | 200 per 1000 | 206 per 1000 (95 to 390) | OR 1.04 (0.42 to 2.56) | 104 (3 studies) | ⊕⊕⊝⊝ low1,2 | |
| Pregnancy rate per couple | 315 per 1000 | 344 per 1000 (225 to 489) | OR 1.14 (0.63 to 2.08) | 206 (4 studies) | ⊕⊕⊝⊝ low1,2 | |
| Multiple pregnancy rate per pregnancy | 33 per 1000 | 5 per 1000 (1 to 45) | OR 0.15 (0.02 to 1.38) | 74 (4 studies) | ⊕⊝⊝⊝ very low1,3 | |
| Miscarriage rate per pregnancy | 124 per 1000 | 196 per 1000 (64 to 467) | OR 1.72 (0.48 to 6.2) | 74 (4 studies) | ⊕⊝⊝⊝ very low1,3 | |
| OHSS per cycle | 0 per 1000 | 0 per 1000 (0 to 0) | OR 2.27 (0.65 to 7.91) | 456 (3 studies) | ⊕⊕⊝⊝ low1,2 | |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Methods used for random sequence generation and allocation concealment were unclear. 2There was serious imprecision: findings were compatible with substantial benefit in either group, or with no effect.
3There was very serious imprecision, with very few events and wide confidence intervals.
Summary of findings 5. Early hCG compared to late hCG for intrauterine insemination in subfertile couples.
| Early hCG compared to late hCG for intrauterine insemination in subfertile couples | ||||||
| Population: women undergoing intrauterine insemination Intervention: Early hCG Comparison: Late hCG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Late hCG | Early hCG | |||||
| Pregnancy rate per couple | 86 per 1000 | 110 per 1000 (68 to 175) | OR 1.32 (0.77 to 2.25) | 612 (1 study) | ⊕⊕⊝⊝ low1,2 | |
| Miscarriage rate | 103 per 1000 | 55 per 1000 (9 to 274) | OR 0.51 (0.08 to 3.28) | 65 (1 study) | ⊕⊝⊝⊝ very low1,3 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Unclear risk of attrition bias.
2There was serious imprecision: findings were compatible with substantial benefit in the early hCG group, or with no effect.
3There was very serious imprecision, with very few events and wide confidence intervals.
Summary of findings 6. Differing dosages of hCG for intrauterine insemination in subfertile couples.
| Differing dosages of hCG for intrauterine insemination in subfertile couples | ||||||
| Population: women undergoing intrauterine insemination Intervention: differing dosages of hCG: 500 µg hCG versus 250 µg hCG | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| 250 µg hCG | 500 µg hCG | |||||
| Pregnancy rate per couple | 91 per 1000 | 121 per 1000 (27 to 402) | OR 1.38 (0.28 to 6.71) | 66 (1 study) | ⊕⊝⊝⊝ very low1,2 | |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Methods of random sequence generation and allocation concealment were unclear, high risk of attrition bias.
2There was very serious imprecision, with very few events and wide confidence intervals.
Background
Description of the condition
Subfertility is usually defined as the inability of a couple to conceive after at least one year of unprotected intercourse. This is approximately 10% of couples who try to conceive. Subfertility is considered to be unexplained when an infertility work up consisting of cycle analysis, semen analysis and analysis of at least one patent Fallopian tube was unable to detect any abnormality. Couples with male subfertility have repeated semen analyses below the criteria for normal semen as defined by the World Health Organization (WHO) (WHO 2010). Couples suspected of cervical hostility used to be diagnosed by a well‐timed non‐progressive postcoital test, defined as the absence of spermatozoa moving in a straight direction and at a functional speed. However, nowadays the accuracy of this test and the existence of the diagnosis have been questioned. Finally, mild endometriosis is defined as grade I or II at diagnostic laparoscopy. When one of these causes for subfertility has been identified and the probability of a spontaneous pregnancy is low, the first treatment option is often intrauterine insemination (IUI), although couples with a good prognosis might benefit from expectant management (Steures 2006). The final goal of this treatment is to achieve a pregnancy and deliver a healthy (singleton) live birth. The probability of conceiving with IUI depends on various confounding factors including age of the couple, type of subfertility, ovarian stimulation and the timing of insemination (Rahman 2011).
As spermatozoa and oocytes survive for only a limited period of time, correct timing of IUI seems essential. Therefore, IUI should logically be performed as close to ovulation as possible.
Description of the intervention
There are several options for timing IUI including luteinising hormone (LH) testing, ultrasound scanning, human chorionic gonadotropin (hCG) injection, recombinant LH and gonadotropin‐releasing hormone (GnRH) agonist administration, and basal body temperature (BBT) charts.
LH levels in urine or blood are one of the most precise predictors of ovulation. According to the WHO, ovulation in natural cycles takes place from 24 to 56 hours after the onset of the LH surge, with a mean time of 32 hours (WHO 1980).
In stimulated cycles, when the dominant follicle(s) reaches a certain mean diameter hCG is given to induce ovulation; which occurs approximately 36 to 40 hours after hCG injection (Andersen 1995).
GnRH agonist can also be used for final oocyte maturation and ovulation. GnRH agonists induce an endogenous surge of LH and follicle‐stimulating hormone (FSH), giving a more physiologic approach than with exogenous hCG. The use of GnRH agonists is less widespread because of the high costs (Andrés‐Oros 2008).
How the intervention might work
Each of these interventions is seeking to predict or synchronise ovulation, or both, in order to time the IUI to provide the best pregnancy outcomes.
Why it is important to do this review
Difficulties exist with the different methods of prediction and synchronisation of ovulation. The usefulness of urinary LH monitoring is hampered by the possibility of false‐negative results, which may occur in up to 23% to 35% of ovulatory cycles. The LH peak values may be below the limit of detection for the urine ovulation prediction kit, or the duration of the LH surge is too short to be easily detected. This can cause inaccurate timing and significantly lower pregnancy rates. On the other hand, the ease of performing a test at home, the lower costs and the non‐invasiveness are advantages of urinary LH monitoring (Lewis 2006). Timing by ultrasound combined with hCG administration is time consuming and limited by the possible occurrence of premature LH surges and the possibility of triggering ovulation in the presence of an immature egg (Cantineau 2007; Cohlen 1998; Martinez 1991a). The major advantage of this hCG method is the clinical predictability of the ovulation. A combination of LH surge and hCG administration may minimise the limitations mentioned above (Kosmas 2006).
This review investigates which approach for synchronisation of ovulation results in the highest pregnancy and live birth rates for subfertile couples undergoing IUI.
Objectives
To evaluate the effectiveness of different synchronisation methods in natural and stimulated cycles for IUI in subfertile couples.
Methods
Criteria for considering studies for this review
Types of studies
We included both published and unpublished randomised controlled trials (RCTs). The method of randomisation was assessed to determine whether the studies were truly randomised. Cross‐over trials will be included, but only data from the first phase will be included in the meta analysis. There were no restrictions based on trial duration.
Types of participants
Subfertile couples were eligible for inclusion. We included all types of subfertility where IUI is the first treatment option (for example unexplained subfertility, male subfertility, mild endometriosis, cervical hostility and cycle disturbances).
Routine fertility evaluation should have consisted of confirmed ovulatory status (by a biphasic basal body temperature chart, mid‐luteal progesterone, or sonographic evidence of ovulation), tubal patency (by hysterosalpingography or laparoscopy, or both) and normal results in semen analysis. Subfertility was regarded as due to male factor when at least two separate semen samples did not meet the WHO criteria of normality. A normal quality semen sample was described as having a sperm concentration of 20 x 106 per mL, total motility 50%, normal morphology in 50%, and no sperm antibodies (WHO 1987). In 1992, the WHO changed its criteria for sperm morphology from 50% to 30% (WHO 1992) and for recent trials we used the 1992 definition of normality. Trials before 1992 should have used the WHO criteria of 1987. When strict criteria for morphology were used > 14% was considered normal (Kruger 1993). Since 2010 the reference values have been adapted and the most important changes are: semen volume of 1.5 mL, a sperm concentration of 15 x 106 per mL, total motility 40% and normal morphology in 4% (Cooper 2010; WHO 2010). For future trials these criteria will be applied.
Mild endometriosis was defined as grade I or II at diagnostic laparoscopy. Cervical factor was defined as a negative result with well‐timed postcoital testing. We reported in the review the differences between trials in defining the types of subfertility. Slight differences did not lead to exclusion.
Types of interventions
RCTs comparing any two of the following interventions in couples undergoing IUI were eligible for inclusion:
LH detection in urine or blood, single test;
hCG administration;
a combination of LH detection and hCG administration;
the use of basal body temperature charts;
ultrasound detection of ovulation;
GnRH agonist administration;
other timing methods.
We included both natural cycles and stimulated cycles and considered them separately. We included all types of ovarian stimulation. We excluded trials comparing synchronisation methods using insemination techniques other than IUI, such as timed intercourse, intracervical insemination, gamete intrafallopian transfer (GIFT) and fallopian tube sperm perfusion.
Types of outcome measures
Primary outcomes
Live birth rate per couple
Secondary outcomes
Clinical pregnancy rate per couple (pregnancy rate per couple)
Ongoing pregnancy rate per couple
Optimal time interval from the hCG injection to IUI
Costs of each method of timing (per treatment cycle)
Adverse outcomes
Multiple pregnancies (multiple pregnancy rate per couple and per pregnancy)
Miscarriage rate (miscarriage rate per couple and per pregnancy)
Ovarian hyperstimulation syndrome (OHSS) per couple
Tubal pregnancy (tubal pregnancy rate per couple)
Dropouts (dropout rate per couple)
Clinical pregnancy was established by a positive hCG test in blood or urine and confirmed by ultrasound at around seven weeks of gestation. Ongoing pregnancy was defined as a pregnancy that extended beyond 12 weeks of gestation, confirmed by ultrasound.
Multiple pregnancies were confirmed by ultrasound or delivery. We included pregnancies in which selective reduction was performed, mentioning the original number of fetuses.
We defined a dropout as a couple leaving the study protocol after randomisation.
Not all outcome measures needed to be available to include a study.
Search methods for identification of studies
Electronic searches
We searched for all publications which described (or might describe) RCTs of synchronisation of ovulation with IUI in natural and stimulated cycles. No language restrictions were made and the search was performed in consultation with the Menstrual Disorders and Subfertility Group (MDSG) Trials Search Co‐ordinator.
The Cochrane Menstrual Disorders and Subfertility Group Specialised Register of controlled trials (from inception to October 2014) (Appendix 1).
The Cochrane Central Register of Controlled Trials (CENTRAL; October 2014) (Appendix 2).
The electronic databases of MEDLINE (inception to October 2014) (Appendix 3).
EMBASE (inception to October 2014) (Appendix 4).
PsycINFO (inception to October 2014) (Appendix 5).
The MEDLINE search was combined with the Cochrane highly sensitive search strategy for identifying RCTs, which appears in the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0; Chapter 6, 6.4.11) (Higgins 2011). The EMBASE search was combined with the trial filter developed by the Scottish Intercollegiate Guidelines Network (SIGN) (www.sign.ac.uk/mehodology/filters.html#random).
Other electronic sources of trials included the following.
Trial registers for ongoing and registered trials: 'ClinicalTrials.gov' a service of the US National Institutes of Health (http://clinicaltrials.gov/ct2/home), and the WHO International Clinical Trials Registry Platform search portal (http://www.who.int/trialsearch/Default.aspx).
Conference abstracts in the Web of Knowledge (http://wokinfo.com/).
LILACS database, as a source of trials from the Portuguese and Spanish speaking world (htpp://regional.bvsalud.org/php/index.php?lang=en) (choose ’LILACS’ in ’all sources’ drop‐down box).
PubMed (http://www.ncbi.nlm.nih.gov/pubmed/).
OpenSIGLE database for grey literature from Europe (http://opensigle.inist.fr/).
We searched the databases using the medical subject headings (MsSH terms) and keywords in Appendix 6.
Searching other resources
We checked the reference lists of all identified studies for relevant articles.
We performed a handsearch of abstracts of the American Society for Reproductive Medicine (1999 to October 2014) and the European Society for Human Reproduction and Embryology (1997 to October 2014) meetings.
When important information was lacking from the original publications we tried to contact the authors. We incorporated additional information in the review.
Data collection and analysis
Selection of studies
After screening the titles and abstracts retrieved by the search, full texts of all potentially eligible studies were obtained. MJ Janssen and AEP Cantineau independently selected the trials to be included according to the above mentioned criteria. We resolved disagreements by consensus or through arbitration by BJ Cohlen. We performed an analysis of agreement for inclusion between the two review authors using the crude percentage agreement. This analysis was performed on the primary comparison, the method of randomisation and concealment of allocation. If it was not clear whether a criterion was met, we tried to contact the authors.
Data extraction and management
The same two review authors independently used a data extraction form to extract the data from published reports. We resolved disagreement as described above. This data extraction form includes information on the type of study, quality of the selected studies, types of participants, types of interventions and the types of outcome measures. An analysis of agreement between the two review authors on assessment of the method of randomisation and study design resulted in 100% agreement.
Type of studies
Randomised controlled trials (RCTs) only.
Trial quality
1. Randomisation:
truly randomised, e.g. blocked randomisation list, on‐site computer system, centralised randomisation scheme, random number tables or drawing lots;
stated without further description, or not stated.
Studies which claimed to be randomised but the method of randomisation was not described or not described in detail were placed in the category 'stated without further description'. We included these studies in the 'waiting for assessment' group and contacted the authors for additional information.
2. Concealment of allocation:
adequate (low risk of bias), e.g. sealed opaque envelopes or third party randomisation;
inadequate (high risk of bias), e.g. open list of random numbers, open envelopes, tables;
stated without further description or not stated (unclear risk of bias).
Studies with an allocation low risk of bias or unclear risk of bias were included in the meta‐analysis.
3. Study design:
parallel design, cross‐over design or not clear (we included only parallel group studies or data before cross over, we designated studies that were unclear as 'awaiting assessment');
single centre or multi‐centre;
inclusion criteria, exclusion criteria;
groups similar at baseline regarding the most important prognostic indicators, yes (included), no (excluded), not stated.
4. Blinding:
were the couple, the care provider and the outcome assessor blinded?
5. Analysis:
by intention to treat (ITT);
power calculation (prospective power calculation, no power calculation or not stated).
6. Dropouts:
percentage of dropouts;
reasons for and details on dropouts (selective dropout?).
7. Cancelled cycles:
percentage of cancelled cycles < 10% (> 10% cancelled cycles then mentioned but excluded from meta‐analysis);
reasons for cancelled cycles.
8. Follow up:
duration of follow up;
losses to follow up.
Study participants
9. Prognostic factors:
woman's age;
type of subfertility;
primary or secondary subfertility;
duration of subfertility;
semen quality;
body mass index.
10. Basic fertility work up:
regular menstrual cycles with biphasic body temperature charts or normal luteal progesterone;
patent tubes on hysterosalpingography or laparoscopy, or both.
11. Previous fertility treatment:
tubal surgery;
controlled ovarian hyperstimulation without insemination;
other.
Type of interventions
12. Stimulation protocols:
type and dosage of drugs for mild ovarian hyperstimulation;
days of ovarian stimulation;
number of dominant follicles (> 10 mm);
cancellation criteria, risk of multiple pregnancies or OHSS;
use of luteal support;
allowance of unprotected intercourse during treatment.
13. Semen sample preparation techniques:
type of semen injected, e.g. cryopreserved donor, partner's fresh semen;
amount of semen injected, number of motile spermatozoa;
method of sperm preparation (washing and centrifugation technique, swim up technique, other).
14. Insemination characteristics:
type of insemination catheter;
use of single or double insemination;
number of treatment cycles;
actual timing of IUI (time from LH detection to IUI, time from hCG administration to IUI).
Type of outcome measures
15. Primary outcomes:
the number of live births.
16. Secondary outcomes:
the number of clinical (total and ongoing) pregnancies.
17. Adverse outcomes:
incidence of miscarriage, multiple pregnancies, OHSS, tubal pregnancy.
18. Best time interval for insemination.
19. Costs of each method.
Assessment of risk of bias in included studies
Data for trial characteristics which have been recognised as potential sources of bias, such as the method used in generating the allocation sequence, how allocation was concealed, comparability of participants' baseline variables, and differences in dropout rates between study arms, were independently determined by MJ Janssen and AEP Cantineau as part of the data collection process. The criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011) were used. Where there was uncertainty, authors were contacted to clarify aspects of study design. Differences in agreement between review authors were resolved as described above.
Two review authors independently assessed the included studies for risk of bias using the Cochrane risk of bias assessment tool (www.cochrane‐handbook.org) using the following domains:
selection bias (random sequence generation and allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessment);
attrition bias (incomplete outcome data);
reporting bias (selective reporting);
other bias.
These domains were assessed to have:
high risk of bias;
unclear risk of bias;
low risk of bias.
Disagreements were resolved by discussion or by a third review author. We described all judgements fully and presented the conclusions in the risk of bias table, which was incorporated into the interpretation of review findings by means of sensitivity analyses.
We judged that blinding of the researcher, the personnel or the participants could not influence the outcomes live birth rate, clinical pregnancy rate, miscarriage rate or any of the other outcomes. All included trials were therefore assessed as low risk of bias for blinding.
According to the Cochrane Handbook for Systematic Reviews of Interventions, a trial with missing data was judged as low risk of bias if the missing data were addressed adequately, there was no imbalance between intervention groups and the missing data were not related to the outcome.
Measures of treatment effect
We performed statistical analyses in accordance with the guidelines for statistical analysis developed by The Cochrane Collaboration, outlined in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Higgins 2011).
For dichotomous data, we expressed results for each included study as Mantel‐Haenszel odds ratios (OR) with 95% confidence intervals (CI).
Unit of analysis issues
The primary analysis was per woman randomised. If an included study only reported per cycle data, the author was contacted for additional information. Studies that could not provide us with per woman data were included in the review but not in the meta‐analysis, and were described separately. We included both parallel group and cross‐over trials in the analysis. For cross‐over trials we used only the first cycle(s) before 'crossing over' when the data required were available.
Furthermore, multiple live births were counted as one live birth event.
Dealing with missing data
For missing data, we attempted to contact the investigators. When we could not obtain the missing data from the investigators, we explained the assumptions we made in the extraction and analysis of the data.
Assessment of heterogeneity
We noted statistical heterogeneity between the results of different studies by visually inspecting the scatter in the data points on the graphs and the overlap in their CIs and using the I² statistic. According to the Cochrane Handbook for Systematic Reviews of Interventions, an I² value greater than 50% was judged to indicate substantial heterogeneity. In the case of statistical heterogeneity, we planned to use a random‐effects model instead of the fixed‐effect model, and to explore the original trials for clinical and methodological heterogeneity.
Assessment of reporting biases
Besides statistical and clinical heterogeneity, publication bias might influence the interpretation of the pooled results. To detect publication bias we planned to construct a funnel plot, plotting sample size versus effect size, if there were sufficient studies. This plot is only relevant when five or more studies per comparison are included. The graph is symmetrical when bias is absent.
Data synthesis
If appropriate, we combined the data in a meta‐analysis with RevMan software (RevMan 5), using a fixed‐effect model.
We considered live birth rate and pregnancy outcomes as a positive consequence of treatment. Therefore, a higher proportion achieving these outcomes was considered a benefit. For adverse outcomes such as multiple pregnancy rate, miscarriage rate and OHSS rate, which are negative consequences, higher numbers were considered to be detrimental (increased odds signify relative harm). This needs to be taken into consideration when interpreting the meta‐analyses.
Subgroup analysis and investigation of heterogeneity
A priori, we planned to perform separate subgroup analyses if there were more than two studies in each subgroup, for trials which differed in the following.
Subfertility causes: male factor, unexplained, cervical hostility, mild endometriosis.
Ovarian stimulation protocols: oral ovulation induction agents (anti‐estrogens) versus gonadotropins (follicle‐stimulating hormone (FSH), human menopausal gonadotropin (HMG)).
LH monitoring: once or twice daily, serum LH versus urinary LH.
Sensitivity analysis
We conducted the following sensitivity analyses for the primary outcome, to examine stability regarding the pooled outcomes.
Restriction to studies without high risk of bias.
Use of a random‐effects model.
Use of relative risk rather than odds ratio.
Overall quality of the body of evidence: summary of findings table
We prepared a summary of findings table using GRADEPRO software. This table evaluated the overall quality of the body of evidence for the review outcomes using GRADE criteria (study limitations that is risk of bias, consistency of effect, imprecision, indirectness and publication bias). Judgements about evidence quality (high, moderate or low) were justified, documented and incorporated into reporting of results for each outcome.
Results
Description of studies
Results of the search
When this review was first published, we identified 95 articles relating to the subject. Of these, 39 were excluded as their title and abstract very clearly did not meet the basic inclusion criteria. The remaining 56 articles were analysed in detail, of which 10 studies were included, 2 studies were awaiting assessment and 1 study was defined as ongoing.
When updating the review in 2014 we performed the search again and 113 additional articles were found with the adapted search strategy; 21 studies were identified which potentially provided data comparing different timing modalities. Of these, 11 were excluded when analysed in detail by two review authors (AC and MJ) (Casadei 2006; Gerrits 2011; Ghanem 2011; Ghazizadeh 2009; Ghosh Dastidar 2009; Panchal 2009; Propst 2012; Ramon 2009; Ramon 2009a; Tonguc 2010). Further evaluation based on the inclusion criteria showed six new trials were eligible for inclusion in the review (AboulGheit 2010; da Silva 2012; Kyrou 2012; Nikbakht 2012; Rahman 2011; Sharma 2011). Furthermore, one study was included from the awaiting assessment category of 2009 (Schmidt‐Sarosi 1995) and one study was included from the ongoing trial section (Weiss 2010). The remaining study in the awaiting assessment category (Propst 2007) was excluded. Four studies have been added to the awaiting assessment category (Aydin 2013; Blockeel 2014; Dehghani 2014; Mostafa 2014). One study is ongoing (OVO R&D 2012). Thus, eight studies were included in addition to the results of the first published version. Full agreement was obtained regarding all trials (see Figure 1).
1.
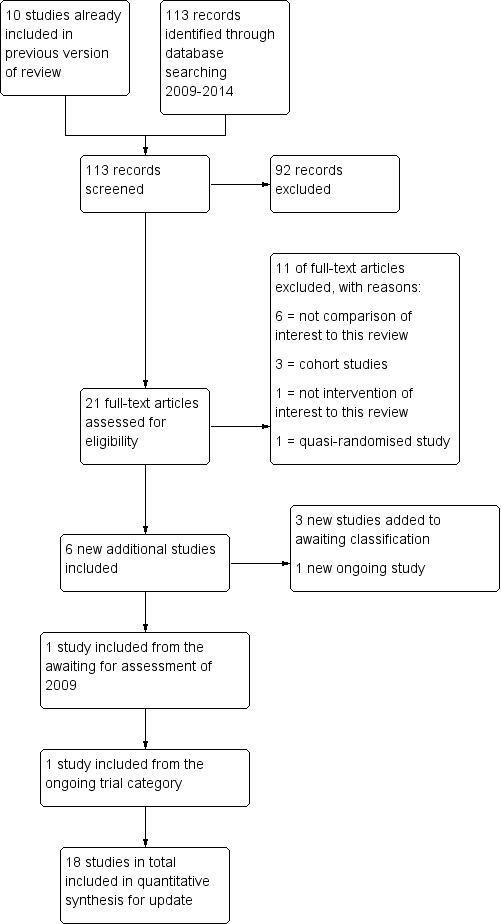
Study flow diagram for 2009 to 2013 literature searches.
The study characteristics and inclusion and exclusion criteria for each study are described in the tables Characteristics of included studies and Characteristics of excluded studies.
Included studies
Eighteen studies were included in total (AboulGheit 2010; Andrés‐Oros 2008; Claman 2004; da Silva 2012; Kyrou 2012; Lewis 2006; Lorusso 2008; Martinez 1991a; Martinez 1991b; Nikbakht 2012; Rahman 2011; Sakhel 2007; Scott 1994; Shalev 1995; Sharma 2011; Schmidt‐Sarosi 1995; Weiss 2010; Zreik 1999) (see Characteristics of included studies). Twelve compared different synchronisation approaches, four compared the optimum time interval from the onset of hCG injection to IUI (AboulGheit 2010; Claman 2004; Rahman 2011; Weiss 2010), one study compared different dosages of hCG injection (Nikbakht 2012) and one study compared early hCG injection (dominant follicle of 16.0 to 16.9 mm) with late hCG injection (dominant follicle 18.0 to 18.9 mm) (da Silva 2012). The study of Lewis 2006, both studies of Martinez 1991a, and the study of Zreik 1999 were used in a meta‐analysis to compare the methods of urinary LH surge versus hCG injection (264 women, 242 first cycle treatments). The study of Kyrou 2012 compared the methods of serum LH detection versus hCG injection in natural cycles. All other studies used some form of ovarian stimulation. Two studies (Lorusso 2008; Sakhel 2007) compared the use of recombinant hCG versus urinary hCG (409 women, 441 cycles) and five studies (Andrés‐Oros 2008; Schmidt‐Sarosi 1995; Scott 1994; Shalev 1995; Sharma 2011) compared the use of hCG versus a GnRH agonist for timing IUI (4 studies, 206 women, 486 cycles). The abstract of Sharma 2011 reported 450 included women but the number of cycles was unclear and the pregnancy rates were expressed in percentages only. Therefore the study was not included in the meta‐analysis. The study of Claman 2004 was not used in a meta‐analysis because only per cycle data were available (75 women, 189 cycles). The study of Kyrou 2012 was not used in the meta‐analysis since more than half of the women underwent insemination for other reasons than subfertility, and there were no data available for the group with subfertility alone (Kyrou 2012). Finally, the study of Weiss 2010 was not included in the meta‐analysis since data per cycle were available with couples who dropped out after randomisation excluded from the analysis (see Characteristics of included studies).
Participants
The age of the participants was stated in all but one trial (Sharma 2011) as either a mean with the standard deviation (SD) for each treatment group or overall. The mean age ranged from 26 to 34 years. There were no statistical differences recorded between the various treatment groups based on age.
All studies included different types of subfertility: unexplained subfertility, mild endometriosis, male factor, cervical factor and tubal or pelvic factor. The study population of Kyrou 2012 contained 58% of women without subfertility (lesbian, single mother) as stated above. Seven studies (Claman 2004; Nikbakht 2012; Sakhel 2007; Schmidt‐Sarosi 1995; Shalev 1995; Weiss 2010; Zreik 1999) also included women with ovulatory disorders. In the studies of Claman 2004 and Zreik 1999 the women with ovulatory disorders comprised less than 15% of all women. In the studies of Sakhel 2007 and Schmidt‐Sarosi 1995 these women comprised around 25% of the total group. In the study of Shalev 1995 69% of the total group of participants had cycle disorders. In all five studies they were equally distributed between the two treatment arms. In the studies of Nikbakht 2012 and Weiss 2010 the number and distribution of these women were not described. Finally, the study of da Silva 2012 included a category 'female factor' (23.4%) without describing details of this group.
The duration of subfertility was given in 10 trials (AboulGheit 2010; da Silva 2012; Lorusso 2008; Martinez 1991a; Martinez 1991b; Nikbakht 2012; Rahman 2011; Sakhel 2007; Weiss 2010; Zreik 1999). In two studies (AboulGheit 2010; Sakhel 2007) the duration was significantly different between the treatment groups. AboulGheit 2010 reported a mean duration of subfertility of 5.6 years in the 24 hours after hCG group compared to a mean of 3.1 and 3.5 years in the 34 hours and 48 hours after hCG groups. Although the pregnancy rates in the first group were lower compared to the other groups, this was not significant. Sakhel 2007 reported a longer duration of subfertility in the group treated with urinary hCG. This difference still remained a factor after analysing the data using logistic regression analysis with clinical pregnancy rate as the dependent variable and controlling for duration of infertility. They did not state if the difference was of any clinical relevance. In the studies of Martinez and co‐workers the mean duration of subfertility was 5.6 and 6.3 years, which was quite long and could have negatively influenced their outcome parameters.
Four studies (da Silva 2012; Nikbakht 2012; Sakhel 2007; Weiss 2010) mentioned the number of couples with primary versus secondary subfertility. Their populations contained between 36% and 68.5% with primary subfertility.
Eight studies (da Silva 2012; Kyrou 2012; Lewis 2006; Martinez 1991a; Schmidt‐Sarosi 1995; Shalev 1995; Sharma 2011; Zreik 1999) stated that they had included women who had undergone previous fertility treatment. Most of the women in the studies of Lewis 2006, Schmidt‐Sarosi 1995 and Zreik 1999 had been treated with clomiphene citrate without IUI. Three studies (da Silva 2012; Martinez 1991a; Sharma 2011) included women who previously had undergone IUI treatment cycles. Kyrou 2012 and Shalev 1995 did not mention the type of previous fertility treatment.
Interventions
Three (Lewis 2006; Martinez 1991b; Zreik 1999) of the four studies comparing urinary LH versus hCG injection used clomiphene citrate as a method of ovarian stimulation. Clomiphene citrate was used either from cycle days three to seven or cycle days five to nine. The fourth study used HMG (Martinez 1991a). One study compared serum LH versus hCG injection in a natural cycle (Kyrou 2012). The studies Lorusso 2008 and Sakhel 2007 comparing recombinant hCG (r‐hCG) with urinary hCG (u‐hCG) both used recombinant FSH (r‐FSH) for ovarian stimulation. However, Sakhel 2007 also added hMG and when the E2 level exceeded 300 pg/mL, or a leading follicle of more than 14 mm diameter was present, a gonadotropin‐releasing hormone antagonist was applied. The studies comparing hCG with a GnRH agonist (Andrés‐Oros 2008; Schmidt‐Sarosi 1995; Scott 1994; Shalev 1995; Sharma 2011) used different ovarian stimulation protocols including clomiphene citrate (Schmidt‐Sarosi 1995; Scott 1994; Sharma 2011), FSH (Andrés‐Oros 2008) and hMG (Shalev 1995). Different stimulation protocols were also used in the studies trying to define the optimal timing of IUI. Rahman 2011 used clomiphene citrate as a method of ovarian stimulation, Claman 2004 and Weiss 2010 used hMG or r‐FSH. Only AboulGheit 2010 compared the optimal timing of IUI after hCG in natural cycles. The study of da Silva 2012 compared early hCG with late hCG depending on the size of the dominant follicle, stimulated with highly purified HMG. Finally, the study of Nikbakht 2012 comparing two doses of r‐hCG achieved ovarian hyperstimulation with clomiphene citrate or letrozole and HMG
Urinary LH versus hCG injection
The use of the technique for timing IUI was one of the comparisons of interest in this review. Lewis 2006 included one group of women which used a home ovulation predictor kit once a day: in the afternoon, starting on day 12. Insemination was scheduled the morning after the first positive test. The women in the hCG group started ultrasound monitoring on day 12 and 10,000 IU hCG was given when there was at least one follicle with a mean diameter of 20 mm and the endometrial thickness was at least 8 mm. A single IUI was scheduled 33 to 42 hours later. Any woman who did not satisfy criteria for hCG administration was instructed to perform home monitoring for an LH surge until their next ultrasound, and to schedule an insemination if her predictor kit gave a positive result. There were no details on how often LH surges were detected in the ultrasound group before a follicle reached the size of 20 mm.
Martinez 1991a started daily ultrasound scanning when total urinary estradiol excretion exceeded 200 mmol/24 hours. When the largest follicle reached a diameter between 18 and 20 mm on ultrasound and the total estradiol excretion was between 300 and 1200 nmol/24 hours women received 10,000 IU hCG. LH detection in the urine was done twice daily from the moment the dominant follicle reached the size of 15 mm. A single IUI was performed 36 to 40 hours after hCG administration or 16 to 28 hours after urinary LH surge detection.
Martinez 1991b started urinary LH monitoring twice a day when the dominant follicle had reached 15 mm in diameter. Women were inseminated 21 hours after an evening positive urine or 24 hours after a morning positive urine. The other treatment group received 10,000 IU hCG when the dominant follicle reached a diameter size between 18 and 22 mm, measured daily by ultrasound when a dominant follicle had reached the size of 15 mm. From 37 to 40 hours after hCG a single IUI was performed.
Zreik 1999 started urinary LH monitoring in the morning on day 10 of the cycle. Ultrasound monitoring in the hCG group started on day 10 and 10,000 IU hCG was given when a leading follicle with diameter 18 mm diameter was noted. In both groups IUI was performed daily for the next two days.
Serum LH versus hCG injection
Kyrou 2012 was the only study using serum LH testing instead of urinary LH testing. The daily monitoring of serum LH levels could start from day 6 of the cycle until the LH rise. When LH started to rise, a second assessment was performed the next day to confirm the LH rise. Criteria for detection were an LH rise of 180% above the latest serum value. In the hCG group women received 5000 IU of hCG as soon as a follicle reached a diameter of ≥ 17 mm. A single IUI was performed 36 h after initiation of the LH rise or 36 h after the hCG injection. In the case where the serum LH suggested an imminent ovulation (LH rise and rise in progesterone) the insemination was performed after 24 h.
Recombinant hCG (r‐hCG) versus urinary hCG (u‐hCG)
Lorusso 2008 monitored ovarian response by ultrasound only. Urinary or recombinant hCG was given when one follicle with a mean diameter of 18 mm or more was present or no more than three follicles had a mean diameter of 16 mm. Double IUI was carried out 24 and 48 hours after administration, except when ovulation had occurred after 24 hours.
Sakhel 2007 monitored ovarian response by ultrasound and serum PGE2. When two or more follicles were 16 mm, with 200 pg/mL E2 per follicle, 10,000 IU u‐hCG or 250 mg r‐hCG was used to induce ovulation. A single IUI was performed 42 hours after the injection but this could be delayed by four hours when there was no collapse of the leading follicle observed on ultrasound. Luteal support was added with progesterone.
hCG versus GnRH agonist (GnRH‐a)
Andrés‐Oros 2008 administered a single injection of triptorelin (0.2 mg) or a single injection of r‐hCG (250 µg) when at least one follicle, and not more than three, reached the size 18 mm or more. A single IUI was performed 36 hours after the injection. Luteal support with progesterone was applied.
Schmidt‐Sarosi 1995 began ultrasound monitoring from cycle day 11. When the largest follicle was > 20 mm, 400 µg nafarelin intranasally (IN) was given on this and the following day, IUI was performed 48h after the first dose. The hCG group received an intramuscular injection of 5000 IU when the largest follicle reached > 20 mm and IUI was performed after 36 h. Luteal support in the GnRH‐a group was given as seven doses of 400 µg nafarelin every 16 hours started 6 days after the first dose. Women in the hCG group received one injection of 2500 IU hCG six days after the primary injection.
Scott 1994 started daily pelvic ultrasound on cycle day 12. When the dominant follicle reached a diameter of 20 to 21 mm the women received GnRH‐a (2 mg leuprolide acetate) subcutaneously or 10,000 IU hCG intramuscularly. Approximately 40 hours after injection, these women underwent a single IUI after a pelvic ultrasound was performed.
Shalev 1995 administered a single injection of triptorelin (0.1 mg) or single injection hCG (10,000 IU) when at least one follicle attained a diameter of 16 mm. Double IUI was performed 24 and 48 hours after the injection.
Sharma 2011 started follicle monitoring from cycle day 10. Urinary hCG (5000 IU) or GnRH‐a (leuprolide 1 mg) was given when a follicular diameter was between 18 and 20 mm with endometrial thickness ≥ 7 mm. A single IUI was performed only after confirmation of ovulation with ultrasound. Luteal support was given with 300 mg vaginal micronized progesterone daily for 15 days.
Optimal time interval
Four studies compared the optimum time interval from ovulation induction to IUI. AboulGheit 2010 triggered ovulation with highly purified hCG (Choriomon, 10,000 IU) intramuscular injection when the leading follicle reached ≥ 18 mm and when at least two follicles reached ≥ 16 mm. Timing of IUI was 24 hours, 34 hours and 48 hours after hCG.
In the study of Claman 2004 the women received 5000 IU hCG intramuscularly or 10,000 IU hCG subcutaneously when two to five follicles were seen on ultrasound with a mean diameter of 17 to 21 mm. Timing of IUI was between 32 and 34 hours or 38 and 40 hours after hCG.
Rahman 2011 started ultrasound monitoring from cycle day 11 or earlier depending on the women’s cycles. An ovulation trigger was given with injection of 5000 IU hCG when at least one follicle reached 18 mm or more and endometrial thickness was at least 7 mm. Single insemination was performed 24 or 36 hours after hCG injection.
Weiss 2010 administered hCG after a cycle with mild ovarian stimulation using gonadotropins and GnRH antagonist. The time and amount of hCG administered was not mentioned, but if five or more follicles over 15 mm were developed, or if ovulation took place before administration of the GnRH antagonist, the couple was excluded. Insemination took place 36 h, 42 h or 48 h after hCG administration. Luteal support was given with endometrin 100 mg twice a day from insemination until eight weeks of gestation.
Size of follicle at hCG injection
da Silva 2012 administered HMG from cycle day 4. Dose adjustments were made according to ovarian response until the criteria for hCG administration were met; 5000 IU of hCG was injected when the dominant follicle was between 16.0 and 16.9 mm diameter and 18.0 and 18.9 mm, respectively, and approximately 36 hours later IUI was performed. Luteal support was obtained with natural micronized progesterone 600 mg/day vaginally.
Two doses of recombinant hCG
In Nikbakht 2012 clomiphene or letrozole and HMG (Pergonal) were administered. When two or more follicles were 16 mm, r‐hCG 250 or 500 ug was used to induce ovulation. A single IUI was performed 42 hours after r‐hCG injection.
The studies used partners' semen, although this was not noted explicitly in all studies. Three studies noted donor cycles (Kyrou 2012; Lewis 2006; Weiss 2010). Semen preparation techniques, the amount of semen fluid injected, the number of motile semen injected and the type of insemination catheter were poorly described or not described at all (see table Characteristics of included studies).
Outcomes
Seven trials (Martinez 1991a; Rahman 2011; Sakhel 2007; Schmidt‐Sarosi 1995; Scott 1994; Shalev 1995; Weiss 2010) reported live birth rates. All but one trial (Claman 2004) assessed pregnancy rate per couple. In one study (Weiss 2010) the couples who dropped out after inclusion were not included in the calculation of the live birth rate and pregnancy rate per couple. Therefore, the latter study was excluded from the meta‐analysis.
Multiple pregnancy rates and miscarriage rates were reported in 11 studies (Andrés‐Oros 2008; da Silva 2012; Lewis 2006; Lorusso 2008; Martinez 1991a; Martinez 1991b; Sakhel 2007; Schmidt‐Sarosi 1995; Scott 1994; Shalev 1995; Weiss 2010). AboulGheit 2010 reported chemical pregnancies and clinical pregnancies separately. The OHSS rate was stated in five studies (Lorusso 2008; Martinez 1991a; Sakhel 2007; Schmidt‐Sarosi 1995; Shalev 1995) and the ectopic pregnancy rate was stated in two publications (Sakhel 2007; Weiss 2010).
One of the studies assessed the costs of the treatment (Lewis 2006). The cost per pregnancy in the LH group was estimated to be USD 3695 and the cost per pregnancy in the hCG group was USD 4830.
Four studies (AboulGheit 2010; Lewis 2006; Nikbakht 2012; Sakhel 2007) diagnosed pregnancy by a rising concentration of hCG. In two studies (Lewis 2006; Rahman 2011) the pregnancy was called viable when a fetal pole with cardiac activity was noted on ultrasound. Five studies (AboulGheit 2010; da Silva 2012; Martinez 1991a; Martinez 1991b; Nikbakht 2012) stated that an ultrasound detection of fetal heart rate activity was performed four weeks after conception and in the study of Kyrou and co‐workers ultrasound detection of fetal heart rate activity was performed 10 weeks after conception. Five studies (Andrés‐Oros 2008; Lorusso 2008; Shalev 1995; Sharma 2011; Weiss 2010) defined clinical pregnancy by the presence of a gestational sac in the uterus, determined by transvaginal ultrasound. Three studies (Schmidt‐Sarosi 1995; Scott 1994; Zreik 1999) did not mention the method of confirming pregnancy.
Studies awaiting assessment
All studies previously awaiting assessment were included (noting that the risk of bias was high, see table Characteristics of included studies).
Attempts have been made to contact authors to get further information about the methods of randomisation, to retrieve unpublished data and for details about published data. Eight replies have been received, resulting in exclusion of four trials (Diaz 2003a; Diaz 2003b; Lewis 2003; Pierson 2002) and inclusion of three trials (Scott 1994; Shalev 1995; Weiss 2010).
Four new studies (Aydin 2013; Blockeel 2014; Dehghani 2014; Mostafa 2014) that were identified will be assessed when this review is next updated.
Ongoing trials
One trial with the comparison of interest is registered on the ClinicalTrials.gov database and is still recruiting couples (OVO R&D 2012) (see Characteristics of ongoing studies). One of the ongoing trials of the 2009 review has been included (Weiss 2010).
Excluded studies
Fifty‐five studies were excluded (see table Characteristics of excluded studies). Reasons for exclusion were: failure to use a truly randomised design (n = 19) (Agarwal 1995; Cedrin‐Durnerin 1993; Check 1994; Costa Franco 2006; Diaz 2003a; Diaz 2008; Fondop 2005; Gerris 1995; Ghanem 2011; Khattab 2005; Kossoy 1989; Martinez 1994; Meherji 2004; Panchal 2009; Romeu 1997a; Romeu 1997b; Shanis 1995; Tavaniotou 2003; Tonguc 2010), not performing the comparison of interest (n = 18) (Arici 1994; Baroni 2001; Casadei 2006; Federman 1990; Fischer 1993; Gerrits 2011; Ghazizadeh 2009; Ghosh Dastidar 2009; Kotecki 2005; Nulsen 1993; Papageorgiou 1995; Pierson 2002; Pirard 2005; Ragni 1999; Ramon 2009; Robinson 1992; Silverberg 1991; Wang 2006), not performing IUI (n = 5) (Barratt 1989; Claraz 1989; George 2007; Odem 1991; Scarpellini 1991), did not meet the inclusion criteria for types of participants (n = 2) (Egbase 2003; Int rhCG study group 2001), or duplicate publications of abstracts or full text articles (n = 8) (Claman 2000; Claman 2004a; Diaz 2003b; Lewis 2002; Lewis 2003; Ramon 2009a; Sakhel 2004; Wang 2001). Finally, one study was excluded from the awaiting assessment category since we did not receive the information we needed about the randomisation method (n = 1) (Propst 2007). The same authors published an abstract in 2012 on the same subject. The research population described seems to be the same group as published before. Additional information was lacking, thus this abstract was excluded as well (Propst 2012).
Risk of bias in included studies
Figure 2 presents our judgements about each methodological quality item, presented as percentages across all included studies, and Figure 3 summarises our judgements about each methodological quality item for each included study.
2.
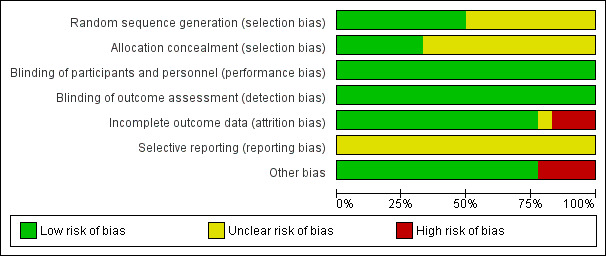
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
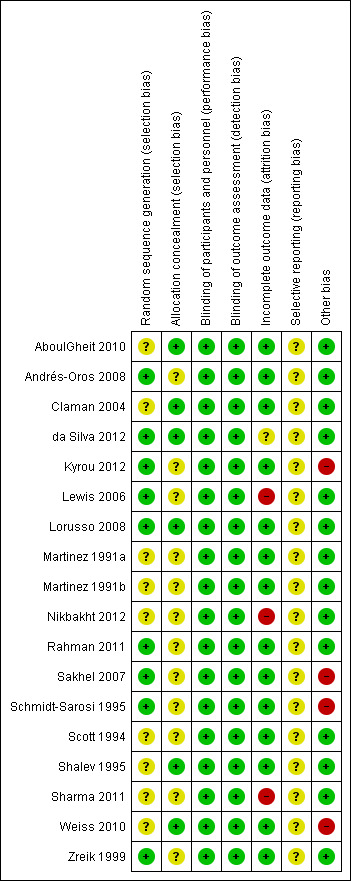
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Study design
Four studies (Martinez 1991a; Martinez 1991b; Scott 1994; Zreik 1999) used a cross‐over design, with pre‐cross over data available. For the meta‐analysis we only included the first cycle data from these cross‐over studies. The trial design was parallel group in the other included studies.
Allocation
The description of methods for randomisation or allocation concealment was generally poor in the published information, which might increase the risk for selection bias. However, additional information was received about allocation methods for most studies.
Random sequence generation
Nine studies mentioned the use of a computer generated program for randomisation (Andrés‐Oros 2008; da Silva 2012; Kyrou 2012; Lewis 2006; Lorusso 2008; Rahman 2011; Sakhel 2007; Shalev 1995; Weiss 2010; Zreik 1999). Five studies (Claman 2004; Martinez 1991a; Martinez 1991b; Schmidt‐Sarosi 1995; Scott 1994) used a random number table, not further specified. Two studies (Nikbakht 2012; Sharma 2011) reported a random assignment without further specification.
Allocation concealment
Concealment of allocation was stated explicitly in six studies (AboulGheit 2010; da Silva 2012; Lewis 2006; Lorusso 2008; Weiss 2010; Zreik 1999). After additional information about allocation had been received, seven other trials (Andrés‐Oros 2008; Claman 2004; Martinez 1991a; Martinez 1991b; Sakhel 2007; Scott 1994; Shalev 1995) could be deemed at low risk of bias in this domain. Concealment of allocation was done by the use of sealed opaque envelopes or a third party (Figure 2; Figure 3). Two studies (Nikbakht 2012; Sharma 2011) were deemed at high risk of this bias. Concealment of allocation was done with sealed envelopes in the latter study.
Blinding
In two studies (Scott 1994; Shalev 1995) blinding was performed. Scott and co‐workers used blinding of the sonographer to minimise the risk of observer bias in determining if ovulation had taken place after injection of hCG or GnRH‐a. None of the trials had details on blinded analysis of the results. All studies were rated at low risk of bias with respect to blinding as we determined that it was unlikely to influence our review outcomes.
Incomplete outcome data
Nine studies reported information on dropouts (Claman 2004; da Silva 2012; Kyrou 2012; Lewis 2006; Martinez 1991a; Martinez 1991b; Weiss 2010; Zreik 1999). The number of dropouts varied from 0% to 31%. Additional information on dropouts was received from four studies (Andrés‐Oros 2008; Sakhel 2007; Shalev 1995; Weiss 2010). The first study (Andrés‐Oros 2008) reported the dropping out of 18 couples who did not meet the criteria to induce ovulation (too many follicles, or no follicles). The main reason for dropout in the study of Weiss and co‐workers was a transfer to in vitro fertilisation (IVF) because of overstimulation. The other five studies reported no dropouts.
Claman and co‐workers stated that the most important reasons for dropping out were a spontaneous LH surge or an inadequate follicular response. Lewis and co‐workers noted failure to detect an LH surge in 23% of the participants in the LH group. In the hCG group 5.3% of the participants dropped out due to personal reasons, especially because of time commitment. An ITT analysis was performed resulting in no significant difference between the treatment groups. In the study of Zreik and co‐workers only one couple out of 54 was excluded, due to failure in compliance. None of the included women in the studies by Martinez 1991b and Kyrou 2012 dropped out. The other study of Martinez (Martinez 1991a) reported that five women decided to stop after the second cycle, and five did not complete the third cycle. Finally, the study of da Silva 2012 reported major protocol deviations in 117/635 couples, no hCG due to insufficient follicular growth in 61/635 couples, and serum estradiol (E2) > 1500 pg/ml or premature LH peak (LH > 10 mIU/ml). No explanation for protocol deviation was reported.
Selective reporting
A total of 44% of the included studies reported live birth rates. The remaining studies defined clinical pregnancy rates (see table Characteristics of included studies).
Other potential sources of bias
Sakhel and co‐workers reported that the included women in the u‐hCG group had a greater mean duration of infertility than the r‐hCG group, which may have been a source of bias in this study. The same applies to the study of AboulGheit 2010 where the couples in the IUI 24 hours after hCG group had a longer mean duration of infertility. Weiss and co‐workers reported significantly more miscarriages in the group with a time interval of 36 hours, and the study of Kyrou and co‐workers included a high percentage of non‐subfertile women. da Silva 2012 did not report the exact size of the dominant follicles per group, which might have introduced bias. Finally, Sharma 2011 excluded 20 couples before randomisation for unclear reasons.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Overall 18 studies with a total of 2279 couples were included in the review.
1. hCG versus LH surge
Four studies compared hCG with LH surge for timing IUI (Lewis 2006; Martinez 1991a; Martinez 1991b; Zreik 1999).
1.1 Live birth rate
One study (Martinez 1991a) reported live birth rate. There was no evidence of a difference between hCG and LH surge (odds ratio (OR) 1.0, 95% confidence interval (CI) 0.06 to 18.08; 1 trial, 24 women, very low quality evidence) (Analysis 1.1).
1.1. Analysis.
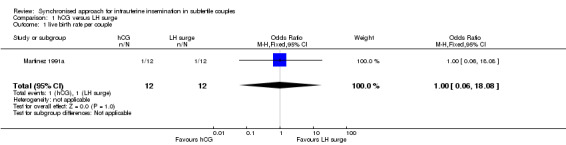
Comparison 1 hCG versus LH surge, Outcome 1 live birth rate per couple.
1.2 Pregnancy rate
All trials included for this comparison reported pregnancy rate per couple. The result revealed no evidence of a difference in pregnancy rate per couple (OR 1.33, 95% CI 0.72 to 2.45; 4 trials, 275 women, I2 = 0%, low quality evidence) (Analysis 1.2, Figure 4).
1.2. Analysis.
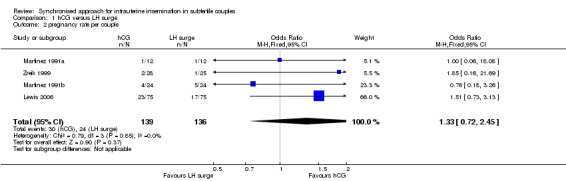
Comparison 1 hCG versus LH surge, Outcome 2 pregnancy rate per couple.
4.
Forest plot of comparison: 1 hCG versus LH surge, outcome: 1.2 pregnancy rate per couple.
1.3 Multiple pregnancy rate
The meta‐analysis of two studies (Lewis 2006; Martinez 1991a) revealed no evidence of a difference in multiple pregnancy rates (OR 1.12, 95% CI 0.17 to 7.6; 2 trials, 42 pregnancies, very low quality evidence) (Analysis 1.3).
1.3. Analysis.
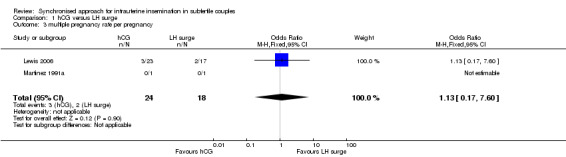
Comparison 1 hCG versus LH surge, Outcome 3 multiple pregnancy rate per pregnancy.
2. u‐hCG versus r‐hCG
Two studies (Lorusso 2008; Sakhel 2007) compared u‐hCG with r‐hCG for timing IUI.
2.1 Live birth rate
One study (Sakhel 2007) reported live birth rate, which showed no evidence of a difference between u‐hCG and r‐hCG (OR 1.17, 95% CI 0.68 to 2.03; 1 trial, 284 women, low quality evidence) (Analysis 2.1).
2.1. Analysis.
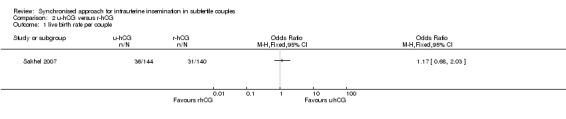
Comparison 2 u‐hCG versus r‐hCG, Outcome 1 live birth rate per couple.
2.2 Pregnancy rate
All trials included in this comparison reported pregnancy rate per couple. The result revealed no evidence of a difference in pregnancy rate per couple (OR 1.02, 95% CI 0.65 to 1.57; 2 trials, 409 women, I2 = 0%, low quality evidence) (Analysis 2.2, Figure 5).
2.2. Analysis.
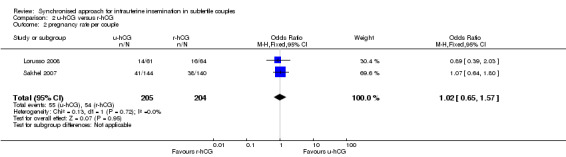
Comparison 2 u‐hCG versus r‐hCG, Outcome 2 pregnancy rate per couple.
5.
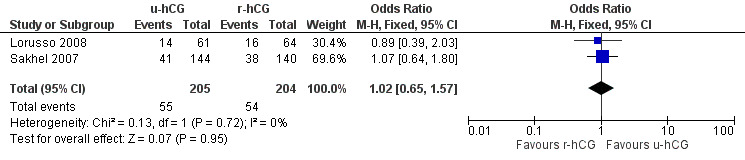
Forest plot of comparison: 2 u‐hCG versus r‐hCG, outcome: 2.2 pregnancy rate per couple.
2.3 Multiple pregnancy rate
No evidence of a difference in multiple pregnancy rates was reported (OR 0.99, 95% CI 0.4 to 2.47; 2 trials, 109 pregnancies, low quality evidence) (Analysis 2.3).
2.3. Analysis.
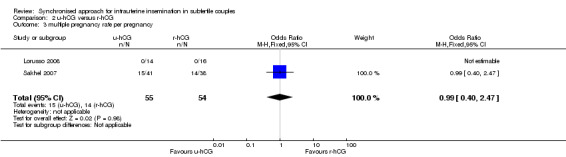
Comparison 2 u‐hCG versus r‐hCG, Outcome 3 multiple pregnancy rate per pregnancy.
2.4 Miscarriage rate
Miscarriages per treatment group showed no evidence of a difference between groups (OR 0.57, 95% CI 0.13 to 2.47; 2 trials, 109 pregnancies, I2 = 0%, very low quality evidence) (Analysis 2.4, Figure 6).
2.4. Analysis.
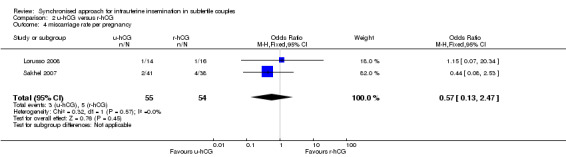
Comparison 2 u‐hCG versus r‐hCG, Outcome 4 miscarriage rate per pregnancy.
6.
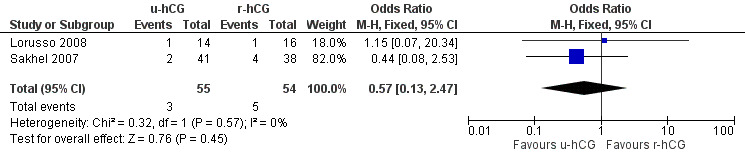
Forest plot of comparison: 2 u‐hCG versus r‐hCG, outcome: 2.4 miscarriage rate per pregnancy.
2.5 OHSS rate
Both studies reported no cases of (severe) OHSS in a total of 468 cycles (moderate quality evidence) (Analysis 2.5).
2.5. Analysis.
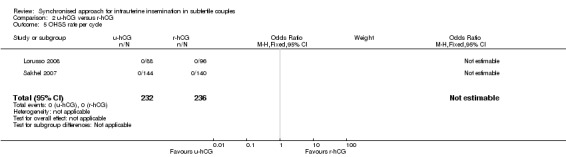
Comparison 2 u‐hCG versus r‐hCG, Outcome 5 OHSS rate per cycle.
3. Short versus long interval
Two studies (AboulGheit 2010; Rahman 2011) compared a short interval (24 hours) with a long interval (34 to 36 hours) after hCG. AboulGheit 2010 included a third group (IUI 48 hours after hCG).
3.1 Live birth rate
One study (Rahman 2011) reported live birth rate, which showed no evidence of a difference between IUI after 24 hours and 34 hours (OR 0.52, 95% CI 0.27 to 1.00; 1 trial, 204 couples, low quality evidence) (Analysis 3.1).
3.1. Analysis.
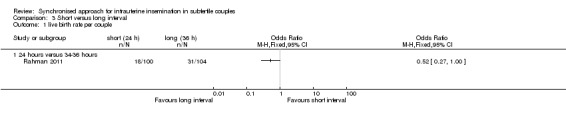
Comparison 3 Short versus long interval, Outcome 1 live birth rate per couple.
3.2 Pregnancy rate
Both studies reported pregnancy rate per couple. The meta‐analysis revealed a lower pregnancy rate in the 24 hour group, when IUI was after 24 hours compared with IUI after 34 to 36 hours (OR 0.55, 95% CI 0.31 to 0.98; 2 trials, 234 women, I2 = 0%, low quality evidence) (Analysis 3.2). AboulGheit 2010 also compared IUI after 24 hours with IUI after 48 hours and found no evidence of a difference between the groups (OR 0.44, 95% CI 0.10 to 1.92; 1 trial, 30 women, low quality evidence) (Analysis 3.2). Nor was there a diffference between IUI after 34 to 36 hours and IUI after 48 hours (OR 0.58, 95% CI 0.14 to 2.48; 1 trial, 30 women, low quality evidence) (Analysis 3.2, Figure 7).
3.2. Analysis.
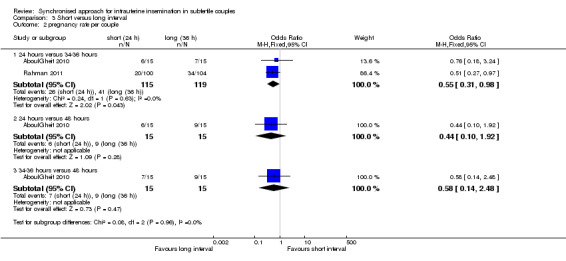
Comparison 3 Short versus long interval, Outcome 2 pregnancy rate per couple.
7.
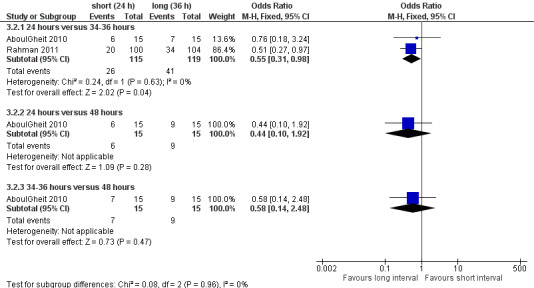
Forest plot of comparison: 3 short versus long interval, outcome: 3.2 pregnancy rate per couple.
3.3 Miscarriage rate
Both studies reported miscarriage rates, with no evidence of a difference between the groups of 24 hours versus 34 to 36 hours (OR 1.58, 95% CI 0.35 to 7.16; 2 trials, 67 pregnancies, I2 = 0%, very low quality evidence); 24 hours versus 48 hours (OR 4.0, 95% CI 0.27 to 58.56; 1 trial, 15 women, very low quality evidence); 34 to 36 hours versus 48 hours (OR 1.33, 95% CI 0.07 to 25.91; 1 trial, 16 women, very low quality evidence) (Analysis 3.3) respectively. Two studies (Claman 2004; Weiss 2010) were excluded from the meta‐analysis since they reported results as pregnancy rates per cycle only. The former did not report a difference between 32 to 34 hours and 38 to 40 hours after hCG, and the latter study was stopped prematurely because of an unusual number of multi‐fetal pregnancies; the study reported a higher pregnancy rate for 42 hours after hCG compared to 36 hours or 48 hours (see table 'Characteristics of included studies' for details, Figure 8).
3.3. Analysis.
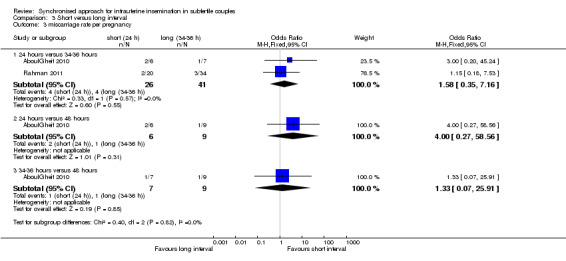
Comparison 3 Short versus long interval, Outcome 3 miscarriage rate per pregnancy.
8.
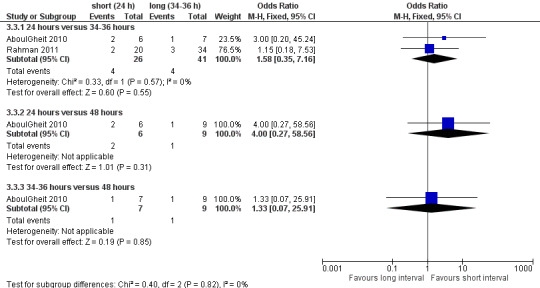
Forest plot of comparison: 3 short versus long interval, outcome: 3.3 miscarriage rate per pregnancy.
4. hCG versus GnRH‐a
Four studies (Andrés‐Oros 2008; Schmidt‐Sarosi 1995; Scott 1994; Shalev 1995) compared hCG versus GnRH‐a.
4.1 Live birth rate
The results for live birth rate per couple revealed no evidence of a difference between the groups (OR 1.04, 95% CI 0.42 to 2.56; 3 trials, 104 women, I2 = 0%, low quality evidence) (Analysis 4.1, Figure 9).
4.1. Analysis.
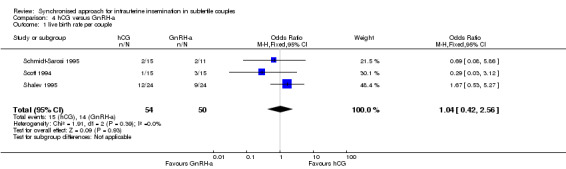
Comparison 4 hCG versus GnRH‐a, Outcome 1 live birth rate per couple.
9.
Forest plot of comparison: 4 hCG versus GnRH‐a, outcome: 4.1 live birth rate per couple.
4.2 Pregnancy rate
All trials reported the pregnancy rate per couple revealing no evidence of a difference between groups (OR 1.14, 95% CI 0.63 to 2.08; 4 trials, 206 women, I2 = 48%, low quality evidence) (Analysis 4.2, Figure 10).
4.2. Analysis.
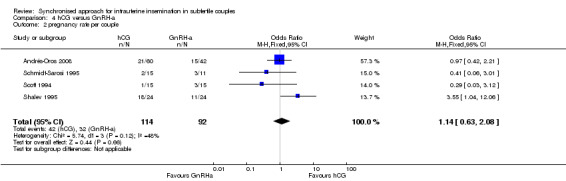
Comparison 4 hCG versus GnRH‐a, Outcome 2 pregnancy rate per couple.
10.
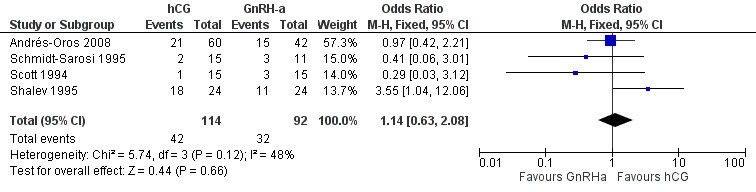
Forest plot of comparison: 4 hCG versus GnRH‐a, outcome: 4.2 pregnancy rate per couple.
4.3 Multiple pregnancy rate
The studies reported three twin pregnancies in the GnRH‐a group and none in the hCG group. There was no evidence of a difference in multiple pregnancy rates between hCG and GnRH‐a (OR 0.15, 95% CI 0.02 to 1.38; 4 trials, 74 pregnancies, I2 = 0%, very low quality evidence) (Analysis 4.3, Figure 11).
4.3. Analysis.
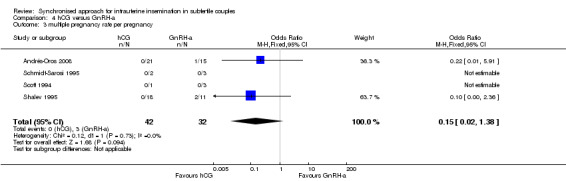
Comparison 4 hCG versus GnRH‐a, Outcome 3 multiple pregnancy rate per pregnancy.
11.
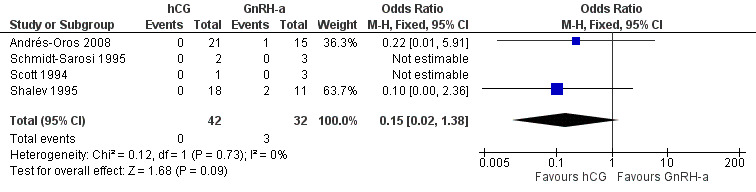
Forest plot of comparison: 4 hCG versus GnRH‐a, outcome: 4.3 multiple pregnancy rate per pregnancy.
4.4 Miscarriage rate
There was no evidence of a difference in the miscarriage rate between the GnRH‐a and hCG group (OR 1.72, 95% CI 0.48 to 6.2; 4 trials, 74 pregnancies, I2 = 0%, very low quality evidence) (Analysis 4.4, Figure 12).
4.4. Analysis.
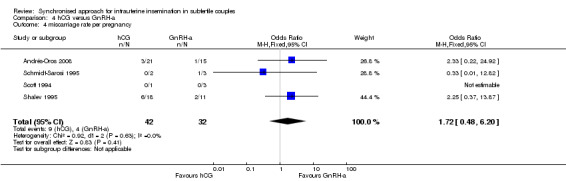
Comparison 4 hCG versus GnRH‐a, Outcome 4 miscarriage rate per pregnancy.
12.
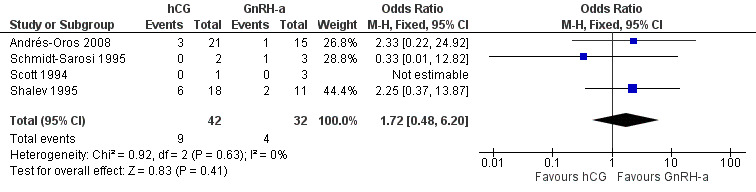
Forest plot of comparison: 4 hCG versus GnRH‐a, outcome: 4.4 miscarriage rate per pregnancy.
4.5 OHSS rate
OHSS rates were compared and there was no evidence of a difference between groups (OR 2.27, 95% CI 0.65 to 7.91; 3 trials, 456 women, low quality evidence) (Analysis 4.5). Shalev 1995 reported four treatment cycles with grade three to grade four OHSS in the GnRH‐a group, and eight treatment cycles with OHSS in the hCG group; the other two studies in this meta‐analysis reported none in either group.
4.5. Analysis.
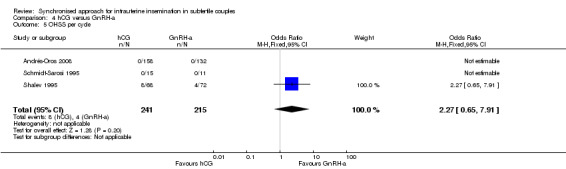
Comparison 4 hCG versus GnRH‐a, Outcome 5 OHSS per cycle.
5. Early versus late hCG
One study (da Silva 2012) compared early hCG versus late hCG.
5.1 Pregnancy rate
No evidence of a difference was reported between both treatment groups in the pregnancy rate per couple (OR 1.32, 95% CI 0.77 to 2.25; 1 trial, 612 women, low quality evidence) (Analysis 5.1).
5.1. Analysis.
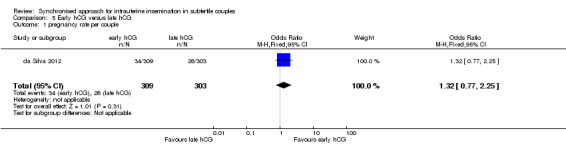
Comparison 5 Early hCG versus late hCG, Outcome 1 pregnancy rate per couple.
5.2 Miscarriage rate
No evidence of a difference between miscarriages rates was reported (OR 0.51, 95% CI 0.08 to 3.28; 1 trial, 65 pregnancies, very low quality evidence) (Analysis 5.2).
5.2. Analysis.
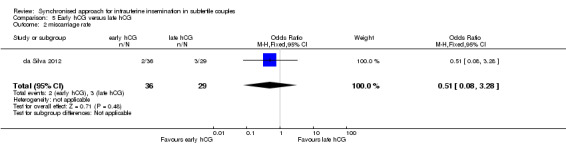
Comparison 5 Early hCG versus late hCG, Outcome 2 miscarriage rate.
The authors reported two multiple pregnancies in the early hCG group and none in the late hCG group.
6. Different dosages of hCG
One trial (Nikbakht 2012) compared 250 ug r‐hCG with 500 ug r‐hCG.
6.1 Pregnancy rate
No evidence of a difference in pregnancy rate per couple was reported (OR 1.38, 95% CI 0.28 to 6.71; 1 trial, 66 women, very low quality evidence) (Analysis 6.1).
6.1. Analysis.
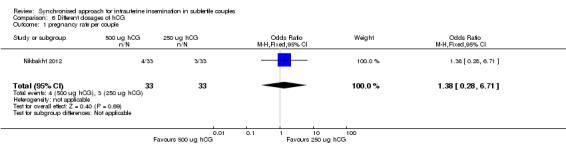
Comparison 6 Different dosages of hCG, Outcome 1 pregnancy rate per couple.
Discussion
Summary of main results
The aim of this review was to investigate the optimal synchronisation of ovulation with intrauterine insemination (IUI) in subfertile couples undergoing natural and stimulated cycles with regard to live birth rates. The trials in this review revealed that not one of the available methods is superior to another. However, the available evidence is scarce due to small sample sizes and lack of data concerning the primary outcome.
hCG injection versus LH surge detection
Although the dropout rate in the LH surge group was much higher than in the hCG group (due to no detection of a LH surge in 23% of the cycles) there was no evidence of a difference in live birth or pregnancy rates between these treatment groups (OR 1.5, 95% CI 0.73 to 3.1) (Lewis 2006).
The cause of dropouts in the LH surge group could be the absence of detection of LH surges in urine samples. This has been reported in other studies as well, due to a short LH surge or incorrect use of the intervention by the woman (Miller 1996). When counselling couples, the advantages of home ovulation predictor tests (no difference in pregnancy outcomes compared to hCG injection, convenience and low costs) and disadvantages (high number of false‐negative results) should be considered in relationship to the advantages (low number of false‐negative results) and disadvantages (expensive and time consuming) of ultrasound detection combined with hCG injection. No data on the occurrences of premature LH surges in the hCG group have been reported in the pooled studies. This might negatively influence the treatment outcome in the hCG group, resulting in lower pregnancy rates and no perceptible difference between timing using LH surge detection and hCG injection (Cantineau 2007).
The general quality of the evidence was estimated to be low or very low, meaning that further research is likely or very likely to have an important impact on our confidence in the estimate of effect and is likely to change this estimate (Table 1).
Urinary hCG (u‐hCG) versus recombinant hCG (r‐hCG)
No evidence of a difference in pregnancy rates was found between u‐hCG and r‐hCG. Other reasons such as costs, injection site reactions and possible batch‐to‐batch inconsistencies should be considered in deciding which to use.
The general quality of the evidence was estimated to be low or very low (Table 2).
Short (24 hours) versus long interval (36 hours)
The evidence provided by prospective studies (AboulGheit 2010; Rahman 2011) comparing different hCG to IUI intervals after ovarian stimulation revealed more live births when an interval of 34 to 36 hours was used. However, this difference was not statistically significant. A higher number of pregnancies was reported when IUI was performed 34 to 36 hours after hCG compared to IUI 24 hours after hCG injection. This might be in part due to a significant difference in the duration of subfertility (significantly longer in the 24 hours group in the study of AboulGheit 2010). This study and other studies that only reported pregnancy rate per cycle suggest a more flexible approach in timing IUI after hCG, which allows women to inject hCG in the early evening when pharmacies are still open, in case of problems (Claman 2004).
The general quality of the evidence was estimated to be low or very low (Table 3).
hCG versus GnRH agonist (GnRH‐a)
No evidence of a difference was found, when analysing live birth rates and pregnancy rates, between the timing methods using hCG and GnRH‐a. More evidence is needed to determine the place of GnRH‐a as a timing method for IUI, also considering costs and secondary outcomes such as the OHSS rate.
The general quality of the evidence was estimated to be low or very low (Table 4).
Early hCG versus late hCG depending on the size of the dominant follicle
As well as the ITT analysis, the per protocol analysis reported no advantage of hCG injection with a dominant follicle between 16.0 and 16.9 mm compared to a dominant follicle between 18.0 and 18.9 mm (da Silva 2012). Significantly more dominant follicles and significantly higher estradiol levels were seen in the late group without significantly increased numbers of premature LH surges or clinical pregnancies. No information was reported on the exact sizes of the dominant follicles. For example, when a dominant follicle was 17 mm in the early group it was unclear whether it was stated as a major protocol deviation. Since the day of hCG administration and the total dose of HMG did not differ, it is questionable how different the groups really were.
The general quality of the evidence was estimated to be low or very low (Table 5).
Different dosages of hCG
No evidence of a difference was found between 250 µg r‐hCG and 500 µg r‐hCG. Significantly more dominant follicles were seen in the 250 µg r‐hCG group, which might be a confounding factor.
The quality of the evidence overall was estimated to be low or very low (Table 6).
Overall completeness and applicability of evidence
Definite answers could not be given for most comparisons. When performing IUI, small numbers show a positive effect of insemination around 34 to 36 hours compared to 24 hours after hCG injection.
Quality of the evidence
The quality of the evidence for most comparisons was low or very low. The main limitations in the evidence were failure to describe study methods, serious imprecision and attrition bias.
Potential biases in the review process
Our searches aimed to identify all potentially eligible studies.
Agreements and disagreements with other studies or reviews
No other reviews were available concerning the difference between hCG injection and the LH detection test for timing IUI. Other retrospective studies revealed conflicting results.
Authors' conclusions
Implications for practice.
There is insufficient evidence to determine whether different methods of synchronization of ovulation and insemination differ in safety and effectiveness. More research is needed.
There is no evidence to advise one of the treatment options over another (ultrasound combined with hCG injection versus urinary LH surge detection, medication to time the insemination, dose of medication, time interval between medication and insemination) since live births and pregnancy rates do not differ significantly. The choice should be based on hospital facilities, convenience for the couple, medical staff, costs and dropout levels.
The choice of urinary hCG or recombinant hCG should be based on costs and couples' preferences since pregnancy rates are not significantly different.
Since the evidence suggested an advantage of insemination 34 to 36 hours after hCG, this could be advised until more reliable evidence is available from well‐powered RCTs.
The results suggest that no advice could be given on the timing of hCG injection in relationship to the size of the dominant follicles nor on the dosages of recombinant hCG.
Implications for research.
Large prospective multi‐centre trials with adequate concealment of allocation comparing ultrasound monitoring combined with hCG injection and LH surge detection in urinary samples should be performed with special attention to costs and the convenience of the treatments.
Large prospective multi‐centre trials with adequate concealment of allocation and comparing different time intervals between hCG and IUI should be performed, with special attention to convenience for the patient. Data should be adequately reported as the live birth rate per couple or at least as the ongoing pregnancy rate per couple. Adverse effects should also be reported.
What's new
| Date | Event | Description |
|---|---|---|
| 15 October 2014 | New search has been performed | Eight new trials were included in the review (AboulGheit 2010; da Silva 2012; Kyrou 2012; Nikbakht 2012; Rahman 2011; Sharma 2011; Schmidt‐Sarosi 1995; Weiss 2010). Four studies were placed in awaiting classification (Aydin 2013; Blockeel 2014; Dehghani 2014; Mostafa 2014). |
| 15 October 2014 | New citation required but conclusions have not changed | Based on the new meta‐analysis the conclusions are not changed. |
History
Protocol first published: Issue 1, 2008 Review first published: Issue 4, 2010
| Date | Event | Description |
|---|---|---|
| 6 July 2009 | Amended | The protocol stated that no couples with cycle disturbances should be included, however almost all studies, apart from in unexplained subfertility and male subfertility, also included a category of women with cycle disturbances. We accepted this when only a some of the included couples belonged to this category. |
| 10 November 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Dr R Bernardus as co‐author of the publications with Dr Martinez for the additional information. The same applies to Dr Abuzeid for the additional information provided about the article of Sakhel and co‐workers, and to Dr Lewis, Dr Andrés Oros, Dr Claman, Dr Scott, Dr Shalev, Dr Weiss, Dr García‐Velasco and Dr Pierson for information on their publications.
Appendices
Appendix 1. MDSG search strategy
Keywords CONTAINS "artificial insemination" or "IUI" or "IUI timing" or "Intrauterine Insemination" or Title CONTAINS "artificial insemination" or "IUI" or "IUI timing" or "Intrauterine Insemination"
AND
Keywords CONTAINS "human chorionic gonadotrophin" or "human chorionic gonadotropin" or "human menopausal gonadotrophin" or "HCG" or "chorionic gonadotrophins" or "GnRH agonist" or "GnRH agonists" or "GnRH analog" or "GnRH analogue" or "GnRH analogues" or "GnRHa" or "GnRHa‐gonadotropin" or "Luteinising hormone releasing hormone" or "luteinizing hormone" or "Lutenising hormone releasing hormone" or "luteinizing hormone supplementation" or "lh" or "basal body temp" or "hMG" or "Profasi" or "BBT" or "ultrasonography" or "ultrasound" or "timing LH surge" or "timing of insemination" or "timing ovulation" or "timing of administration" or "timed intercourse" or "time of insemination"
Appendix 2. CENTRAL search strategy
Central Register of Controlled Trials <1st Quarter 2009> 1 human chorionic gonadotropin.tw. (311) 2 hCG.tw. (829) 3 choriogon$.tw. (5) 4 (pregnyl or chorulon or gonabion).tw. (2) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1775) 6 (LH or ICSH).tw. (1829) 7 exp Chorionic Gonadotropin/ (524) 8 body temperature$.tw. (1125) 9 GnRH agonist.tw. (503) 10 GnRH analogue.tw. (110) 11 gonadotropin releasing hormone agonist.tw. (245) 12 GnRHa.tw. (169) 13 HMG.tw. (1026) 14 human menopausal gonadotropin.tw. (169) 15 (profasi or ovitrelle).tw. (9) 16 gonadotrophin releasing hormone agonist.tw. (111) 17 BBT.tw. (26) 18 ultrasound$.tw. (4652) 19 ultrasonograph$.tw. (2199) 20 time$.tw. (99572) 21 timing.tw. (2259) 22 or/1‐21 (110066) 23 Artificial Insemination.tw. (51) 24 intrauter$ inseminat$.tw. (329) 25 (intra‐uter$ adj2 inseminat$).tw. (25) 26 iui.tw. (227) 27 AIH.tw. (23) 28 exp artificial insemination/ or exp ovulation induction/ (858) 29 or/23‐28 (1085) 30 22 and 29 (711) 31 (ivf or icsi).tw. (1833) 32 (in vitro fertilization or intracytoplasmic sperm injection).tw. (1271) 33 or/31‐32 (2318) 34 30 not 33 (392) 35 from 34 keep 1‐392 (392)
Cochrane Central Register of Controlled Trials <April 2012>
1 human chorionic gonadotropin.tw. (371)
2 hCG.tw. (981)
3 choriogon$.tw. (5)
4 (pregnyl or chorulon or gonabion).tw. (2)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1964)
6 (LH or ICSH).tw. (2030)
7 exp Chorionic Gonadotropin/ (587)
8 body temperature$.tw. (1279)
9 GnRH agonist.tw. (579)
10 GnRH analogue.tw. (123)
11 gonadotropin releasing hormone agonist.tw. (270)
12 GnRHa.tw. (190)
13 HMG.tw. (1112)
14 human menopausal gonadotropin.tw. (182)
15 (profasi or ovitrelle).tw. (10)
16 gonadotrophin releasing hormone agonist.tw. (117)
17 BBT.tw. (30)
18 ultrasound$.tw. (5976)
19 ultrasonograph$.tw. (2664)
20 time$.tw. (120256)
21 timing.tw. (2821)
22 or/1‐21 (132789)
23 exp Insemination, Artificial/ (269)
24 Artificial Insemination.tw. (53)
25 intrauter$ inseminat$.tw. (386)
26 (intra‐uter$ adj2 inseminat$).tw. (27)
27 iui.tw. (284)
28 AIH.tw. (26)
29 or/23‐28 (558)
30 22 and 29 (264)
31 limit 30 to yr="2009 ‐Current" (38)
Central register of controlled trials < dec 2012>
1 human chorionic gonadotropin.tw. (374) 2 hCG.tw. (995) 3 choriogon$.tw. (5) 4 (pregnyl or chorulon or gonabion).tw. (2) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1965) 6 (LH or ICSH).tw. (2040) 7 exp Chorionic Gonadotropin/ (588) 8 body temperature$.tw. (1285) 9 GnRH agonist.tw. (589) 10 GnRH analogue.tw. (124) 11 gonadotropin releasing hormone agonist.tw. (271) 12 GnRHa.tw. (190) 13 HMG.tw. (1116) 14 human menopausal gonadotropin.tw. (182) 15 (profasi or ovitrelle).tw. (10) 16 gonadotrophin releasing hormone agonist.tw. (118) 17 BBT.tw. (30) 18 ultrasound$.tw. (6038) 19 ultrasonograph$.tw. (2674) 20 time$.tw. (121198) 21 timing.tw. (2855) 22 or/1‐21 (133854) 23 exp Insemination, Artificial/ (270) 24 Artificial Insemination.tw. (54) 25 intrauter$ inseminat$.tw. (399) 26 (intra‐uter$ adj2 inseminat$).tw. (28) 27 iui.tw. (293) 28 AIH.tw. (26) 29 or/23‐28 (580) 30 22 and 29 (269) 31 limit 30 to yr="2012 ‐Current" (3)
Appendix 3. MEDLINE search strategy
MEDLINE ( 1966 to March 2009)
1 human chorionic gonadotropin.tw. (9408)
2 hCG.tw. (17711)
3 choriogon$.tw. (806)
4 (pregnyl or chorulon or gonabion).tw. (49)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (48292)
6 (LH or ICSH).tw. (39220)
7 exp Chorionic Gonadotropin/ (26357)
8 body temperature$.tw. (17493)
9 GnRH agonist.tw. (2074)
10 GnRH analogue.tw. (610)
11 gonadotropin releasing hormone agonist.tw. (1062)
12 GnRHa.tw. (819)
13 HMG.tw. (10035)
14 human menopausal gonadotropin.tw. (1057)
15 (profasi or ovitrelle).tw. (27)
16 gonadotrophin releasing hormone agonist.tw. (302)
17 BBT.tw. (291)
18 ultrasound$.tw. (99121)
19 ultrasonograph$.tw. (55953)
20 time$.tw. (1545099)
21 timing.tw. (50623)
22 or/1‐21 (1789257)
23 exp Insemination, Artificial/ (8561)
24 Artificial Insemination.tw. (3649)
25 intrauter$ inseminat$.tw. (1295)
26 (intra‐uter$ adj2 inseminat$).tw. (140)
27 iui.tw. (808)
28 AIH.tw. (902)
29 or/23‐28 (10983)
30 22 and 29 (2844)
31 randomised controlled trial.pt. (266031)
32 controlled clinical trial.pt. (78661)
33 (randomised or randomised).ab. (210367)
34 placebo.ab. (110319)
35 drug therapy.fs. (1291549)
36 randomly.ab. (128228)
37 trial.ab. (183721)
38 groups.ab. (888340)
39 or/31‐38 (2358832)
40 (animals not (humans and animals)).sh. (3251132)
41 39 not 40 (1999305)
42 30 and 41 (469)
43 (ivf or icsi or intracytoplasmic sperm injection$).tw. (13661)
44 42 not 43 (394)
45 from 44 keep 1‐394 (394)
MEDLINE (January 2009 to 07 May 2012)
1 human chorionic gonadotropin.tw. (10613)
2 hCG.tw. (19802)
3 choriogon$.tw. (862)
4 (pregnyl or chorulon or gonabion).tw. (65)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (51259)
6 (LH or ICSH).tw. (43606)
7 exp Chorionic Gonadotropin/ (28013)
8 body temperature$.tw. (20554)
9 GnRH agonist.tw. (2496)
10 GnRH analogue.tw. (726)
11 gonadotropin releasing hormone agonist.tw. (1204)
12 GnRHa.tw. (984)
13 HMG.tw. (11574)
14 human menopausal gonadotropin.tw. (1093)
15 (profasi or ovitrelle).tw. (30)
16 gonadotrophin releasing hormone agonist.tw. (341)
17 BBT.tw. (347)
18 ultrasound$.tw. (131852)
19 ultrasonograph$.tw. (68456)
20 time$.tw. (2066122)
21 timing.tw. (69281)
22 or/1‐21 (2365234)
23 exp Insemination, Artificial/ (9448)
24 Artificial Insemination.tw. (4477)
25 intrauter$ inseminat$.tw. (1616)
26 (intra‐uter$ adj2 inseminat$).tw. (165)
27 iui.tw. (1036)
28 AIH.tw. (1276)
29 or/23‐28 (12947)
30 22 and 29 (3554)
31 randomized controlled trial.pt. (327017)
32 controlled clinical trial.pt. (84075)
33 randomized.ab. (242418)
34 placebo.tw. (139743)
35 clinical trials as topic.sh. (159870)
36 randomly.ab. (178062)
37 trial.ti. (104315)
38 (crossover or cross‐over or cross over).tw. (53309)
39 or/31‐38 (801493)
40 exp animals/ not humans.sh. (3712811)
41 39 not 40 (739736)
42 30 and 41 (294)
43 (2009$ or 2010$ or 2011$ or 2012$).ed. (3080766)
44 42 and 43 (56
MEDLINE (May 2012 to 04 Feb 2013) 1 human chorionic gonadotropin.tw. (10853) 2 hCG.tw. (20241) 3 choriogon$.tw. (869) 4 (pregnyl or chorulon or gonabion).tw. (71) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (51761) 6 (LH or ICSH).tw. (44399) 7 exp Chorionic Gonadotropin/ (28368) 8 body temperature$.tw. (21082) 9 GnRH agonist.tw. (2630) 10 GnRH analogue.tw. (754) 11 gonadotropin releasing hormone agonist.tw. (1260) 12 GnRHa.tw. (1028) 13 HMG.tw. (11735) 14 human menopausal gonadotropin.tw. (1131) 15 (profasi or ovitrelle).tw. (31) 16 gonadotrophin releasing hormone agonist.tw. (353) 17 BBT.tw. (359) 18 ultrasound$.tw. (138745) 19 ultrasonograph$.tw. (71151) 20 time$.tw. (2158722) 21 timing.tw. (72720) 22 or/1‐21 (2468620) 23 exp Insemination, Artificial/ (9640) 24 Artificial Insemination.tw. (4650) 25 intrauter$ inseminat$.tw. (1692) 26 (intra‐uter$ adj2 inseminat$).tw. (173) 27 iui.tw. (1092) 28 AIH.tw. (1347) 29 or/23‐28 (13345) 30 22 and 29 (3735) 31 randomized controlled trial.pt. (339054) 32 controlled clinical trial.pt. (85098) 33 randomized.ab. (256457) 34 placebo.tw. (144132) 35 clinical trials as topic.sh. (162088) 36 randomly.ab. (187749) 37 trial.ti. (109412) 38 (crossover or cross‐over or cross over).tw. (55285) 39 or/31‐38 (833381) 40 exp animals/ not humans.sh. (3754079) 41 39 not 40 (768269) 42 30 and 41 (306) 43 (2012$ or 2013$).ed. (1118579) 44 42 and 43 (17)
Appendix 4. EMBASE search strategy
EMBASE (1974 to March 2009)
1 human chorionic gonadotropin.tw. (7671)
2 hCG.tw. (14541)
3 choriogon$.tw. (657)
4 (pregnyl or chorulon or gonabion).tw. (1250)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (35470)
6 (LH or ICSH).tw. (30151)
7 exp Chorionic Gonadotropin/ (21583)
8 body temperature$.tw. (13145)
9 GnRH agonist.tw. (2019)
10 GnRH analogue.tw. (547)
11 gonadotropin releasing hormone agonist.tw. (1044)
12 GnRHa.tw. (810)
13 HMG.tw. (9668)
14 human menopausal gonadotropin.tw. (970)
15 (profasi or ovitrelle).tw. (1323)
16 gonadotrophin releasing hormone agonist.tw. (303)
17 BBT.tw. (216)
18 ultrasound$.tw. (92192)
19 ultrasonograph$.tw. (49842)
20 time$.tw. (1321475)
21 timing.tw. (42682)
22 or/1‐21 (1529635)
23 Artificial Insemination.tw. (1376)
24 intrauter$ inseminat$.tw. (1274)
25 (intra‐uter$ adj2 inseminat$).tw. (144)
26 iui.tw. (845)
27 AIH.tw. (970)
28 exp artificial insemination/ or exp ovulation induction/ (10395)
29 or/23‐28 (12514)
30 22 and 29 (6018)
31 Clinical Trial/ (534452)
32 Randomized Controlled Trial/ (166828)
33 exp randomisation/ (26635)
34 Single Blind Procedure/ (8034)
35 Double Blind Procedure/ (71767)
36 Crossover Procedure/ (21100)
37 Placebo/ (124638)
38 Randomi?ed controlled trial$.tw. (32668)
39 Rct.tw. (2683)
40 random allocation.tw. (637)
41 randomly allocated.tw. (10170)
42 allocated randomly.tw. (1350)
43 (allocated adj2 random).tw. (560)
44 Single blind$.tw. (7444)
45 Double blind$.tw. (84622)
46 ((treble or triple) adj blind$).tw. (140)
47 placebo$.tw. (109819)
48 prospective study/ (80677)
49 or/31‐48 (702424)
50 case study/ (5962)
51 case report.tw. (118966)
52 abstract report/ or letter/ (493672)
53 or/50‐52 (616305)
54 49 not 53 (677956)
55 30 and 54 (1225)
56 limit 55 to yr="2008 ‐ 2009" (88)
57 from 56 keep 1‐88 (88)
Ovid EMBASE (January 2010 to 07 May 2012)
EMBASE is only searched two years back as the UKCC has hand searched EMBASE to this point and these trials are already in CENTRAL.
1 human chorionic gonadotropin.tw. (10712)
2 hCG.tw. (22067)
3 choriogon$.tw. (881)
4 (pregnyl or chorulon or gonabion).tw. (1485)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (54337)
6 (LH or ICSH).tw. (45662)
7 exp Chorionic Gonadotropin/ (34988)
8 body temperature$.tw. (22592)
9 GnRH agonist.tw. (3159)
10 GnRH analogue.tw. (881)
11 gonadotropin releasing hormone agonist.tw. (1335)
12 GnRHa.tw. (1226)
13 HMG.tw. (13943)
14 human menopausal gonadotropin.tw. (1157)
15 (profasi or ovitrelle).tw. (1503)
16 gonadotrophin releasing hormone agonist.tw. (372)
17 BBT.tw. (364)
18 ultrasound$.tw. (171377)
19 ultrasonograph$.tw. (86331)
20 time$.tw. (2414703)
21 timing.tw. (79935)
22 or/1‐21 (2776077)
23 Artificial Insemination.tw. (4325)
24 intrauter$ inseminat$.tw. (2100)
25 (intra‐uter$ adj2 inseminat$).tw. (267)
26 iui.tw. (1532)
27 AIH.tw. (1935)
28 exp artificial insemination/ or exp ovulation induction/ (20846)
29 or/23‐28 (25228)
30 22 and 29 (10464)
31 Clinical Trial/ (864714)
32 Randomized Controlled Trial/ (320860)
33 exp randomization/ (57970)
34 Single Blind Procedure/ (15808)
35 Double Blind Procedure/ (108521)
36 Crossover Procedure/ (33692)
37 Placebo/ (197302)
38 Randomi?ed controlled trial$.tw. (73975)
39 Rct.tw. (9062)
40 random allocation.tw. (1134)
41 randomly allocated.tw. (16989)
42 allocated randomly.tw. (1796)
43 (allocated adj2 random).tw. (705)
44 Single blind$.tw. (12061)
45 Double blind$.tw. (126812)
46 ((treble or triple) adj blind$).tw. (265)
47 placebo$.tw. (173231)
48 prospective study/ (202252)
49 or/31‐48 (1240932)
50 case study/ (15388)
51 case report.tw. (223558)
52 abstract report/ or letter/ (829909)
53 or/50‐52 (1064356)
54 49 not 53 (1206183)
55 30 and 54 (2195)
56 (2010$ or 2011$ or 2012$).em. (2405704)
57 55 and 56 (386)
Ovid Embase (May 2012 to 04 Feb 2013)
1 human chorionic gonadotropin.tw. (11139) 2 hCG.tw. (23106) 3 choriogon$.tw. (896) 4 (pregnyl or chorulon or gonabion).tw. (1535) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (55784) 6 (LH or ICSH).tw. (47444) 7 exp Chorionic Gonadotropin/ (36305) 8 body temperature$.tw. (23776) 9 GnRH agonist.tw. (3359) 10 GnRH analogue.tw. (934) 11 gonadotropin releasing hormone agonist.tw. (1394) 12 GnRHa.tw. (1315) 13 HMG.tw. (14461) 14 human menopausal gonadotropin.tw. (1174) 15 (profasi or ovitrelle).tw. (1555) 16 gonadotrophin releasing hormone agonist.tw. (386) 17 BBT.tw. (391) 18 ultrasound$.tw. (187297) 19 ultrasonograph$.tw. (91634) 20 time$.tw. (2601310) 21 timing.tw. (86897) 22 or/1‐21 (2985880) 23 Artificial Insemination.tw. (4492) 24 intrauter$ inseminat$.tw. (2229) 25 (intra‐uter$ adj2 inseminat$).tw. (277) 26 iui.tw. (1647) 27 AIH.tw. (2159) 28 exp artificial insemination/ or exp ovulation induction/ (21522) 29 or/23‐28 (26288) 30 22 and 29 (10961) 31 Clinical Trial/ (876466) 32 Randomized Controlled Trial/ (336673) 33 exp randomization/ (60661) 34 Single Blind Procedure/ (16967) 35 Double Blind Procedure/ (112989) 36 Crossover Procedure/ (36118) 37 Placebo/ (212667) 38 Randomi?ed controlled trial$.tw. (83349) 39 Rct.tw. (10867) 40 random allocation.tw. (1206) 41 randomly allocated.tw. (18256) 42 allocated randomly.tw. (1863) 43 (allocated adj2 random).tw. (716) 44 Single blind$.tw. (13000) 45 Double blind$.tw. (133772) 46 ((treble or triple) adj blind$).tw. (300) 47 placebo$.tw. (184418) 48 prospective study/ (224710) 49 or/31‐48 (1305860) 50 case study/ (18516) 51 case report.tw. (238003) 52 abstract report/ or letter/ (857366) 53 or/50‐52 (1108963) 54 49 not 53 (1269963) 55 30 and 54 (2283) 56 (2012$ or 2013$).em. (1409980) 57 55 and 56 (146)
Appendix 5. PsycINFO search strategy
PsycINFO <1806 to March Week 3 2009>
1 human chorionic gonadotropin.tw. (44) 2 hCG.tw. (42) 3 choriogon$.tw. (0) 4 (pregnyl or chorulon or gonabion).tw. (0) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (968) 6 (LH or ICSH).tw. (1789) 7 exp Chorionic Gonadotropin/ (0) 8 body temperature$.tw. (2652) 9 GnRH agonist.tw. (22) 10 GnRH analogue.tw. (3) 11 gonadotropin releasing hormone agonist.tw. (25) 12 GnRHa.tw. (9) 13 HMG.tw. (70) 14 human menopausal gonadotropin.tw. (3) 15 (profasi or ovitrelle).tw. (0) 16 gonadotrophin releasing hormone agonist.tw. (1) 17 BBT.tw. (23) 18 ultrasound$.tw. (1090) 19 ultrasonograph$.tw. (271) 20 time$.tw. (310399) 21 timing.tw. (13204) 22 or/1‐21 (323545) 23 Artificial Insemination.tw. (198) 24 intrauter$ inseminat$.tw. (4) 25 (intra‐uter$ adj2 inseminat$).tw. (0) 26 iui.tw. (10) 27 AIH.tw. (8) 28 exp artificial insemination/ or exp ovulation induction/ (861) 29 or/23‐28 (970) 30 22 and 29 (121) 31 from 30 keep 1‐121 (121)
Database: PsycINFO <1806 to May Week 1 2012>
1 human chorionic gonadotropin.tw. (65)
2 hCG.tw. (64)
3 (pregnyl or chorulon or gonabion).tw. (1)
4 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1161)
5 (LH or ICSH).tw. (2202)
6 body temperature$.tw. (3106)
7 GnRH agonist.tw. (33)
8 GnRH analogue.tw. (7)
9 gonadotropin releasing hormone agonist.tw. (28)
10 GnRHa.tw. (19)
11 HMG.tw. (126)
12 human menopausal gonadotropin.tw. (3)
13 (profasi or ovitrelle).tw. (0)
14 gonadotrophin releasing hormone agonist.tw. (1)
15 BBT.tw. (35)
16 ultrasound$.tw. (1801)
17 ultrasonograph$.tw. (514)
18 time$.tw. (410417)
19 timing.tw. (18060)
20 or/1‐19 (427843)
21 Artificial Insemination.tw. (211)
22 intrauter$ inseminat$.tw. (13)
23 iui.tw. (19)
24 AIH.tw. (20)
25 or/21‐24 (253)
26 20 and 25 (38)
27 random.tw. (35121)
28 control.tw. (273455)
29 double‐blind.tw. (15930)
30 clinical trials/ (6006)
31 placebo/ (3203)
32 exp Treatment/ (514585)
33 or/27‐32 (779748)
34 26 and 33 (10)
35 limit 34 to yr="2009 ‐Current" (4)
PsycINFO <1806 to January Week 5 2013>
1 human chorionic gonadotropin.tw. (66) 2 hCG.tw. (65) 3 (pregnyl or chorulon or gonabion).tw. (1) 4 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1200) 5 (LH or ICSH).tw. (2299) 6 body temperature$.tw. (3205) 7 GnRH agonist.tw. (37) 8 GnRH analogue.tw. (7) 9 gonadotropin releasing hormone agonist.tw. (30) 10 GnRHa.tw. (19) 11 HMG.tw. (143) 12 human menopausal gonadotropin.tw. (3) 13 (profasi or ovitrelle).tw. (0) 14 gonadotrophin releasing hormone agonist.tw. (2) 15 BBT.tw. (37) 16 ultrasound$.tw. (1970) 17 ultrasonograph$.tw. (580) 18 time$.tw. (435066) 19 timing.tw. (19256) 20 or/1‐19 (453493) 21 Artificial Insemination.tw. (215) 22 intrauter$ inseminat$.tw. (13) 23 iui.tw. (19) 24 AIH.tw. (22) 25 or/21‐24 (259) 26 20 and 25 (39) 27 random.tw. (37054) 28 control.tw. (287829) 29 double‐blind.tw. (16599) 30 clinical trials/ (6539) 31 placebo/ (3372) 32 exp Treatment/ (536873) 33 or/27‐32 (816147) 34 26 and 33 (11) 35 limit 34 to yr="2012 ‐ 2013" (1)
Appendix 6. Other electronic sources search strategy (PubMed)
intrauterine; intra uterine; intra‐uterine; insemination; inseminate; IUI; artificial insemination; AI; Artificial insemination husband; AIH; timing; hCG; human chorionic gonadotropin; human chorionic gonadotrophin; gonadotrophins; Pregnyl; Ovitrelle; Profasi; GnRH agonist; GnRH agonists; GnRH analogue; GnRH analogue; GnRH analogues; GnRHa; GnRHa‐gonadotropin; Luteinising hormone; Luteinising hormone releasing hormone; LH; LH surge; LH determination; LH rise; LH detection kit; urinary LH; basal body temp; BBT; hMG; ultrasonography; ultrasound; timing of insemination; timing ovulation; timing of administration; subfertile; subfertility; infertility; (randomised controlled trial [Publication Type], controlled clinical trial [Publication Type], randomised controlled trials, random allocation, double‐blind method, single‐blind method, clinical trial [Publication Type], clinical trials, (clinical AND trial*)).
Appendix 7. Search string
MEDLINE (1966 to March 2009)
1 human chorionic gonadotropin.tw. (9408)
2 hCG.tw. (17711)
3 choriogon$.tw. (806)
4 (pregnyl or chorulon or gonabion).tw. (49)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (48292)
6 (LH or ICSH).tw. (39220)
7 exp Chorionic Gonadotropin/ (26357)
8 body temperature$.tw. (17493)
9 GnRH agonist.tw. (2074)
10 GnRH analogue.tw. (610)
11 gonadotropin releasing hormone agonist.tw. (1062)
12 GnRHa.tw. (819)
13 HMG.tw. (10035)
14 human menopausal gonadotropin.tw. (1057)
15 (profasi or ovitrelle).tw. (27)
16 gonadotrophin releasing hormone agonist.tw. (302)
17 BBT.tw. (291)
18 ultrasound$.tw. (99121)
19 ultrasonograph$.tw. (55953)
20 time$.tw. (1545099)
21 timing.tw. (50623)
22 or/1‐21 (1789257)
23 exp Insemination, Artificial/ (8561)
24 Artificial Insemination.tw. (3649)
25 intrauter$ inseminat$.tw. (1295)
26 (intra‐uter$ adj2 inseminat$).tw. (140)
27 iui.tw. (808)
28 AIH.tw. (902)
29 or/23‐28 (10983)
30 22 and 29 (2844)
31 randomised controlled trial.pt. (266031)
32 controlled clinical trial.pt. (78661)
33 (randomised or randomised).ab. (210367)
34 placebo.ab. (110319)
35 drug therapy.fs. (1291549)
36 randomly.ab. (128228)
37 trial.ab. (183721)
38 groups.ab. (888340)
39 or/31‐38 (2358832)
40 (animals not (humans and animals)).sh. (3251132)
41 39 not 40 (1999305)
42 30 and 41 (469)
43 (ivf or icsi or intracytoplasmic sperm injection$).tw. (13661)
44 42 not 43 (394)
45 from 44 keep 1‐394 (394)
MEDLINE (January 2009 to 07 May 2012)
1 human chorionic gonadotropin.tw. (10613)
2 hCG.tw. (19802)
3 choriogon$.tw. (862)
4 (pregnyl or chorulon or gonabion).tw. (65)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (51259)
6 (LH or ICSH).tw. (43606)
7 exp Chorionic Gonadotropin/ (28013)
8 body temperature$.tw. (20554)
9 GnRH agonist.tw. (2496)
10 GnRH analogue.tw. (726)
11 gonadotropin releasing hormone agonist.tw. (1204)
12 GnRHa.tw. (984)
13 HMG.tw. (11574)
14 human menopausal gonadotropin.tw. (1093)
15 (profasi or ovitrelle).tw. (30)
16 gonadotrophin releasing hormone agonist.tw. (341)
17 BBT.tw. (347)
18 ultrasound$.tw. (131852)
19 ultrasonograph$.tw. (68456)
20 time$.tw. (2066122)
21 timing.tw. (69281)
22 or/1‐21 (2365234)
23 exp Insemination, Artificial/ (9448)
24 Artificial Insemination.tw. (4477)
25 intrauter$ inseminat$.tw. (1616)
26 (intra‐uter$ adj2 inseminat$).tw. (165)
27 iui.tw. (1036)
28 AIH.tw. (1276)
29 or/23‐28 (12947)
30 22 and 29 (3554)
31 randomized controlled trial.pt. (327017)
32 controlled clinical trial.pt. (84075)
33 randomized.ab. (242418)
34 placebo.tw. (139743)
35 clinical trials as topic.sh. (159870)
36 randomly.ab. (178062)
37 trial.ti. (104315)
38 (crossover or cross‐over or cross over).tw. (53309)
39 or/31‐38 (801493)
40 exp animals/ not humans.sh. (3712811)
41 39 not 40 (739736)
42 30 and 41 (294)
43 (2009$ or 2010$ or 2011$ or 2012$).ed. (3080766)
44 42 and 43 (56
MEDLINE (May 2012 to 04 Feb 2013) 1 human chorionic gonadotropin.tw. (10853) 2 hCG.tw. (20241) 3 choriogon$.tw. (869) 4 (pregnyl or chorulon or gonabion).tw. (71) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (51761) 6 (LH or ICSH).tw. (44399) 7 exp Chorionic Gonadotropin/ (28368) 8 body temperature$.tw. (21082) 9 GnRH agonist.tw. (2630) 10 GnRH analogue.tw. (754) 11 gonadotropin releasing hormone agonist.tw. (1260) 12 GnRHa.tw. (1028) 13 HMG.tw. (11735) 14 human menopausal gonadotropin.tw. (1131) 15 (profasi or ovitrelle).tw. (31) 16 gonadotrophin releasing hormone agonist.tw. (353) 17 BBT.tw. (359) 18 ultrasound$.tw. (138745) 19 ultrasonograph$.tw. (71151) 20 time$.tw. (2158722) 21 timing.tw. (72720) 22 or/1‐21 (2468620) 23 exp Insemination, Artificial/ (9640) 24 Artificial Insemination.tw. (4650) 25 intrauter$ inseminat$.tw. (1692) 26 (intra‐uter$ adj2 inseminat$).tw. (173) 27 iui.tw. (1092) 28 AIH.tw. (1347) 29 or/23‐28 (13345) 30 22 and 29 (3735) 31 randomized controlled trial.pt. (339054) 32 controlled clinical trial.pt. (85098) 33 randomized.ab. (256457) 34 placebo.tw. (144132) 35 clinical trials as topic.sh. (162088) 36 randomly.ab. (187749) 37 trial.ti. (109412) 38 (crossover or cross‐over or cross over).tw. (55285) 39 or/31‐38 (833381) 40 exp animals/ not humans.sh. (3754079) 41 39 not 40 (768269) 42 30 and 41 (306) 43 (2012$ or 2013$).ed. (1118579) 44 42 and 43 (17)
EMBASE (1974 to March 2009)
1 human chorionic gonadotropin.tw. (7671)
2 hCG.tw. (14541)
3 choriogon$.tw. (657)
4 (pregnyl or chorulon or gonabion).tw. (1250)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (35470)
6 (LH or ICSH).tw. (30151)
7 exp Chorionic Gonadotropin/ (21583)
8 body temperature$.tw. (13145)
9 GnRH agonist.tw. (2019)
10 GnRH analogue.tw. (547)
11 gonadotropin releasing hormone agonist.tw. (1044)
12 GnRHa.tw. (810)
13 HMG.tw. (9668)
14 human menopausal gonadotropin.tw. (970)
15 (profasi or ovitrelle).tw. (1323)
16 gonadotrophin releasing hormone agonist.tw. (303)
17 BBT.tw. (216)
18 ultrasound$.tw. (92192)
19 ultrasonograph$.tw. (49842)
20 time$.tw. (1321475)
21 timing.tw. (42682)
22 or/1‐21 (1529635)
23 Artificial Insemination.tw. (1376)
24 intrauter$ inseminat$.tw. (1274)
25 (intra‐uter$ adj2 inseminat$).tw. (144)
26 iui.tw. (845)
27 AIH.tw. (970)
28 exp artificial insemination/ or exp ovulation induction/ (10395)
29 or/23‐28 (12514)
30 22 and 29 (6018)
31 Clinical Trial/ (534452)
32 Randomized Controlled Trial/ (166828)
33 exp randomisation/ (26635)
34 Single Blind Procedure/ (8034)
35 Double Blind Procedure/ (71767)
36 Crossover Procedure/ (21100)
37 Placebo/ (124638)
38 Randomi?ed controlled trial$.tw. (32668)
39 Rct.tw. (2683)
40 random allocation.tw. (637)
41 randomly allocated.tw. (10170)
42 allocated randomly.tw. (1350)
43 (allocated adj2 random).tw. (560)
44 Single blind$.tw. (7444)
45 Double blind$.tw. (84622)
46 ((treble or triple) adj blind$).tw. (140)
47 placebo$.tw. (109819)
48 prospective study/ (80677)
49 or/31‐48 (702424)
50 case study/ (5962)
51 case report.tw. (118966)
52 abstract report/ or letter/ (493672)
53 or/50‐52 (616305)
54 49 not 53 (677956)
55 30 and 54 (1225)
56 limit 55 to yr="2008 ‐ 2009" (88)
57 from 56 keep 1‐88 (88)
Ovid EMBASE (January 2010 to 07 May 2012)
EMBASE is only searched two years back as the UKCC has hand searched EMBASE to this point and these trials are already in CENTRAL.
1 human chorionic gonadotropin.tw. (10712)
2 hCG.tw. (22067)
3 choriogon$.tw. (881)
4 (pregnyl or chorulon or gonabion).tw. (1485)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (54337)
6 (LH or ICSH).tw. (45662)
7 exp Chorionic Gonadotropin/ (34988)
8 body temperature$.tw. (22592)
9 GnRH agonist.tw. (3159)
10 GnRH analogue.tw. (881)
11 gonadotropin releasing hormone agonist.tw. (1335)
12 GnRHa.tw. (1226)
13 HMG.tw. (13943)
14 human menopausal gonadotropin.tw. (1157)
15 (profasi or ovitrelle).tw. (1503)
16 gonadotrophin releasing hormone agonist.tw. (372)
17 BBT.tw. (364)
18 ultrasound$.tw. (171377)
19 ultrasonograph$.tw. (86331)
20 time$.tw. (2414703)
21 timing.tw. (79935)
22 or/1‐21 (2776077)
23 Artificial Insemination.tw. (4325)
24 intrauter$ inseminat$.tw. (2100)
25 (intra‐uter$ adj2 inseminat$).tw. (267)
26 iui.tw. (1532)
27 AIH.tw. (1935)
28 exp artificial insemination/ or exp ovulation induction/ (20846)
29 or/23‐28 (25228)
30 22 and 29 (10464)
31 Clinical Trial/ (864714)
32 Randomized Controlled Trial/ (320860)
33 exp randomization/ (57970)
34 Single Blind Procedure/ (15808)
35 Double Blind Procedure/ (108521)
36 Crossover Procedure/ (33692)
37 Placebo/ (197302)
38 Randomi?ed controlled trial$.tw. (73975)
39 Rct.tw. (9062)
40 random allocation.tw. (1134)
41 randomly allocated.tw. (16989)
42 allocated randomly.tw. (1796)
43 (allocated adj2 random).tw. (705)
44 Single blind$.tw. (12061)
45 Double blind$.tw. (126812)
46 ((treble or triple) adj blind$).tw. (265)
47 placebo$.tw. (173231)
48 prospective study/ (202252)
49 or/31‐48 (1240932)
50 case study/ (15388)
51 case report.tw. (223558)
52 abstract report/ or letter/ (829909)
53 or/50‐52 (1064356)
54 49 not 53 (1206183)
55 30 and 54 (2195)
56 (2010$ or 2011$ or 2012$).em. (2405704)
57 55 and 56 (386)
Ovid EMBASE (May 2012 to 04 Feb 2013)
1 human chorionic gonadotropin.tw. (11139) 2 hCG.tw. (23106) 3 choriogon$.tw. (896) 4 (pregnyl or chorulon or gonabion).tw. (1535) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (55784) 6 (LH or ICSH).tw. (47444) 7 exp Chorionic Gonadotropin/ (36305) 8 body temperature$.tw. (23776) 9 GnRH agonist.tw. (3359) 10 GnRH analogue.tw. (934) 11 gonadotropin releasing hormone agonist.tw. (1394) 12 GnRHa.tw. (1315) 13 HMG.tw. (14461) 14 human menopausal gonadotropin.tw. (1174) 15 (profasi or ovitrelle).tw. (1555) 16 gonadotrophin releasing hormone agonist.tw. (386) 17 BBT.tw. (391) 18 ultrasound$.tw. (187297) 19 ultrasonograph$.tw. (91634) 20 time$.tw. (2601310) 21 timing.tw. (86897) 22 or/1‐21 (2985880) 23 Artificial Insemination.tw. (4492) 24 intrauter$ inseminat$.tw. (2229) 25 (intra‐uter$ adj2 inseminat$).tw. (277) 26 iui.tw. (1647) 27 AIH.tw. (2159) 28 exp artificial insemination/ or exp ovulation induction/ (21522) 29 or/23‐28 (26288) 30 22 and 29 (10961) 31 Clinical Trial/ (876466) 32 Randomized Controlled Trial/ (336673) 33 exp randomization/ (60661) 34 Single Blind Procedure/ (16967) 35 Double Blind Procedure/ (112989) 36 Crossover Procedure/ (36118) 37 Placebo/ (212667) 38 Randomi?ed controlled trial$.tw. (83349) 39 Rct.tw. (10867) 40 random allocation.tw. (1206) 41 randomly allocated.tw. (18256) 42 allocated randomly.tw. (1863) 43 (allocated adj2 random).tw. (716) 44 Single blind$.tw. (13000) 45 Double blind$.tw. (133772) 46 ((treble or triple) adj blind$).tw. (300) 47 placebo$.tw. (184418) 48 prospective study/ (224710) 49 or/31‐48 (1305860) 50 case study/ (18516) 51 case report.tw. (238003) 52 abstract report/ or letter/ (857366) 53 or/50‐52 (1108963) 54 49 not 53 (1269963) 55 30 and 54 (2283) 56 (2012$ or 2013$).em. (1409980) 57 55 and 56 (146)
Central Register of Controlled Trials <1st Quarter 2009> 1 human chorionic gonadotropin.tw. (311) 2 hCG.tw. (829) 3 choriogon$.tw. (5) 4 (pregnyl or chorulon or gonabion).tw. (2) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1775) 6 (LH or ICSH).tw. (1829) 7 exp Chorionic Gonadotropin/ (524) 8 body temperature$.tw. (1125) 9 GnRH agonist.tw. (503) 10 GnRH analogue.tw. (110) 11 gonadotropin releasing hormone agonist.tw. (245) 12 GnRHa.tw. (169) 13 HMG.tw. (1026) 14 human menopausal gonadotropin.tw. (169) 15 (profasi or ovitrelle).tw. (9) 16 gonadotrophin releasing hormone agonist.tw. (111) 17 BBT.tw. (26) 18 ultrasound$.tw. (4652) 19 ultrasonograph$.tw. (2199) 20 time$.tw. (99572) 21 timing.tw. (2259) 22 or/1‐21 (110066) 23 Artificial Insemination.tw. (51) 24 intrauter$ inseminat$.tw. (329) 25 (intra‐uter$ adj2 inseminat$).tw. (25) 26 iui.tw. (227) 27 AIH.tw. (23) 28 exp artificial insemination/ or exp ovulation induction/ (858) 29 or/23‐28 (1085) 30 22 and 29 (711) 31 (ivf or icsi).tw. (1833) 32 (in vitro fertilization or intracytoplasmic sperm injection).tw. (1271) 33 or/31‐32 (2318) 34 30 not 33 (392) 35 from 34 keep 1‐392 (392)
Cochrane Central Register of Controlled Trials <April 2012>
1 human chorionic gonadotropin.tw. (371)
2 hCG.tw. (981)
3 choriogon$.tw. (5)
4 (pregnyl or chorulon or gonabion).tw. (2)
5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1964)
6 (LH or ICSH).tw. (2030)
7 exp Chorionic Gonadotropin/ (587)
8 body temperature$.tw. (1279)
9 GnRH agonist.tw. (579)
10 GnRH analogue.tw. (123)
11 gonadotropin releasing hormone agonist.tw. (270)
12 GnRHa.tw. (190)
13 HMG.tw. (1112)
14 human menopausal gonadotropin.tw. (182)
15 (profasi or ovitrelle).tw. (10)
16 gonadotrophin releasing hormone agonist.tw. (117)
17 BBT.tw. (30)
18 ultrasound$.tw. (5976)
19 ultrasonograph$.tw. (2664)
20 time$.tw. (120256)
21 timing.tw. (2821)
22 or/1‐21 (132789)
23 exp Insemination, Artificial/ (269)
24 Artificial Insemination.tw. (53)
25 intrauter$ inseminat$.tw. (386)
26 (intra‐uter$ adj2 inseminat$).tw. (27)
27 iui.tw. (284)
28 AIH.tw. (26)
29 or/23‐28 (558)
30 22 and 29 (264)
31 limit 30 to yr="2009 ‐Current" (38)
Central register of controlled trials < dec 2012>
1 human chorionic gonadotropin.tw. (374) 2 hCG.tw. (995) 3 choriogon$.tw. (5) 4 (pregnyl or chorulon or gonabion).tw. (2) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1965) 6 (LH or ICSH).tw. (2040) 7 exp Chorionic Gonadotropin/ (588) 8 body temperature$.tw. (1285) 9 GnRH agonist.tw. (589) 10 GnRH analogue.tw. (124) 11 gonadotropin releasing hormone agonist.tw. (271) 12 GnRHa.tw. (190) 13 HMG.tw. (1116) 14 human menopausal gonadotropin.tw. (182) 15 (profasi or ovitrelle).tw. (10) 16 gonadotrophin releasing hormone agonist.tw. (118) 17 BBT.tw. (30) 18 ultrasound$.tw. (6038) 19 ultrasonograph$.tw. (2674) 20 time$.tw. (121198) 21 timing.tw. (2855) 22 or/1‐21 (133854) 23 exp Insemination, Artificial/ (270) 24 Artificial Insemination.tw. (54) 25 intrauter$ inseminat$.tw. (399) 26 (intra‐uter$ adj2 inseminat$).tw. (28) 27 iui.tw. (293) 28 AIH.tw. (26) 29 or/23‐28 (580) 30 22 and 29 (269) 31 limit 30 to yr="2012 ‐Current" (3)
PsycINFO <1806 to March Week 3 2009>
1 human chorionic gonadotropin.tw. (44) 2 hCG.tw. (42) 3 choriogon$.tw. (0) 4 (pregnyl or chorulon or gonabion).tw. (0) 5 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (968) 6 (LH or ICSH).tw. (1789) 7 exp Chorionic Gonadotropin/ (0) 8 body temperature$.tw. (2652) 9 GnRH agonist.tw. (22) 10 GnRH analogue.tw. (3) 11 gonadotropin releasing hormone agonist.tw. (25) 12 GnRHa.tw. (9) 13 HMG.tw. (70) 14 human menopausal gonadotropin.tw. (3) 15 (profasi or ovitrelle).tw. (0) 16 gonadotrophin releasing hormone agonist.tw. (1) 17 BBT.tw. (23) 18 ultrasound$.tw. (1090) 19 ultrasonograph$.tw. (271) 20 time$.tw. (310399) 21 timing.tw. (13204) 22 or/1‐21 (323545) 23 Artificial Insemination.tw. (198) 24 intrauter$ inseminat$.tw. (4) 25 (intra‐uter$ adj2 inseminat$).tw. (0) 26 iui.tw. (10) 27 AIH.tw. (8) 28 exp artificial insemination/ or exp ovulation induction/ (861) 29 or/23‐28 (970) 30 22 and 29 (121) 31 from 30 keep 1‐121 (121)
Database: PsycINFO <1806 to May Week 1 2012>
1 human chorionic gonadotropin.tw. (65)
2 hCG.tw. (64)
3 (pregnyl or chorulon or gonabion).tw. (1)
4 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1161)
5 (LH or ICSH).tw. (2202)
6 body temperature$.tw. (3106)
7 GnRH agonist.tw. (33)
8 GnRH analogue.tw. (7)
9 gonadotropin releasing hormone agonist.tw. (28)
10 GnRHa.tw. (19)
11 HMG.tw. (126)
12 human menopausal gonadotropin.tw. (3)
13 (profasi or ovitrelle).tw. (0)
14 gonadotrophin releasing hormone agonist.tw. (1)
15 BBT.tw. (35)
16 ultrasound$.tw. (1801)
17 ultrasonograph$.tw. (514)
18 time$.tw. (410417)
19 timing.tw. (18060)
20 or/1‐19 (427843)
21 Artificial Insemination.tw. (211)
22 intrauter$ inseminat$.tw. (13)
23 iui.tw. (19)
24 AIH.tw. (20)
25 or/21‐24 (253)
26 20 and 25 (38)
27 random.tw. (35121)
28 control.tw. (273455)
29 double‐blind.tw. (15930)
30 clinical trials/ (6006)
31 placebo/ (3203)
32 exp Treatment/ (514585)
33 or/27‐32 (779748)
34 26 and 33 (10)
35 limit 34 to yr="2009 ‐Current" (4)
PsycINFO <1806 to January Week 5 2013>
1 human chorionic gonadotropin.tw. (66) 2 hCG.tw. (65) 3 (pregnyl or chorulon or gonabion).tw. (1) 4 (Luteinizing Hormone or interstitial cell stimulating hormone or lutropin or luteoz?man).ti,ab,sh. (1200) 5 (LH or ICSH).tw. (2299) 6 body temperature$.tw. (3205) 7 GnRH agonist.tw. (37) 8 GnRH analogue.tw. (7) 9 gonadotropin releasing hormone agonist.tw. (30) 10 GnRHa.tw. (19) 11 HMG.tw. (143) 12 human menopausal gonadotropin.tw. (3) 13 (profasi or ovitrelle).tw. (0) 14 gonadotrophin releasing hormone agonist.tw. (2) 15 BBT.tw. (37) 16 ultrasound$.tw. (1970) 17 ultrasonograph$.tw. (580) 18 time$.tw. (435066) 19 timing.tw. (19256) 20 or/1‐19 (453493) 21 Artificial Insemination.tw. (215) 22 intrauter$ inseminat$.tw. (13) 23 iui.tw. (19) 24 AIH.tw. (22) 25 or/21‐24 (259) 26 20 and 25 (39) 27 random.tw. (37054) 28 control.tw. (287829) 29 double‐blind.tw. (16599) 30 clinical trials/ (6539) 31 placebo/ (3372) 32 exp Treatment/ (536873) 33 or/27‐32 (816147) 34 26 and 33 (11) 35 limit 34 to yr="2012 ‐ 2013" (1)
Data and analyses
Comparison 1. hCG versus LH surge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 live birth rate per couple | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 18.08] |
| 2 pregnancy rate per couple | 4 | 275 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.72, 2.45] |
| 3 multiple pregnancy rate per pregnancy | 2 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.17, 7.60] |
Comparison 2. u‐hCG versus r‐hCG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 live birth rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 pregnancy rate per couple | 2 | 409 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.65, 1.57] |
| 3 multiple pregnancy rate per pregnancy | 2 | 109 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.40, 2.47] |
| 4 miscarriage rate per pregnancy | 2 | 109 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.13, 2.47] |
| 5 OHSS rate per cycle | 2 | 468 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Short versus long interval.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 live birth rate per couple | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 24 hours versus 34‐36 hours | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 pregnancy rate per couple | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 24 hours versus 34‐36 hours | 2 | 234 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.31, 0.98] |
| 2.2 24 hours versus 48 hours | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.10, 1.92] |
| 2.3 34‐36 hours versus 48 hours | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.14, 2.48] |
| 3 miscarriage rate per pregnancy | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 24 hours versus 34‐36 hours | 2 | 67 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.35, 7.16] |
| 3.2 24 hours versus 48 hours | 1 | 15 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.27, 58.56] |
| 3.3 34‐36 hours versus 48 hours | 1 | 16 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.07, 25.91] |
Comparison 4. hCG versus GnRH‐a.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 live birth rate per couple | 3 | 104 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.42, 2.56] |
| 2 pregnancy rate per couple | 4 | 206 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.63, 2.08] |
| 3 multiple pregnancy rate per pregnancy | 4 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.02, 1.38] |
| 4 miscarriage rate per pregnancy | 4 | 74 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.48, 6.20] |
| 5 OHSS per cycle | 3 | 456 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.65, 7.91] |
Comparison 5. Early hCG versus late hCG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pregnancy rate per couple | 1 | 612 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.77, 2.25] |
| 2 miscarriage rate | 1 | 65 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.08, 3.28] |
Comparison 6. Different dosages of hCG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 pregnancy rate per couple | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.28, 6.71] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AboulGheit 2010.
| Methods | Single centre, parallel prospective randomised trial. Concealment of allocation: third party Blinding not stated. Follow up not stated. Duration study: January 2008 to July 2009 Power calculation not stated. |
|
| Participants | 45 couples, 125 cycles, duration of subfertility not stated Exclusion criteria: couples with bilateral tubal block and women with endocrinological disorders Mean age of women, 24 h after hCG: 28.7 yrs ± 6.1, 34 h after hCG: 26.4 ± 4.5 and 48 h after hCG: 26.8 yrs ± 4.3 Type of subfertility: unexplained |
|
| Interventions | IUI 24 hours, 34 hours or 48 hours after hCG Stimulation method: was not stated except 10.000 IU hCG when the leading follicle reached ≥ 18 mm and at least two follicles reached ≥ 16 mm Type of semen: partner semen. Semen prepared with a swim up technique Insemination procedure: IUI catheter, one insemination per cycle |
|
| Outcomes | Clinical pregnancy rate per couple: 24 h group 6/15 (40%), 34 h group 7/15 (46%), 48 h group 9/15 (60%) Clinical pregnancy defined as gestational sac and later evidence of fetal heart activity on transvaginal ultrasound |
|
| Notes | Method of randomisation unclear. Signifcantly different in mean of subfertility between treatment groups Duration of subfertility was significantly longer in the group where IUI was performed after 24 hours The author did not have an explanation for this difference Setting: Obstetric and Gynaecology Department Cairo University, Egypt Funding not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about sequence generation |
| Allocation concealment (selection bias) | Low risk | Couples were randomised into three groups by a third party (nurse) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding stated, but outcome is not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding stated, but outcome is not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Andrés‐Oros 2008.
| Methods | Single centre, parallel prospective randomised trial. Computer generated list of random numbers. Concealment of allocation: third party Blinding not stated. Follow up not stated. Duration study not stated Power calculation not stated |
|
| Participants | 120 couples, 290 cycles, at least 2 years of subfertility Exclusion criteria: women with PCOS or other cycle disturbances, semen analysis < 5 million after work up Mean age of women: r‐hCG group: 32.2 yrs ± 2.5 and GnRH‐a group: 32.3 yrs ± 2.5 Type of subfertility: unexplained, endometriosis stage 1 or 2, male factor (WHO 1992), unilateral tubal factor |
|
| Interventions | GnRH‐a versus r‐hCG for triggering ovulation in IUI Stimulation method: 75 IU FSH, 250 ug r‐hCG sc or 0.2 mg GnRH‐a sc (triptorelin 0.2 mg) IUI 36 hours after injection of hCG or GnRH‐a Type of semen not explicitly stated. Semen prepared with a swim up technique Insemination procedure: Gynetics catheter, one insemination per cycle |
|
| Outcomes | Clinical pregnancy rate per couple: r‐hCG group 21/60 (35%), GnRH‐a group 15/42 (35.7%) Number of miscarriages: r‐hCG group 3/21 (14%), GnRH‐a group 1/15 (6.7%) Multiple pregnancy rates: r‐hCG group 0/21 (0%), GnRH‐a group 1/15 (6.7%) Pregnancy diagnosed: transvaginal ultrasound demonstrating heart activity |
|
| Notes | 60 couples received r‐hCG and only 42 couples received GnRH‐a. The other 18 couples did not reach the point to induce ovulation due to too many, or no follicles. The author did not have an explanation for this difference Setting: Assisted Reproduction Service. Miguel Servet University Hospital, Zaragoza, Spain No funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | None stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding stated, outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding stated, outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Claman 2004.
| Methods | Single centre, parallel design with random number table. Concealment of allocation: third party No blinding used. Duration of the study and follow up not stated Power calculation: sample size of 190 with a power of 0.8 to detect an increase in pregnancy rate from 15% to 30% between groups with an alpha of 0.05. ITT: no |
|
| Participants | 75 women, 189 cycles, > 2 years subfertility Exclusion criteria: cycles with endogenous LH surge Mean age of women: short hCG‐IUI interval: 34.4 yrs ± 3.6 and long hCG‐IUI interval: 34.3 yrs ± 3.6 Type of subfertility: unexplained, endometriosis stage 1 or 2, male factor (WHO 1992), clomiphene resistant oligo‐ovulation, or combination of factors |
|
| Interventions | Stimulation method: 100 to 225 IU FSH, 5000 IU hCG im or 10,000 IU hCG sc IUI either 32 to 34 hours or 38 to 40 hours after injection of hCG Type of semen not explicitly stated. Semen prepared with a two‐layer density gradient separation technique, final sample suspended in 0.35 ml of culture medium Insemination procedure: Tomcat catheter high up in the uterine fundus, one insemination per cycle |
|
| Outcomes | Pregnancy rate per cycle: short interval 20/96 (20%), long interval group 14/93 (15%) Secondary outcomes not stated Pregnancy diagnosed: transvaginal ultrasound demonstrating heart activity |
|
| Notes | Inclusion of couples with oligo‐ovulation Setting: Division of Reproductive Medicine, Department of Obstetrics and Gynecology, The Ottawa Hospital, Canada No funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | None stated; the author comment ‘next random number in the table’ does not state the random sequence generation |
| Allocation concealment (selection bias) | Low risk | Third party (a nurse) in the clinical care team picked the next random number in the table and crossed it |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data addressed adequately |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
da Silva 2012.
| Methods | Multi‐centre trial, parallel design with automatically generated randomisation. Concealment of allocation: third party (centralised telephonic system). Blinding was not stated. Duration of the study 3 years Power calculation: sample size of 260 in each group with a power of 0.8. ITT: stated, Per protocol group stated separately |
|
| Participants | 635 women, cycles not stated, 2 to 5 years of subfertility Exclusion criteria: tubal obstruction, endometriosis grade III and IV, metrorrhagia of unknown origin, present or past malignant or metabolic or endocrine diseases, cervical infection, positive serology for hepatitis B, C, HIV or syphilis, anti‐spermatozoa antibodies, positive sperm culture, ejaculation disorders, alcohol or drug addiction, participation in another clinical trial in the previous month. Occurence of a spontaneous LH surge during COS before the day of hCG administration Mean age of women: early hCG: 30.9 yrs ± 3.8 and late hCG: 31.0 yrs ± 3.8 Type of subfertility: unexplained, endometriosis stage 1 or 2, male factor, female factor or combination of factors |
|
| Interventions | Stimulation method: 75 IU FSH/day from day 4 (maximum dose 300 IU), 5000 IU hCG im IUI 36 hours after injection of hCG. hCG when DF 16.0 to 16.9 mm or within 18.0 to 18.9 mm Type of semen: husband. Semen prepared with double centrifugation technique using standardized protocols Insemination procedure: not stated. Luteal support with progesterone vaginally |
|
| Outcomes | Clinical pregnancy rate: early 36/309 (11.7%), late group 29/303 (9.6%) Secondary outcomes: ongoing intrauterine pregnancy rate (>10 weeks) and incidence of premature LH surge before hCG administration Pregnancy diagnosed: transvaginal ultrasound demonstrating heart activity |
|
| Notes | 117 major protocol deviations | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Groups of randomisation were automatically generated using a centralized telephonic system |
| Allocation concealment (selection bias) | Low risk | The use of a centralized telephonic system |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only states 'treatment not initiated in 23 participants', does not state reason |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Kyrou 2012.
| Methods | Single centre, parallel design with randomisation on the basis of a computer generated list. Concealment of allocation: not stated No blinding used. Duration of the study: April 2009 until October 2010. Duration of follow up not stated Power calculation: sample size of 2943 couples in each group to achieve 80% power of at a 5% significance level to detect a difference of 3%. No ITT |
|
| Participants | 300 women, 300 cycles Inclusion criteria: age ≤ 36 years, regular menstrual cycles, BMI between 18 and 29 kg/m2, basal concentration of FSH (≤ 12 IU/L), estradiol (≤ 80 pg/ml) and progesterone (≤ 1.6 ng/ml) on cycle day 1 and normal hysterosalpingography. Husband semen with more than 5 million spermatozoa per ejaculation and morphology > 4% normal. Donor semen Exclusion criteria: PCO and endometriosis Mean age of women: LH surge group: 31.5 ± 3.7 yrs, and hCG group: 31.4 ± 3.7 yrs Mean duration of subfertility not stated Type of subfertility: unexplained, male factor |
|
| Interventions | Stimulation method: natural cycle LH surge group: daily serum testing of LH from cycle day 6. hCG group: 10,000 IU hCG at follicle size of ≥ 17mm. IUI 24 to 36 hours later Husband semen and donor semen Insemination procedure: 0.3 ml of semen into the uterine cavity through a Friedman catheter, bed rest for 10 min. One insemination |
|
| Outcomes | Pregnancy rate per couple: LH surge group 34/150 (22.7%) and hCG group 16/150 (10.7%) Secondary outcome measures: not stated Costs: not stated Pregnancy diagnosed: ultrasound at 12 weeks gestation |
|
| Notes | Large group without subfertility (lesbian couples, single mother); LH group 58%, hCG group 58.7% Setting: Centre for Reproductive Medicine, University Hospital Brussels, Belgium No funding stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised on the basis of a computer generated list |
| Allocation concealment (selection bias) | Unclear risk | None stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Comment: high percentage of non‐subfertile women included |
Lewis 2006.
| Methods | Single centre, parallel design. Randomisation order was assigned by computer program Blinding until first ultrasound after informed consent. Duration of the study and follow up not stated Power calculation: a sample size of 75 women in each group was needed to detect differences in cumulative pregnancy rates of 22% versus 49% after 3 cycles. ITT was performed |
|
| Participants | 150 women, 129 completed at least one cycle Inclusion criteria: > 1 year subfertility or three failed cycles of donor IUI. At least one patent tube and a functional ipsilateral ovary. Four million motile spermatozoa with normal morphology Exclusion criteria: elevated FSH levels on cycle day 3, severe endometriosis, recurrent pregnancy loss, previous use of superovulation and IUI Age of women: LH surge group: 33.5 ± 3.9 yrs and hCG group: 34.0 ± 3.9 yrs Type of subfertility: unexplained, mild endometriosis, male factor, cervical factor, tubal or pelvic factor |
|
| Interventions | Stimulation method: 100 mg clomiphene citrate from day 5 through day 9 LH surge group: home monitoring u‐LH and IUI morning after positive test. hCG group: 10,000 IU hCG and IUI 33 to 42 hours later Husband semen and probably donor semen Insemination procedure: not stated. One insemination per cycle |
|
| Outcomes | Pregnancy rate per couple: LH surge group 25% and hCG group: 31% Multiple pregnancy rate per couple: LH surge group 11.1% and hCG group: 12.9%. Miscarriage rate per couple: LH surge group: 34% and hCG group: 18% Costs: stated in the abstract. Cost per pregnancy LH group USD 3695; cost per pregnancy hCG group USD 4830 Pregnancy diagnosed: rising concentration of hCG. Viable pregnancy is defined as a fetal pole with heart activity by ultrasound |
|
| Notes | The abstract used different pregnancy rates as did the full text article Setting: Department of Obstetrics and Gynecology, University of Rochester School of Medicine and Dentistry, Rochester, New York, USA Funding by product donation by Serono, Inc, Rockland, Massachusetts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation order was assigned by computer program |
| Allocation concealment (selection bias) | Unclear risk | Only states ‘Treatment group assignment was not known’, but method of concealment is not stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reason of missing data not stated; imbalance in numbers across intervention groups, possibly related to true outcome |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Lorusso 2008.
| Methods | Single centre, parallel design for three cycles. Randomisation order was assigned by computer generated table. Concealment of allocation: sealed opaque envelopes Blinding unclear. Follow up until pregnancy was beyond 12th week of gestation. Power calculation: at least 61 couples in each group would be required to achieve 80% power to detect an increase of 20% in progesterone levels in the r‐hCG group. ITT was not stated Duration: IUI treatment between October 2005 and December 2007 |
|
| Participants | 125 women, 184 cycles were completed Inclusion criteria: endometriosis grade I or II according to the AFS, infertility due to sexual dysfunction, a normal uterine cavity and tubal patency assessed by HSG and/or laparoscopy, primary or secondary infertility lasting for at least 24 months, no infection of semen in last 6 months, normal semen analysis according to the WHO or at least 5 million motile spermatozoa after semen preparation, willingness to participate in the study and to comply with the procedure Exclusion criteria: maternal age > 40 years, severe male‐factor infertility, endometriosis grade III or IV, previous IVF attempts, positive hepatitis B virus, hepatitis C virus or HIV serology, PCOS or recurrent miscarriage Age of women: r‐hCG group: 33 ± 3.6 yrs and u‐hCG group: 32.0 ± 4.4 yrs Duration of subfertility: r‐hCG group: 4 ± 1.7 yrs and u‐hCG group: 3 ± 2.4 yrs Type of subfertility: mild endometriosis, mild male factor, unexplained infertility |
|
| Interventions | Stimulation method: daily dose of 37.5 IU r‐FSH starting from cycle day 2 to 3 for 5 days according to a low‐dose, step up protocol 250 µg sc r‐hCG or 5000 IU u‐hCG IM when one follicle with mean diameter > 17 mm was present and no more than 3 follicles with a mean diameter > 15 mm IUI was carried out 24 hr and 48 hr after hCG administration Husband's semen Insemination procedure: not stated; two inseminations |
|
| Outcomes | Pregnancy rate per couple: r‐hCG group 29.7% and u‐hCG group: 24.6% Clinical pregnancy rate per couple: r‐hCG group: 25% and u‐hCG group: 22.9% Multiple pregnancy rate per couple: none. Miscarriage rate per pregnancy: r‐hCG group: 6.3% and u‐hCG group: 7.1% Costs: not stated Pregnancy diagnosed: serum hCG testing 14 days after IUI. Clinical pregnancy was defined as fetal cardiac activity on transvaginal sonography |
|
| Notes | Primary endpoint was the ovulation rate Setting: Centre for Physiopathology of Human Reproduction and Gametes Cryopreservation, Gynaecology and Obstetrics, University of Bari, Italy No funding |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Couples were randomised by a computer generated table |
| Allocation concealment (selection bias) | Low risk | Concealment by use of sealed opaque envelopes, each containing a unique study number and prepared independently by a secretary |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Martinez 1991a.
| Methods | Single centre, cross‐over. Random number table, sealed envelopes Blinding not stated. Follow up not stated. Power calculation not stated. ITT not stated Duration: trial was conducted between January and November 1990 |
|
| Participants | 12 women, 12 cycles (we only used pre‐cross over first cycle data). Total study group: 48 women, 160 cycles Inclusion criteria: male subfertility or unexplained infertility Exclusion criteria: not stated Mean age for the total group of 48 women: 33 ± 2.9 yrs Mean duration of subfertility for the subfertility for the total group of 48 women: 6.3 ± 2.8 yrs Type of subfertility: male or idiopathic |
|
| Interventions | Stimulation method: 75 to 150 IU HMG IM LH surge group: u‐LH detection kit two times a day, IUI 16 to 28 hours after a positive test. hCG group; 10,000 IU hCG, IUI after 36 to 40 hours Husband semen. Semen prepared with a two‐layer Percoll gradient centrifugation, final sample suspended in 0.2 ml of culture media Insemination procedure: 0.5 cm from the uterine fundus with the use of a Makler’s device, one insemination |
|
| Outcomes | Live birth rate: LH group 17%, hCG group 17% Clinical pregnancy rate: LH group: 17% , hCG group: 17% No secondary outcomes stated: no multiple pregnancies, no miscarriages, no costs Pregnancy diagnosed: hCG in urine 14 days after IUI |
|
| Notes | This study also compares IUI to timed intercourse. Because of the double comparison and the cross‐over design, we only used the pre‐cross over IUI data Setting: Department of Reproductive Endocrinology and Fertility, Free University Hospital, Amsterdam, the Netherlands Funding: supported by Organon International, Oss, the Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | None stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed, no imbalance |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Martinez 1991b.
| Methods | Single centre, cross‐over. Random number table, sealed opaque envelopes Blinding not stated. Follow up not stated. Power calculation not stated. No ITT Study duration not stated |
|
| Participants | 48 women, 48 first cycles Inclusion criteria: idiopathic, male or cervical factor infertility Exclusion criteria: not stated. Mean age of women: 31.2 ± 3.8 yrs for the total group of women Mean duration of subfertility: 5.6 ± 2.6 yrs Type of subfertility: idiopathic, male or cervical factor infertility |
|
| Interventions | Stimulation method: 100 mg clomiphene citrate from day three through day seven LH group; home monitoring u‐LH and IUI 21 to 24 hours after a positive test. hCG group: 10,000 IU hCG and IUI 37 to 40 hours later Husband semen. Semen prepared with a Percoll density gradient centrifugation, final sample suspended in 0,2 ml of culture media Insemination procedure: 0.5 cm from the uterine fundus with the use of a Makler's device, one insemination |
|
| Outcomes | Live birth rate: 21% LH group, 17% hCG group Pregnancy rate: 21% LH group, 17% hCG group Multiple pregnancy rate: not known in the LH group, 25% hCG group Costs: not stated Pregnancy diagnosed: hCG in urine 14 days after IUI |
|
| Notes | Only the first cycle pre‐cross over data were used Setting: Department of Reproductive Endocrinology and Fertility, Free University Hospital, Amsterdam, the Netherlands Funding: Organon International, Oss, the Netherlands |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Author comment: 'sealed opaque envelopes', does not state numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Nikbakht 2012.
| Methods | Single centre, randomised controlled clinical trial Randomisation method and concealment of allocation not stated Blinding not stated. Follow up until 6 weeks pregnancy. Power calculation: not stated Study duration June 2009 to April 2010 |
|
| Participants | 66 women, number of cycles not stated Inclusion criteria: healthy women age 22 to 44 years with > 1 year of non‐tubal infertility Exclusion criteria: not stated Mean age of women: 28.5 ± 3 yrs (250 µg hCG) versus 31.9 ± 3 yrs (500 µg hCG) Mean duration of subfertility: 5.0 ± 4.6 yrs (250 µg hCG) versus 6.9 ± 6.8 yrs (500 µg hCG) |
|
| Interventions | Stimulation method: clomiphene citrate or letrozole and HMG Ovulation trigger: r‐hCG 250 µg or 500 µg Type of semen not stated explicitly, with swim up method Insemination procedure: 0.3 ml inseminated, catheter type not stated. One insemination No luteal support was not stated |
|
| Outcomes | Pregnancy rate per cycle: 9.9% (250 µg) versus 12.1% (500 µg) Pregnancy diagnosed by vaginal ultrasound 4 weeks after IUI |
|
| Notes | Setting: Ahvaz, Iran In group with 250 µg hCG significantly more dominant follicles Funding: research grant from the Ahvaz Jundishapur University of Medical Sciences |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | None stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | States 66 women were randomly assigned to one of two groups at the start of the cycle and that 20 of the women refused to participate to the study; still there are data on 66 participants. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Rahman 2011.
| Methods | Single centre, parallel design. Computer generated random tables Concealment of allocation not stated Blinding not stated. Follow up until delivery. Power calculation: 80 per group was proposed to provide 80% power for the primary comparison of pregnancy rates Study duration not stated |
|
| Participants | 204 women, 461 first cycles Inclusion criteria: idiopathic, mild male factor infertility Exclusion criteria: severe male factor, women > 38 years, PCOS, endometriosis or tubal disease Mean age of women: 28.3 ± 3.2 yrs (36 h after hCG) versus 27.1 ± 2.3 yrs (24 h after hCG) Mean duration of subfertility: 4.5 ± 1.0 yrs (36 h after hCG) versus 4.3 ± 1.5 yrs (24 h after hCG) Type of subfertility: idiopathic, male or cervical factor infertility |
|
| Interventions | Stimulation method: 50 mg clomiphene citrate from day three through day seven Ovulation trigger: hCG 5000 IU, 24 hours or 36 hours later IUI Husband semen. Semen prepared with a density gradient centrifugation Insemination procedure: flexible intrauterine catheter, one insemination No luteal support |
|
| Outcomes | Live birth rate per couple: 31/104 (29.8%) 36 h after hCG, 18/100 (18%) 24 h after hCG Pregnancy rate per cycle: 34/231 (14.7%) 36 h after hCG, 20/230 (8.7%) 24 h after hCG Pregnancy rate per couple: 34/104 (32.7%) 36 h after hCG, 20/100 (20%) 24 h after hCG Pregnancy diagnosed: transvaginal ultrasound demonstrating heart activity |
|
| Notes | Setting: Department of Obstetrics and Gynaecology, All India Institute of Medical Science, New Dehli No funding used. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The use of computer generated random tables |
| Allocation concealment (selection bias) | Unclear risk | None stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Sakhel 2007.
| Methods | Single centre, parallel. Randomly assigned by computer generated numbers, sealed envelopes Blinding not stated. Follow up: not clearly stated. Power calculation: performed afterwards, a power of 63% was achieved. ITT was not performed since no dropouts or cycle cancellations were reported Duration: April 2003 to March 2004 |
|
| Participants | 284 women, 284 cycles Inclusion criteria: healthy women between 22 and 44 years with non‐tubal infertility. One fallopian tube should be patent, unexplained subfertility, ovulatory disorder, mild to moderate male factor, early stages of endometriosis and advanced stages of endometriosis after conservative operative laparoscopy Exclusion criteria: tubal blockage and severe male factor Mean age of women: r‐hCG group: 31.9 ± 4.1 yrs and u‐hCG group: 32.7 ± 4.8 yrs Duration of subfertility: r‐hCG group: 2.3 ± 1.5 yrs and u‐hCG group: 3.0 ± 2.3 yrs Type of subfertility: ovulatory disorders, early stage endometriosis, mild male factor, idiopathic infertility. Primary infertility in 55.8% of couples |
|
| Interventions | Stimulation method: 75 to 150 IU FSH and HMG, GnRH antagonist IUI 42 hours after injection of 10,000 IU u‐hCG or 250 µg r‐hCG Type of semen injected: husband. Semen washed using the double‐density gradient method. Insemination of 0.3 ml Insemination procedure: not stated, one insemination |
|
| Outcomes | Outcome live birth rate per couple: 22.1% r‐hCG, 25% u‐hCG Pregnancy rate per couple: 27.1% r‐HCG, 28.5% u‐hCG Multiple pregnancy rate per cycle: 36.8% r‐hCG, 36.6% u‐hCG Miscarriage rate per cycle: 10.5% r‐hCG, 4.9% u‐hCG OHSS rate: no cases of severe OHSS Ectopic pregnancy rate per cycle: 7.9% r‐hCG, 7.3% u‐hCG Costs: not stated Pregnancy diagnosed: serum hCG level two weeks after the insemination |
|
| Notes | Aggressive stimulation with a mean number of ovulated follicles of 2.3 ± 1.4 r‐hCG group and 3.0 ± 2.0 u‐hCG group, resulting in a high pregnancy and multiple pregnancy rate Setting: IVF Michigan PC, Rochester Hills, MI, USA Funding: supported in part by Serono, Rockland, Massachusetts |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned by computer generated numbers |
| Allocation concealment (selection bias) | Unclear risk | None stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Comment: the included women in the u‐hCG group had a greater mean duration of infertility than the r‐hCG group |
Schmidt‐Sarosi 1995.
| Methods | Single centre, parallel design. Random number table, concealment of allocation not stated Blinding not stated. Follow up until birth characteristics were available Power calculation performed: when assuming a 20% pregnancy rate and defining a clinically important pregnancy rate of at least 10%, 6600 cycles were needed in each treatment group to achieve a power of 80% No ITT |
|
| Participants | 26 women, 26 cycles Inclusion criteria: at least unilateral tubal patency, laboratory values euthyroid and normoprolactinemic and > 5 million motile sperm cells after swim up Exclusion criteria: previously undergone clomiphene citrate/hCG stimulation Mean age of women: hCG group: 30.2 yrs and GnRH‐a group: 34.5 yrs Duration of subfertility: not stated Type of subfertility: anovulation, luteal phase defect or unexplained |
|
| Interventions | Stimulation method: 50 mg clomiphene citrate from cycle day 5 to 9 Intervention: two doses of 400 µg nafarelin intranasal (IN) versus 5000 IU hCG IM injection. IUI 48h after the first dose of nafarelin or 36 h after hCG injection Luteal support was given with nafarelin or hCG in each group Type of semen injected: not stated Insemination procedure: not stated, single insemination |
|
| Outcomes | Live birth rate per couple: GnRH‐a group: 2/11 (18.2%). hCG group: 2/15 (13.3%) Pregnancy rate per couple: GnRH‐a group: 3/11 (27.3%). hCG group: 2/15 (13.3%) Miscarriage rate: GnRH‐a group: 1/3 (33.3%). hCG group: 0/2 (0%) No multiple pregnancies and no OHSS Costs: not stated Pregnancy diagnosed: no definition of pregnancy was stated |
|
| Notes | Concealment of allocation not stated Small groups using different forms of luteal support |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised via a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | None stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data addressed adequately |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Comment: the use of different forms of luteal support in both groups |
Scott 1994.
| Methods | Single centre, cross‐over. Randomisation through random number table. Concealment of allocation: sealed envelopes Blinding was used: the sonographer was blinded to which treatment the woman had received Study duration and follow up not stated. Power calculation: only stated for the incidence of unruptured follicle syndrome. ITT not stated |
|
| Participants | 30 women, 30 first cycles Inclusion criteria: women with subfertility of at least one year and ovulatory cycles Exclusion criteria: not stated Mean age of women: 32.2 ± 1.0 SD Duration of subfertility: at least one year, not further stated Type of subfertility: unexplained (n = 26), male factor ( n = 4) |
|
| Interventions | Stimulation method: clomiphene citrate 100 mg orally each day, from cycle day 5 to 9 Intervention: 2 mg of leuprolide acetate or 10.000 IU hCG. IUI after 40 hours Type of semen: not stated. Insemination procedure: not stated. One insemination |
|
| Outcomes | Pregnancy rate per couple: 20% GnRH‐a group, 6.7% hCG group Live birth rate per couple: 20% GnRH‐a group, 6.7% hCG group Secondary outcome measures: not stated Costs: not stated Pregnancy diagnosed: not stated |
|
| Notes | Primary outcome measure was not pregnancy rate, but the endocrine dynamics during the periovular interval, the incidence of luteinised unruptured follicle syndrome and the characteristics of the adequate luteal phase Setting: Division of Reproductive Endocrinology, Department of Obstetrics and Gynecology, Wilford Hall Medical Center, Lackland Air Force Base, Texas No funding stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | None stated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The sonologists were blinded to which treatment the couples had received to minimize the risk of observer bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Shalev 1995.
| Methods | Trial design: parallel. Randomisation by self made computer program. Concealment of allocation by third party Blinding was used. Follow up: until birth characteristics were available. Power calculation for reduction in rate of OHSS was performed, but not further mentioned. ITT was not performed Study duration not stated |
|
| Participants | 48 women, 140 cycles Inclusion criteria: anovulation, oligo‐ovulation or unexplained infertility Exclusion criteria: women at high risk of developing severe OHSS (> 20 mature pre‐ovulatory follicles and estradiol concentrations > 4000 pg/ml) Mean age of women: hCG group: 30.4 yrs and GnRH‐a group: 29.2 yrs Duration of subfertility: not stated per group, but at least one year Type of subfertility: anovulation, oligo‐ovulation or unexplained infertility |
|
| Interventions | Stimulation method: individualized regime of HMG starting on cycle day five Intervention: 0.1 mg triptorelin or 10.000 IU hCG, IUI 24 and 48 hours after injection Type of semen injected: husband. Semen prepared by discontinuous Percoll gradient and washed twice. A volume of 0.3 to 0.5 ml of sperm suspension containing an average of 19 x 106 per ml of motile spermatozoa Insemination procedure: Tefcat catheter high in uterine cavity Number of inseminations: two |
|
| Outcomes | Outcome live birth rate per cycle: 17.6% hCG group, 12.5% GnRH‐a group Pregnancy rate per cycle: 26.5% hCG group, 15.3% GnRH‐a group Pregnancy rate per couple: 45.8% hCG group, 66.7% GnRH‐a group Multiple pregnancy rate: 0% hCG group, 18% GnRH‐a group Miscarriage rate: 33.3% hCG group, 18% GnRH‐a group OHSS rate: 11.8% hCG group, 5.6% GnRH‐a group Ectopic pregnancy rate: not stated Costs: not stated Pregnancy diagnosed: rising concentration of hCG. Clinical pregnancy was diagnosed by fetal heart beat |
|
| Notes | Very high pregnancy rate per couple Setting: Fertility Unit, Department of Obstetrics and Gynaecology, Central Emek Hospital, Afula, Israel No funding stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Author comment: randomisation was performed using a self made computer program. Adequate sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | Author comment: third party |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding stated, but outcome not likely to be influenced either way |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding stated, but outcome not likely to be influenced either way |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete data adequately addressed |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Sharma 2011.
| Methods | Single centre, prospective randomised study. Concealment of allocation with sealed envelopes No blinding was stated. Follow up until clinical pregnancy. No power calculation was stated. No ITT analysis was stated Study duration: January to October 2010 |
|
| Participants | 505 women were eligible, 450 women included Inclusion criteria: unexplained subfertility with two previous failed clomiphene citrate/IUI cycles, with follicular endometrial dys‐synchrony (follicle ≥ 18 mm, endometrial thickness < 7 mm) Exclusion criteria: women with persistent endometrial thickness < 7 mm. IUI was cancelled when a luteinised unruptured follicle was present or semen collection failed Mean age of women: not state Mean duration of subfertility: not stated Type of subfertility: unexplained infertility |
|
| Interventions | Stimulation method: clomiphene citrate 100 mg Intervention: 1 mg GnRH‐a versus 5000 IU uhCG im injection IUI after confirmation of ovulation (time frame not stated) Type of semen injected:not stated Insemination procedure: not stated, one insemination. Luteal support was given: 300 mg progesterone vaginally |
|
| Outcomes | Ongoing pregnancy rate per couple: 9.8% (GnRH‐a) versus 4.4% (hCG) Clinical pregnancy rate per couple: 10.2% (GnRH‐a) versus 4.9% (hCG) Miscarriage rate: 10% (GnRH‐a) versus 8.7% (hCG) Clinical pregnancy defined as the presence of gestational sac with cardiac activity on ultrasound |
|
| Notes | Unclear why 20 couples were excluded who met the criteria for ovulation triggering Setting: Institute of reproductive medicine, Kolkata, India Results could not be included in the meta‐analysis since it was not clear whether the results were given for the total group of included couples or only those couples who underwent an IUI procedure Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Only states ‘sealed envelopes’, not opaque or numbered |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear why 20 couples were excluded before randomisation |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Weiss 2010.
| Methods | Single centre, parallel. Random number generator, sealed opaque envelopes No blinding used. Follow up until after delivery. No power calculation performed: the study was stopped before reaching significant power following an unusual number of multi‐fetal pregnancies. No ITT analysis: couples were withdrawn from the study if they were transferred to IVF or IUI was cancelled Study duration: from July 2008 to not stated |
|
| Participants | 92 completed cycles Inclusion criteria: ovulatory disorders, male factor, partial mechanical factor, endometriosis, unexplained infertility Exclusion criteria: known allergy to the utilized drugs, No patent tubes, sperm count < 1 million total motile sperm of normal morphology, women who are candidates for mono‐ovulation, failure to receive consent and women with baseline functional cysts (> 12 mm) Mean age of women: 31.6 ± 5.8 yrs (36 h after hCG) versus 31.8 ± 6.5 yrs (42 h after hCG) versus 29.4 ± 5.7 yrs (48 h after hCG) Mean duration of subfertility: 2.2 ± 1.5 yrs (36 h after hCG) versus 2.1 ± 1.4 yrs (42 h after hCG versus 2.1 ± 1.3 yrs (48 h after hCG) Type of subfertility: mild to moderate male infertility, anovulation, unilateral mechanical factor, endometriosis and unexplained infertility |
|
| Interventions | Stimulation method: gonadotropins either recombinant or urinary. Dosing was flexible and based on womens' age Interventions: IUI either 36 hours, 42 hours or 48 hours after hCG (dosage not stated) Type of semen injected: husband or donor Insemination procedure: not stated, one insemination |
|
| Outcomes | Live birth rate per cycle: 5/35 (14%) 36 h after hCG, 9/24 (38%) 42 h after hCG, 7/33 (21%) 48 h after hCG Pregnancy rate per cycle: 10/35 (29%) 36 h after hCG, 9/24 (38%) 42 h after hCG, 8/33 (24%) 48 h after hCG Number of miscarriage: 5/10 (50%) 36 h after hCG, 0/9 (0%) 42 h after hCG, 1/8 (11%) 48 h after hCG Multiple pregnancy rate: 3/10 (30%) 36 h after hCG, 4/9 (44%) 42 h after hCG, 3/8 (38%) 48 h after hCG Tubal pregnancy rate: 0/35 (0%) 36 h after hCG, 0/24 (0%) 42 h after hCG, 1/33 (3%) 48 h after hCG Pregnancy diagnosed: transvaginal ultrasound demonstrating a gestational sac |
|
| Notes | Inclusion of women with ovulatory disorders Number of women included not stated Study stopped prematurely because of an unusual number of multi‐fetal pregnancies Setting: HaEmek Medical Center. Afula, Israel Funding: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Low risk | Author comment: The numbers were placed in consecutively ordered sealed opaque envelopes. At the time of enrolment, the envelope was opened and group assignment was made |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data adequately addressed (9 women were withdrawn for hyperstimulation, premature ovulation or for lack of response to gonadotropins) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | High risk | Comment: there were significantly more miscarriages in the 36 h group |
Zreik 1999.
| Methods | Single centre, cross‐over. Randomisation was performed with the use of a computer generated random number table Blinding until informed consent was obtained. Follow up not clearly stated. ITT was performed Duration: from September 1994 to July 1996 |
|
| Participants | 54 women, 53 first cycles Inclusion criteria: normal hysterosalpingography, a normal endometrium biopsy, history of clomiphene citrate use of < six months' duration Exclusion criteria: not stated Mean age of women: hCG group: 32 range 24 to 41 LH surge group: 33 range 25 to 41 years Duration of subfertility: 2.8 years, range 1 to 8 hCG group, 3.2 years, range 1 to 10 LH group Type of subfertility: unexplained, male factor, anovulation |
|
| Interventions | 50‐100 clomiphene citrate from cycle day three to seven LH group: home monitoring u‐LH, IUI daily after positive test for the next two days. hCG group: 10,000 IU hCG, IUI daily for the next two days type of semen injected not stated Insemination procedure: not stated, double insemination |
|
| Outcomes | Outcome pregnancy rate per couple: 4% LH group, 7.1% hCG group Secondary outcome measures: not stated Costs: not stated Pregnancy diagnosed: no definition of pregnancy was stated |
|
| Notes | Cross‐over study design. Only the first cycle pre‐cross over data were used. Inclusion of 15 women with anovulation. Pregnancy rate very low Setting: Yale Reproductive Medicine Center, New Haven, Conneticut, USA No funding stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The use of a computer generated random number table |
| Allocation concealment (selection bias) | Unclear risk | States that the assignment was not known to the treating physician or the couple until consent was obtained, but does not state method of concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | No blinding, but outcome not likely to be influenced |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data adequately addressed |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agarwal 1995 | Retrospective study |
| Arici 1994 | Compared stimulated with non‐stimulated cycles. Double and single insemination used |
| Baroni 2001 | Compared different timing methods at different follicle sizes at different times to IUI |
| Barratt 1989 | Endo‐cervical and peri‐cervical insemination |
| Casadei 2006 | Comparing single IUI versus double IUI versus TI with IUI |
| Cedrin‐Durnerin 1993 | Quasi‐randomised trial |
| Check 1994 | Prospective non‐randomised study |
| Claman 2000 | Abstract of an included study |
| Claman 2004a | Abstract of an included study |
| Claraz 1989 | Intracervical insemination |
| Costa Franco 2006 | Retrospective study design |
| Diaz 2003a | Inadequate randomisation; random numbers in an open list |
| Diaz 2003b | Abstract of an excluded study |
| Diaz 2008 | Inadequate randomisation; random numbers in an open list. Same study as Diaz 2003a |
| Egbase 2003 | Inclusion of PCOS women only |
| Federman 1990 | Comparing single versus double insemination. Cross‐over study |
| Fischer 1993 | Investigates the time interval from hCG administration to follicular wall rupture |
| Fondop 2005 | Cohort study |
| George 2007 | Timed intercourse |
| Gerris 1995 | Prospective non‐randomised study |
| Gerrits 2011 | Trial to determine the safety of orally administered LH agonists |
| Ghanem 2011 | Cohort study |
| Ghazizadeh 2009 | Comparing the usefulness of GnRH antagonist administration in preventing premature LH surge |
| Ghosh Dastidar 2009 | Comparing the supplementation of LH in the stimulation protocol |
| Int rhCG study group 2001 | Included anovulatory patients only. Used both IUI and timed intercourse |
| Khattab 2005 | Retrospective study design |
| Kossoy 1989 | Cohort study |
| Kotecki 2005 | Comparison of five different ovarian stimulation protocols |
| Lewis 2002 | Abstract of an included study |
| Lewis 2003 | Abstract of an included study |
| Martinez 1994 | Retrospective study |
| Meherji 2004 | Commentary report |
| Nulsen 1993 | Cross‐over study. Comparing stimulated with non‐stimulated cycles. Comparing double versus single insemination |
| Odem 1991 | Quasi‐randomised trial. Insemination through cervical cap |
| Panchal 2009 | Cohort study |
| Papageorgiou 1995 | Comparing stimulated with non‐stimulated cycles |
| Pierson 2002 | Dose finding study |
| Pirard 2005 | Investigated the luteal support between hCG triggered cycles and GnRHa administered cycles |
| Propst 2007 | Cohort study. Not the comparison of interest |
| Propst 2012 | Not comparison of interest |
| Ragni 1999 | Compared a single peri‐ovulatory IUI with two double IUI regimes |
| Ramon 2009 | Ultrasound guided IUI versus blind IUI |
| Ramon 2009a | Abstract of an excluded study |
| Robinson 1992 | Inclusion of donor insemination only |
| Romeu 1997a | Prospective non‐randomised trial |
| Romeu 1997b | Failure to use a truly randomised design |
| Sakhel 2004 | Abstract of an included study |
| Scarpellini 1991 | Also comparing IUI with timed intercourse |
| Shanis 1995 | Not truly randomised |
| Silverberg 1991 | Comparing single versus double insemination |
| Tavaniotou 2003 | Cohort study |
| Tonguc 2010 | Inadequate randomisation; sequentially enrolled into three groups according to their entry |
| Wang 2001 | Abstract of an excluded study |
| Wang 2006 | Ovulation induction at different follicle sizes |
Characteristics of studies awaiting assessment [ordered by study ID]
Aydin 2013.
| Methods | Single centre, parallel. Randomisation was performed with the use of a computer generated random numbers, upon enrolment an opaque envelope was opened Follow up until clinical pregnancy. ITT was performed Duration: from September 2011 to January 2013 |
| Participants | 220 women, 220 first cycles Inclusion criteria: normal hysterosalpingography, normal hormone essay, semen analysis total progressive motile sperm count > 5 million/ml with > 4% morphology after sperm preparation Exclusion criteria: women with endocrinologic disorders, women with any history of surgery on the reproductive system, women < 20 years and > 35 years, women with expected to be poor responders due to day 3 baseline ultrasonography and or FSH > 10 mIU/ml, estradiol > 40 pg/ml and an antral follicle count (AFC < 6), women who had previously smoked, with advanced male factor infertility (referred for IVF) Mean age of women: 34 to 36 hrs after hCG group: 30.6 ± 3.4 IUI with hCG: 30.7 ± 3.3 Duration of subfertility: 4.9 ± 4.9 years 34 to 36 hrs after hCG group, 5.2 ± 4.7 years, IUI with hCG group Type of subfertility: unexplained, male factor |
| Interventions | 75 to 112.5 IU r‐FSH from cycle day 3, with low dose step up. Ovulation triggering 250 µg r‐hCG Type of semen: partner Insemination procedure: two‐layer density gradient separation technique, single IUI |
| Outcomes | Outcome pregnancy rate per couple: 10/106 (9.4%) 34 to 36 hrs after hCG, 12/98 (12.2%) IUI with hCG group Secondary outcome measures: not stated Costs: not stated Pregnancy diagnosed: presence of an embryo with cardiac activity on ultrasound |
| Notes | Setting: Eskisehir Osmangazi University, Center for Reproductive Health, Eskisehir, Turkey Long duration of subfertility No funding stated |
Blockeel 2014.
| Methods | Single centre, parallel designed randomised trial with computer generated list. Concealment of allocation: third party Single blinding used (investigator). Follow up until 12 weeks pregnancy |
| Participants | Women who are candidates for intrauterine insemination in a natural cycle Inclusion criteria: Age between 18 and 39 yrs. Donor semen. Cycle with less then 3 follicles reaching 15 mm or more, basal hormonal values of progesterone Exclusion criteria: after more than 6 intrauterine inseminations, tubal infertility |
| Interventions | IUI 24 or 48 hours after spontaneous LH peak |
| Outcomes | Primary outcome measure: Clinical pregnancy rate per couple. Secondary outcome measure: live birth rate |
| Notes | Inclusion of donor semen |
Dehghani 2014.
| Methods | Single centre, parallel designed randomised trial with computer generated list. Concealment of allocation: third party Single blinding used (investigator). Follow up until 12 weeks pregnancy |
| Participants | 100 infertile couples were divided into two groups Inclusion criteria: 18 to 35 years; open fallopian tubes confirmed by hysterosalpingography Exclusion criteria: tubal factor, severe endometriosis, hypothalamic amenorrhea, or severe oligospermia (sperm count lower than 5 million per ml based on WHO 2012 classification) (Table 1) in their husbands |
| Interventions | HCG injection before IUI and HCG injection after IUI |
| Outcomes | The main outcome measure was the result of an hCG test that was done two weeks after the IUI; if it was positive, transvaginal sonography would be performed in the seventh week for clinical confirmation of pregnancy |
| Notes |
Mostafa 2014.
| Methods | Single centre, parallel designed randomised trial with random computer generated table. Concealment of allocation: not stated Blinding: not stated. Follow up: till pregnancy test |
| Participants | One hundred infertile couples with a diagnosis of unexplained infertility who had been scheduled for intrauterine insemination (IUI) by husband semen Inclusion criteria: age of female partner less than 37 years; a normal basal hormonal profile (FSH, LH, TSH, E2 and prolactin); a satisfactory basal (day 2) transvaginal ultrasound examination Cases with failed previous 3 IUI trials were excluded |
| Interventions | Study group: 50 women in whom hCG (10,000 IU) was injected 3 to 5 min after IUI Control group: 50 women in whom hCG (10,000 IU) was injected 24 to 32 h before IUI |
| Outcomes | Pregnancy rate |
| Notes | All patients gave informed consent and the study was approved by local ethics committee for scientific research |
Characteristics of ongoing studies [ordered by study ID]
OVO R&D 2012.
| Trial name or title | Combining Urinary Luteinizing hormone Testing with ultrasound monitoring in intrauterine insemination cycles |
| Methods | Parallel designed randomised trial |
| Participants | Women who undergo IUI treatment for unexplained infertility, mild male factor or donor insemination Inclusion criteria: women between 18 and 39 years. natural and stimulated cycles with clomiphene citrate or letrozole. At least one patent tube and an antral follicular count ≥ 10 and FSH ≤ 10 |
| Interventions | Ultrasound monitoring with hCG administration at a leading follicle of 18 mm versus ultrasound monitoring with LH testing in urine |
| Outcomes | Primary outcome measure: pregnancy rate. Secondary outcome measure: rate of positive LH testing |
| Starting date | January 2011 |
| Contact information | Harnois M, Levesque C, Ovo fertilite, Montreal, Canada |
| Notes | Inclusion of donor semen. Sponsored study |
Differences between protocol and review
The protocol stated that women with ovulatory disturbances should not be included. Since the available evidence was scarce we decided to include studies where a proportion of the included women suffered from ovulatory disturbances. In the updated version we were more liberal towards whether a study was truly randomised; when the trial design did not mention the allocation concealment certain studies were included, identifying it as at high risk on bias in the table of included studies.
The protocol stated that if more than 10% of the cycles were cancelled, these data would not be incorporated in the meta‐analysis. Since only a few studies were available, higher dropout rates and cancelled cycles were accepted in the published version as well as in the updated version of the review.
The protocol stated that we would report miscarriage and multiple pregnancy results per woman randomised. For the full review and this update we reported miscarriage and multiple pregnancy results per pregnancy.
2014 update: methods sections updated to current Cochrane recommendations.
Contributions of authors
Astrid Cantineau: title registration; substantial contribution to developing protocol; reviewing articles for inclusion in review and update; substantial contribution writing review.
Mirjam Janssen: writing the protocol; performing search, selection of articles; substantial contribution writing review and update.
Ben Cohlen: formulation of research question; critical view on protocol; arbitration with reviewing the articles; substantial contribution writing review and update.
Thomas Allersma: reviewing articles for inclusion in updated review; substantial contribution writing update.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
None known for any of the review authors.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AboulGheit 2010 {published data only}
- AboulGheit S. Pregnancy rates following three different timings of intrauterine insemination for women with unexplained infertility: A randomised controlled trial. Middle East Fertility Society Journal 2010;15:265‐8. [Google Scholar]
Andrés‐Oros 2008 {published and unpublished data}
- Andrés Orós P, Lamarca Ballestero M, García Aguirre S, Ballesteros Moffa ME, Conte Martín P, Navarro Martín R, Duque Gallo JA. Triggering ovulation in intrauterine insemination cycles with gonadotropin releasing hormone agonist (GnRHa) versus human chorionic gonadotropphin (hCG) [Inducción de la ovulación en ciclos de inseminación intrauterina con análogos de la GnRH (a‐GnRH) versus hormona coriogonadotrópica humana (hCG)]. Revista Iberoamericana de Fertilidad 2008;25(4):223‐8. [Google Scholar]
Claman 2004 {published and unpublished data}
- Claman P, Wilkie V, Collins D. Timing intrauterine insemination either 33 or 39 hours after administration of human chorionic gonadotropin yields the same pregnancy rates as after superovulation therapy. Fertility and Sterility 2004;82(1):13‐6. [DOI] [PubMed] [Google Scholar]
da Silva 2012 {published data only}
- Silva ALB, Arbo E, Fanchin R. Early versus late hCG administration to trigger ovulation in mild stimulated IUI cycles: a randomized clinical trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2012;164:156‐60. [DOI] [PubMed] [Google Scholar]
Kyrou 2012 {published data only}
- Kyrou D, Kolibianakis EM, Fatemi HM, Grimbizis GF, Theodoridis TD, Camus M, et al. Spontaneous triggering of ovulation versus HCG administration in patients undergoing IUI: a prospective randomized study. Reproductive BioMedicine Online 2012;25(3):278‐83. [DOI] [PubMed] [Google Scholar]
- Kyrou D, Riva A, Verpoest W, Fatemi HM, Tournaye H, Devroey P. What is the optimal moment for IUI in natural cycles? Human chorionic gonadotropin or luteinizing monitoring? Preliminary results of a randomized study.. Fertility and Sterility 2010;94 Suppl 1:170 Abstract no. P‐265. [Google Scholar]
Lewis 2006 {published data only}
- Lewis V, Queenan J, Hoeger K, Stevens J, Guzick GS. Clomiphene citrate monitoring for intrauterine insemination timing: a randomized trial. Fertility and Sterility 2006;85(2):401‐6. [DOI] [PubMed] [Google Scholar]
Lorusso 2008 {published data only}
- Lorusso F, Palmisano M, Serrati G, Bassi E, Lamanna G, Vacca M, Depalo R. Intrauterine insemination with recombinant or urinary human chorionic gonadotropin: A prospective randomized trial. Gynecological Endocrinology 2008;24(11):644‐8. [DOI] [PubMed] [Google Scholar]
Martinez 1991a {published and unpublished data}
- Martinez AR, Bernardus RE, Voorhorst FJ, Vermeiden JP, Schoemaker J. Pregnancy rates after timed intercourse or intrauterine insemination after human menopausal gonadotropin stimulation of normal ovulatory cycles: a controlled study. Fertility and Sterility 1991;55(2):258‐65. [DOI] [PubMed] [Google Scholar]
Martinez 1991b {published and unpublished data}
- Martinez AR, Bernardus RE, Voorhorst FJ, Vermeiden JP, Schoemaker J. A controlled study of human chorionic gonadotrophin induced ovulation versus urinary luteinizing hormone surge for timing of intrauterine insemination. Human Reproduction 1991;6(9):1247‐51. [DOI] [PubMed] [Google Scholar]
Nikbakht 2012 {published data only}
- Nikbakht R, Hemadi M. Comparison of two doses of recombinant Human Chorionic Gonadotropin (rhCG) during ovulation induction in intrauterine insemination cycles: a prospective randomized clinical trial. International Journal of Pharmacology 2012;8(4):259‐64. [Google Scholar]
Rahman 2011 {published data only}
- Rahman SM, Karmakar D, Malhotra N, Kumar S. Timing of intrauterine insemination: an attempt to unravel the enigma. Archives of Gynecology and Obstetrics 2011;284:1023‐7. [DOI] [PubMed] [Google Scholar]
Sakhel 2007 {published and unpublished data}
- Sakhel K, Khedr M, Schwark S, Ashraf M, Fakih MH, Abuzeid M. Comparison of urinary and recombinant hCG during ovulation induction in IUI cycles: a prospective randomized clinical trial. Fertility and Sterility 2007;87(6):1357‐62. [DOI] [PubMed] [Google Scholar]
Schmidt‐Sarosi 1995 {published data only}
- Schmidt‐Sarosi C, Kaplan DR, Sarosi P, Essig MN, Licciardi FL, Keltz M, Levitz M. Ovulation triggering in clomiphene citrate‐stimulated cycles: human chorionic gonadotropin versus a gonadotropin releasing hormone agonist. Journal of Assisted Reproduction and Genetics 1995;12(3):167‐74. [DOI] [PubMed] [Google Scholar]
Scott 1994 {published and unpublished data}
- Scott RT, Bailey SA, Kost ER, Neal GS, Hofmann GE, Illions EH. Comparison of leuprolide acetate and human chorionic gonadotropin for the induction of ovulation in clomiphene citrate‐stimulated cycles. Fertility and Sterility 1994;61(5):872‐9. [DOI] [PubMed] [Google Scholar]
Shalev 1995 {published and unpublished data}
- Shalev E, Geslevich Y, Matilsky M, Ben‐Ami M. Induction of pre‐ovulatory gonadotrophin surge with gonadotrophin‐releasing hormone agonist compared to pre‐ovulatory injection of human chorionic gonadotrophins for ovulation induction in intrauterine insemination treatment cycles. Human Reproduction 1995;10(9):2244‐7. [DOI] [PubMed] [Google Scholar]
Sharma 2011 {published data only}
- Sharma S, Goswami S, Goswami SK, Ghosh S, Chattopadhyay R, Sarkar A, Chakravarty BN. Efficacy of GnRH agonist vs hCG as ovulation trigger in IUI cycle: a prospective randomised study. Human Reproduction 2011;26 Suppl 1:i 306. Abstract P‐476. [Google Scholar]
Weiss 2010 {published and unpublished data}
- Weiss A, Geslevich Y, Beck R, Lavie M, Ayeli V, Shalev E. Optimal timing of intra‐uterine insemination for controlled ovarian stimulation utilizing gonadotropins with GnRH antagonists. Human Reproduction 2010;25(6 Suppl 1):i45‐6. Abstract O‐116. [Google Scholar]
Zreik 1999 {published data only}
- Zreik TG, García Velasco JA, Habboosh MS, Olive DL, Arici A. Prospective, randomized crossover study to evaluate the benefit of human chorionic gonadotropin‐timed versus urinary luteinizing hormone‐timed intrauterine insemination in clomiphene citrate‐stimulated treatment cycles. Fertility and Sterility 1999;71(6):1070‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agarwal 1995 {published data only}
- Agarwal SK, Buyalos RP. Corpus luteum function and pregnancy rates with clomiphene citrate therapy: Comparison of human chorionic gonadotrophin‐induced versus spontaneous ovulation. Human Reproduction 1995;10(2):328‐31. [DOI] [PubMed] [Google Scholar]
Arici 1994 {published data only}
- Arici A, Byrd W, Bradsha K, Kutteh WH, Marshburn P, Carr BR. Evaluation of clomiphene citrate and human chorionic gonadotropin treatment: a prospective, randomized, crossover study during intrauterine insemination cycles. Fertility and Sterility 1994;61(2):314‐8. [DOI] [PubMed] [Google Scholar]
Baroni 2001 {published data only}
- Baroni E, Ragni G, Guermandi E, Riccaboni A, Arnoldi M, Scarduelli C, Crosignani PG. Timing of intrauterine insemination: the use of GnRH antagonist versus ovulation detection kit in FSH‐stimulated cycles. Human Reproduction 2001;16 Suppl 1:138. [Google Scholar]
Barratt 1989 {published data only}
- Barratt CL, Cooke S, Chauhan M, Cooke ID. A prospective randomized controlled trial comparing urinary luteinizing hormone dipsticks and basal body temperature charts with time donor insemination. Fertility and Sterility 1989;52(3):394‐7. [DOI] [PubMed] [Google Scholar]
Casadei 2006 {published data only}
- Casadei L, Zamaro V, Calcagni M, Ticconi C, Dorrucci M, Piccione E. Homologous intrauterine insemination in controlled ovarian hyperstimulation cycles: A comparison among three different regimes. European Journal of Obstetrics & Gynecology and Reproductive Biology 2006;129:155‐61. [DOI] [PubMed] [Google Scholar]
Cedrin‐Durnerin 1993 {published data only}
- Cedrin‐Durnerin I, Attalah M, Martin B, Fillion C, Tanguy M, Bellais E, et al. Timing of intra‐uterine insemination after induction of ovulation with hCG: randomised study [Programmation de la date de l'insemination intra‐uterine apres declenchement de l'ovulation par hCG. Etude randomisee]. Contraception Fertilite Sexualite 1993;21(5):431. [Google Scholar]
Check 1994 {published data only}
- Check JH, Peymer M, Zaccardo M. Evaluation of whether using hCG to stimulate oocyte release helps or decreases pregnancy rates following intrauterine insemination. Gynecologic and Obstetric Investigation 1994;38(1):57‐9. [DOI] [PubMed] [Google Scholar]
Claman 2000 {published data only}
- Claman P, Wilkie V, Collins D. A short (335 h) compared with a long (395 h) interval between hCG injection and intrauterine insemination after superovulation therapy. Human Reproduction 2000;15:6‐7. [Google Scholar]
Claman 2004a {published data only}
- Claman P, Wilkie V, Collins D. Timing intrauterine insemination either 33 or 39 hours after hCG yields the same pregnancy rates after super‐ovulation therapy. A prospective randomized trial. The 20th Annual Meeting of the European Society of Human Reproduction and Embryology. 2004:i113‐4.
Claraz 1989 {published data only}
- Claraz E, Frobert C, Bremond A, Cottinet D. Significance of HCG injection for ovulation induction and of ovulation prediction factors in the practice of artificial insemination using donor sperm. A randomized study. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 1989;18(8):1049‐54. [PubMed] [Google Scholar]
Costa Franco 2006 {published data only}
- Costa Franco AC, Pongiluppi Herbst M. Analyses from variables involved in the intrauterine insemination process in a human reproduction clinic. Jornal Brasileiro de Reproducao Assistida 2006;10(1):22‐4. [Google Scholar]
Diaz 2003a {published data only}
- Diaz I, Guillen A, Pacheco A, Requena A, García‐Velasco JA. Comparison of hormonal profile at final oocyte maturation with GnRH agonist vs hCG in IVI cycles: preliminary study. Revista Iberoamericana de Fertilidad y Reproduccion Humana 2003;20(3):157‐61. [Google Scholar]
Diaz 2003b {published data only}
- Diaz I, Guillen A, Pacheco A, Requena A, Simon C, García Velasco J. Final oocyte maturation with GnRH agonist versus hCG in intrauterine insemination. Human Reproduction 2003;18 Suppl 1:134‐5. [Google Scholar]
Diaz 2008 {published data only}
- Diaz I, Guillen A, Pacheco A, Requena A, Garcia‐Velasco JA. Endocrine modifications associated with final oocyte maturation with gonadotropin‐releasing hormone agonists vs. human chorionic gonadotropin in women undergoing intrauterine insemination. The Journal of Reproductive Medicine 2008;53(1):33‐9. [PubMed] [Google Scholar]
Egbase 2003 {published data only}
- Egbase P, Grudzinskas J, Al Sharhan M, Ashkenani L. HCG or GnRH agonist to trigger ovulation in GnRH antagonist‐treated intrauterine insemination cycles: a prospective randomized study. Human Reproduction 2002;17(Abstract book 1):2. [Google Scholar]
Federman 1990 {published data only}
- Federman CA, Demesic DA, Boone WR, Shapiro SS. Relative efficiency of therapeutic donor insemination using a luteinizing hormone monitor. Fertility and Sterility 1990;54(3):489‐92. [DOI] [PubMed] [Google Scholar]
Fischer 1993 {published data only}
- Fischer R, Nakajima S, Gibson M, Brumsted J. Ovulation after intravenous and intramuscular human chorionic gonadotropin. Fertility and Sterility 1993;60:418‐22. [PubMed] [Google Scholar]
Fondop 2005 {published data only}
- Fondop JJ, Ventura B, Romoscanu I, Ibecheole V, Stalberg A, Ziegler D. MiniHCG in mild COH responders for limiting multiple pregnancies. The 21st Annual Meeting of the European Society of Human Reproduction and Embryology. 2005:i113.
George 2007 {published data only}
- George K, George S, Chandy A, Raju R, Bala S. hCG administration offers no outcome benefit over spontaneous ovulation in anovulatory women treated with clomiphene citrate. Fertility and Sterility 2007;87(4):985‐7. [DOI] [PubMed] [Google Scholar]
Gerris 1995 {published data only}
- Gerris J, Vits A, Joostens M, Royen E. Triggering of ovulation in human menopausal gonadotrophin‐stimulated cycles: comparison between intravenously administered gonadotrophin releasing hormone (100 and 500 ug), GnRH agonist (buserelin 500 ug) and human chorionic gonadotrophin (10000 IU). Human Reproduction 1995;10(1):56‐62. [DOI] [PubMed] [Google Scholar]
Gerrits 2011 {published data only}
- Gerrits MGF, Heuvel MW, Addo S, Mannaerts B, Peeters PAM. Safety, pharmacokinetics and ovulation induction of the first orally administered low molecular weight LH agonist. Basic & Clinical Pharmacology & Toxicology 2011;109 Suppl 1:70. [Google Scholar]
Ghanem 2011 {published data only}
- Ghanem ME, Bakre NI, Emam MA, Al Boghdady LA, Helal AS, Elmetwally AG, Hassan M, et al. The effect of timing of intrauterine insemination in relation to ovulation and the number of inseminations on cycle pregnancy rate in common infertility etiologies. Human Reproduction 2011;26(3):576‐83. [DOI] [PubMed] [Google Scholar]
Ghazizadeh 2009 {published data only}
- Ghazizadeh S, Pourmatroud E, Shariat M, Masomi M, Bagheri M. Study of positive and negative consequences of using GnRH antagonist in intrauterine insemination cycles. International Journal of Fertility and Sterility 2009;3(2):56‐61. [Google Scholar]
Ghosh Dastidar 2009 {published data only}
- Ghosh Dastidar S, Ghosh Dastidar B. The impact of LH supplementation in controlled ovarian stimulation with recombinant FSH and GnRH antagonist in IUI cycle. Human Reproduction 2009;24 Suppl 1:i43. O‐106. [Google Scholar]
Int rhCG study group 2001 {published data only}
- International Recombinant Human Chorionic Gonadotropin Study Group. Induction of ovulation in World Health Organization group II anovulatory women undergoing follicular stimulation with recombinant human follicle‐stimulating hormone: a comparison of recombinant human chorionic gonadotropin (rhCG) and urinary hCG. Fertility and Sterility 2001;75(6):1111‐8. [DOI] [PubMed] [Google Scholar]
Khattab 2005 {published data only}
- Khattab AF, Mustafa FA, Taylor PJ. The use of urine LH detection kits to time intrauterine insemination with donor sperm. Human Reproduction 2005;20(9):2542‐5. [DOI] [PubMed] [Google Scholar]
Kossoy 1989 {published data only}
- Kossoy LR, Hill GA, Parker RA, Rogers BJ, Dalglish CS, Herbert GM 3rd, Wentz AC. Luteinizing hormone and ovulation timing in a therapeutic donor insemination program using frozen semen. American Journal of Obstetrics and Gynecology 1989;160(5 part 1):1169‐72. [DOI] [PubMed] [Google Scholar]
Kotecki 2005 {published data only}
- Kotecki JA, Dzik A, Freitas GC, Soares JB, Bahamondes LG, Cavagna M. Comparison of Five different ovarian stimulation protocols for intrauterine insemination. A prospective randomized trial. 61st Annual Meeting of the American Society for Reproductive Medicine 2005;84 Suppl 1:93‐4. [Google Scholar]
Lewis 2002 {published data only}
- Lewis V, Guzick D. Clomiphene and intrauterine insemination (IUI) ‐ what is the best way to time insemination?. Fertility and Sterility 2002;78 Suppl 1(3):154. [Google Scholar]
Lewis 2003 {published and unpublished data}
- Lewis V, Guzick D. Is administration of hCG in a clomiphene cycle cost effective?. 59th Annual Meeting of the American Society for Reproductive Medicine 2003;80 Suppl 3:213. [Google Scholar]
Martinez 1994 {published data only}
- Martinez AR, Bernardus RE, Vermeiden JPW, Schoemaker J. Time schedules of intrauterine insemination after urinary luteinizing hormone surge detection and pregnancy results. Gynecological Endocrinology 1994;8(1):1‐5. [DOI] [PubMed] [Google Scholar]
Meherji 2004 {published data only}
- Meherji PK. Intrauterine insemination in the management of infertility. Indian Journal of Medical Research 2004;120(6):507‐9. [PubMed] [Google Scholar]
Nulsen 1993 {published data only}
- Nulsen JC, Walsh S, Dumez S, Metzger DA. A randomized and longitudinal study of human menopausal gonadotropin with intrauterine insemination in the treatment of infertility. Obstetrics and Gynecology 1993;82(5):780‐6. [PubMed] [Google Scholar]
Odem 1991 {published data only}
- Odem RR, Durso NM, Long CA, Pineda JA, Strickler RC, Gast MJ. Therapeutic donor insemination: a prospective randomized study of scheduling methods. Fertility and Sterility 1991;55(5):976‐82. [PubMed] [Google Scholar]
Panchal 2009 {published data only}
- Panchal S, Nagori CB. Pre‐hCG 3D and 3D power Doppler assessment of the follicle for improving pregnancy rates in intrauterine insemination cycles. Journal of Human Reproductive Sciences 2009;2(2):62‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papageorgiou 1995 {published data only}
- Papageorgiou GA, Papageorgiou A, Zafrakas MA. Intrauterine insemination and mild ovarian hyperstimulation as a treatment for male subfertility. Human Reproduction 1995;Abstract book 2, 11th Annual Meeting of ESHRE, Hamburg:85. [Google Scholar]
Pierson 2002 {published and unpublished data}
- Pierson R, Olatunbosun F, Baerwald A, Moustier B, Saunders H, Loumaye E. Recombinant human luteinizing hormone for triggering follicular rupture: a dose finding study in ovulation induction. Fertility and Sterility 2002;78(3):56. [Google Scholar]
Pirard 2005 {published data only}
- Pirard C, Donnez J, Loumaye E. GnRH agonist as novel luteal support: results of a randomized, parallel group, feasibility study using intranasal administration of buserelin. Human Reproduction 2005;20:1798‐804. [DOI] [PubMed] [Google Scholar]
Propst 2007 {published data only}
- Propst AM, Thoppil JJ, Groll JM, Frattarelli JL, Robinson RD, Retzloff MG. Pre‐ovulatory vs ovulatory intrauterine insemination in controlled ovarian hyperstimulation cycles. Fertility and Sterility 2007;88 Suppl 1:172‐3. [Google Scholar]
Propst 2012 {published data only}
- Propst AM, Thoppil JJ, Groll JM, Frattarelli JL, Robinson RD, Retzloff MG. A single pre‐ovulatory IUI at 12 hours after hCG trigger is comparable to a traditional IUI at 36 hours. Fertility and Sterility 2012;3 Suppl:S85‐6. [Google Scholar]
Ragni 1999 {published data only}
- Ragni G, Maggioni P, Guermandi E, Testa A, Baroni E, Colombo M, Crosignani PG. Efficacy of double intrauterine insemination in controlled ovarian hyperstimulation cycles. Fertility and Sterility 1999;72(4):619‐22. [DOI] [PubMed] [Google Scholar]
Ramon 2009 {published data only}
- Ramon O, Matorras R, Corcostegui B, Meabe A, Burgos J, Exposito A, Crisol L. Ultrasound‐guided artificial insemination: a randomized controlled trial. Human Reproduction 2009;24(5):1080‐4. [DOI] [PubMed] [Google Scholar]
Ramon 2009a {published data only}
- Ramon O, Matorras R, Corcostegui B, Meabe A, Burgos J, Exposito A, Crisol L. Ultrasound guided artificial insemination: a randomized controlled trial. Human Reproduction 2009;24 Suppl 1:i45. O‐114 . [DOI] [PubMed] [Google Scholar]
Robinson 1992 {published data only}
- Robinson JN, Lockwood GM, Dalton JD, Franklin PA, Farr MM, Barlow DH. A randomized prospective study to assess the effect of the use of home urinary luteinizing hormone detection on the efficiency of donor insemination. Human Reproduction 1992;7(1):63‐5. [DOI] [PubMed] [Google Scholar]
Romeu 1997a {published data only}
- Romeu A, Monzo A, Diez E, Peiro T, Fernandez P. Triggering ovulation for intrauterine insemination using a GnRH analog or HCG in highly purified FSH‐stimulated cycles. Acta Obstetricia et Gynecologica Scandinavica 1997;76 Suppl:74. [Google Scholar]
Romeu 1997b {published data only}
- Romeu A, Monzo A, Peiro T, Diez E, Peinado JA, Quintero. Endogenous LH surge versus hCG as ovulation trigger after low‐dose highly purified FSH in IUI: a comparison of 761 cycles. Journal of Assisted Reproduction and Genetics 1997;14(9):518‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakhel 2004 {published data only}
- Sakhel K, Khedr M, Schwark S, Ashraf M, Fakih MH, Abuzeid M. Comparison between urinary and recombinant hCG during ovulation induction in IUI cycles: a prospective randomized clinical trial. 60th Annual Meeting of the American Society for Reproductive Medicine 2004;82 Suppl 2:238. [Google Scholar]
Scarpellini 1991 {published data only}
- Scarpellini F, Lufino R, Benvenuto P, Scarpellini L, Sbracia M. Efficacy of biochemical vs biological monitoring during the "timing" of the chorionic gonadotropin administration in FSH and HMG stimulated cycles. Acta Europaea Fertilitatis 1991;22(6):329‐32. [PubMed] [Google Scholar]
Shanis 1995 {published data only}
- Shanis BS, Check JH. Efficacy of gonadotropin‐releasing hormone agonists to induce ovulation following low‐dose human menopausal gonadotropin stimulation. Recent Progress in Hormone Research 1995;50:483‐6. [DOI] [PubMed] [Google Scholar]
Silverberg 1991 {published data only}
- Silverberg KM, Johnson JV, Olive DL, Schenken RS. A prospective randomized trial to determine the optimal timing of intrauterine insemination following hCG administration after controlled ovarian hyperstimulation. Fertility and Sterility 1991;54:100. [DOI] [PubMed] [Google Scholar]
Tavaniotou 2003 {published data only}
- Tavaniotou A, Devroey P. Effect of human chorionic gonadotropin on luteal luteinizing hormone concentration in natural cycles. Fertility and Sterility 2003;80(3):654‐5. [DOI] [PubMed] [Google Scholar]
Tonguc 2010 {published data only}
- Tonguc E, Var T, Onalan G, Altinbas S, Tokmak A, Karakas N, Gulerman C. Comparison of the effectiveness of single versus double intrauterine insemination with three different timing regimes. Fertility and Sterility 2010;94(4):1267‐70. [DOI] [PubMed] [Google Scholar]
Wang 2001 {published data only}
- Wang C, Horng S, Chang C, Huang H, Wang H, Soong Y. Delayed HCG injection and earlier insemination can improve the outcome of IUI ‐ a novel and simplified protocol. 17th World Congress on Fertility and Sterility 2001;Abstract book:189. [Google Scholar]
Wang 2006 {published data only}
- Wang CW, Horng SG, Chen CK, Wang HS, Huang HY, Soong YK. Delayed timing of human chorionic gonadotropin injection in combination with earlier insemination can improve the outcome of intrauterine insemination. Human Reproduction 2006;21 Suppl:i119. [Google Scholar]
References to studies awaiting assessment
Aydin 2013 {published data only}
- Aydin Y, Hassa H, Oge T, Tokgoz Y. A randomized study of simultaneous hCG administration with intrauterine insemination in stimulated cycles. European Journal of Obstetrics & Gynecology and Reproductive Biology 2013;170:444‐8. [DOI] [PubMed] [Google Scholar]
Blockeel 2014 {unpublished data only}
- Blockeel C, Knez J, Polyzos NP, Vos M, Camus M, Tournaye H. Should an intrauterine insemination with donor semen be performed 1 or 2 days after the spontaneous LH rise? A prospective RCT. Human Reproduction 2014;29(4):697‐703. [DOI] [PubMed] [Google Scholar]
- Blockeel C, Polyzos N, Ermini B, Riva A, Stoop D, Tournaye H, Devroey P. Timing of IUI 24 or 48 hours after spontaneous LH peak. ClinicalTrials.gov June 29, 2012. [Google Scholar]
Dehghani 2014 {published data only}
- Dehghani‐Firouzabai R, Aflatoonian A, Davar R, Farid‐Mojtahedi M. A comparison of pregnancy rate before and after the administration of HCG in intrauterine insemination. Archives of Gynecology and Obstetrics 2014;29(2):429‐32. [DOI] [PubMed] [Google Scholar]
Mostafa 2014 {published data only}
- Mostafa MS, Huseiny AM, Soliman BS, Mohammed MM. Effect of postponing hCG injection after intrauterine insemination on pregnancy rate. Middle East Fertility Society Journal 2014;19(3):183‐6. [Google Scholar]
References to ongoing studies
OVO R&D 2012 {unpublished data only}
Additional references
Andersen 1995
- Andersen AG, Als‐Nielsen B, Hornnes PJ, Franch Andersen L. Time interval from human chorionic gonadotrophin (HCG) injection to follicular rupture. Human Reproduction 1995;10(12):3202‐5. [DOI] [PubMed] [Google Scholar]
Cantineau 2007
Cohlen 1998
- Cohlen BJ, Velde ER, Kooij RJ, Looman CW, Habbema JD. Controlled ovarian hyperstimulation and intrauterine insemination for treating male subfertility: a controlled study. Human Reproduction 1998;13(6):1553‐8. [DOI] [PubMed] [Google Scholar]
Cooper 2010
- Cooper TG, Noonan E, von Eckardstein S, Auger J, Baker HW, Behre HM, et al. World Health Organization reference values for human semen characteristics. Human Reproduction Update 2010;16(3):231‐45. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
Kosmas 2006
- Kosmas IP, Tatsioni A, Fatemi HM, Kolibianakis EM, Tournaye H, Devroey P. Human chorionic gonadotropin administration vs. luteinizing monitoring for intrauterine insemination timing, after administration of clomiphene citrate: a meta‐analysis. Fertility and Sterility 2006;13:607‐12. [DOI] [PubMed] [Google Scholar]
Kruger 1993
- Kruger TF, Du Toit TC, Franken DR, Acosta AA, Oehninger SC, Menkveld R, et al. A new computerized method of reading sperm morphology (strict criteria) is as efficient as technician reading. Fertility and Sterility 1993;59(1):202‐9. [DOI] [PubMed] [Google Scholar]
Miller 1996
- Miller PB, Soules MR. The usefulness of a urinary LH kit for ovulation prediction during menstrual cycles of normal women. Obstetrics and Gynecology 1996;87:13‐7. [DOI] [PubMed] [Google Scholar]
Steures 2006
- Steures P, Steeg JW, Hompes PG, Habbema JD, Eijkemans MJ, Broekmans FJ, et al. Collaborative Effort on the Clinical Evaluation in Reproductive Medicine. Intrauterine insemination with controlled ovarian hyperstimulation versus expectant management for couples with unexplained subfertility and an intermediate prognosis: a randomised clinical trial. Lancet 2006;15(368 (9531)):216‐21. [DOI] [PubMed] [Google Scholar]
WHO 1980
- World Health Organization. Temporal relationships between ovulation and defined changes in the concentration of plasma estradiol‐17 beta, luteinizing hormone, follicle‐stimulating hormone, and progesterone. American Journal of Obstetrics and Gynecology 1980;138(4):383‐90. [PubMed] [Google Scholar]
WHO 1987
- World Health Organization. Laboratory Manual for SemenAnalysis and Sperm Cervical Mucus Interaction, revised edition. London/New York: Cambridge University Press, 1987. [Google Scholar]
WHO 1992
- World Health Organization. WHO laboratory manual for the examination of human semen and sperm cervical mucus interaction. Cambridge: Cambridge University Press, 1992. [Google Scholar]
WHO 2010
- World Health Organisation. WHO manual for the examination and processing of human semen. Geneva: World Health Organisation, 2010. [Google Scholar]


