Abstract
Acremonium is acknowledged as a highly ubiquitous genus including saprobic, parasitic, or endophytic fungi that inhabit a variety of environments. Species of this genus are extensively exploited in industrial, commercial, pharmaceutical, and biocontrol applications, and proved to be a rich source of novel and bioactive secondary metabolites. Acremonium has been recognised as a taxonomically difficult group of ascomycetes, due to the reduced and high plasticity of morphological characters, wide ecological distribution and substrate range. Recent advances in molecular phylogenies, revealed that Acremonium is highly polyphyletic and members of Acremonium s. lat. belong to at least three distinct orders of Sordariomycetes, of which numerous orders, families and genera with acremonium-like morphs remain undefined. To infer the phylogenetic relationships and establish a natural classification for acremonium-like taxa, systematic analyses were conducted based on a large number of cultures with a global distribution and varied substrates. A total of 633 cultures with acremonium-like morphology, including 261 ex-type cultures from 89 countries and a variety of substrates including soil, plants, fungi, humans, insects, air, and water were examined. An overview phylogenetic tree based on three loci (ITS, LSU, rpb2) was generated to delimit the orders and families. Separate trees based on a combined analysis of four loci (ITS, LSU, rpb2, tef-1α) were used to delimit species at generic and family levels. Combined with the morphological features, host associations and ecological analyses, acremonium-like species evaluated in the present study are currently assigned to 63 genera, and 14 families in Cephalothecales, Glomerellales and Hypocreales, mainly in the families Bionectriaceae, Plectosphaerellaceae and Sarocladiaceae and five new hypocrealean families, namely Chrysonectriaceae, Neoacremoniaceae, Nothoacremoniaceae, Pseudoniessliaceae and Valsonectriaceae. Among them, 17 new genera and 63 new combinations are proposed, with descriptions of 65 new species. Furthermore, one epitype and one neotype are designated to stabilise the taxonomy and use of older names. Results of this study demonstrated that most species of Acremonium s. lat. grouped in genera of Bionectriaceae, including the type A. alternatum. A phylogenetic backbone tree is provided for Bionectriaceae, in which 183 species are recognised and 39 well-supported genera are resolved, including 10 new genera. Additionally, rpb2 and tef-1α are proposed as potential DNA barcodes for the identification of taxa in Bionectriaceae.
Taxonomic novelties: New families: Chrysonectriaceae L.W. Hou, L. Cai & Crous, Neoacremoniaceae L.W. Hou, L. Cai & Crous, Nothoacremoniaceae L.W. Hou, L. Cai & Crous, Pseudoniessliaceae L.W. Hou, L. Cai & Crous, Valsonectriaceae L.W. Hou, L. Cai & Crous. New genera: Bionectriaceae: Alloacremonium L.W. Hou, L. Cai & Crous, Gossypinidium L.W. Hou, L. Cai & Crous, Monohydropisphaera L.W. Hou, L. Cai & Crous, Musananaesporium L.W. Hou, L. Cai & Crous, Paragliomastix L.W. Hou, L. Cai & Crous, Proliferophialis L.W. Hou, L. Cai & Crous, Proxiovicillium L.W. Hou, L. Cai & Crous, Ramosiphorum L.W. Hou, L. Cai & Crous, Verruciconidia L.W. Hou, L. Cai & Crous, Waltergamsia L.W. Hou, L. Cai & Crous; Clavicipitaceae: Subuliphorum L.W. Hou, L. Cai & Crous; Neoacremoniaceae: Neoacremonium L.W. Hou, L. Cai & Crous; Nothoacremoniaceae: Nothoacremonium L.W. Hou, L. Cai & Crous; Plectosphaerellaceae: Allomusicillium L.W. Hou, L. Cai & Crous, Parafuscohypha L.W. Hou, L. Cai & Crous; Pseudoniessliaceae: Pseudoniesslia L.W. Hou, L. Cai & Crous; Sarocladiaceae: Polyphialocladium L.W. Hou, L. Cai & Crous. New species: Bionectriaceae: Alloacremonium ferrugineum L.W. Hou, L. Cai & Crous, Al. humicola L.W. Hou, L. Cai & Crous, Acremonium aerium L.W. Hou, L. Cai & Crous, A. brunneisporum L.W. Hou, L. Cai & Crous, A. chlamydosporium L.W. Hou, L. Cai & Crous, A. ellipsoideum L.W. Hou, Rämä, L. Cai & Crous, A. gamsianum L.W. Hou, L. Cai & Crous, A. longiphialidicum L.W. Hou, L. Cai & Crous, A. multiramosum L.W. Hou, Rämä, L. Cai & Crous, A. mycoparasiticum L.W. Hou, L. Cai & Crous, A. stroudii K. Fletcher, F.C. Küpper & P. van West, A. subulatum L.W. Hou, L. Cai & Crous, A. synnematoferum L.W. Hou, Rämä, L. Cai & Crous, Bulbithecium ammophilae L.W. Hou, L. Cai & Crous, B. ellipsoideum L.W. Hou, L. Cai & Crous, B. truncatum L.W. Hou, L. Cai & Crous, Emericellopsis brunneiguttula L.W. Hou, L. Cai & Crous, Gliomastix musae L.W. Hou, L. Cai & Crous, Gossypinidium sporodochiale L.W. Hou, L. Cai & Crous, Hapsidospora stercoraria L.W. Hou, L. Cai & Crous, H. variabilis L.W. Hou, L. Cai & Crous, Mycocitrus odorus L.W. Hou, L. Cai & Crous, Nectriopsis ellipsoidea L.W. Hou, L. Cai & Crous, Paracylindrocarpon aurantiacum L.W. Hou, L. Cai & Crous, Pn. foliicola Lechat & J. Fourn., Paragliomastix rosea L.W. Hou, L. Cai & Crous, Proliferophialis apiculata L.W. Hou, L. Cai & Crous, Protocreopsis finnmarkica L.W. Hou, L. Cai, Rämä & Crous, Proxiovicillium lepidopterorum L.W. Hou, L. Cai & Crous, Ramosiphorum echinoporiae L.W. Hou, L. Cai & Crous, R. polyporicola L.W. Hou, L. Cai & Crous, R. thailandicum L.W. Hou, L. Cai & Crous, Verruciconidia erythroxyli L.W. Hou, L. Cai & Crous, Ve. infuscata L.W. Hou, L. Cai & Crous, Ve. quercina L.W. Hou, L. Cai & Crous, Ve. siccicapita L.W. Hou, L. Cai & Crous, Ve. unguis L.W. Hou, L. Cai & Crous, Waltergamsia alkalina L.W. Hou, L. Cai & Crous, W. catenata L.W. Hou, L. Cai & Crous, W. moroccensis L.W. Hou, L. Cai & Crous, W. obpyriformis L.W. Hou, L. Cai & Crous; Chrysonectriaceae: Chrysonectria crystallifera L.W. Hou, L. Cai & Crous; Nectriaceae: Xenoacremonium allantoideum L.W. Hou, L. Cai & Crous; Neoacremoniaceae: Neoacremonium distortum L.W. Hou, L. Cai & Crous, N. flavum L.W. Hou, L. Cai & Crous; Nothoacremoniaceae: Nothoacremonium subcylindricum L.W. Hou, L. Cai & Crous, No. vesiculophorum L.W. Hou, L. Cai & Crous; Myrotheciomycetaceae: Trichothecium hongkongense L.W. Hou, L. Cai & Crous; Plectosphaerellaceae: Brunneomyces polyphialidus L.W. Hou, L. Cai & Crous, Parafuscohypha proliferata L.W. Hou, L. Cai & Crous; Sarocladiaceae: Chlamydocillium acaciae L.W. Hou, L. Cai & Crous, C. antarcticum L.W. Hou, L. Cai & Crous, C. guttulatum L.W. Hou, L. Cai & Crous, C. lolii L.W. Hou, L. Cai & Crous, C. soli L.W. Hou, L. Cai & Crous, C. terrestre L.W. Hou, L. Cai & Crous, Parasarocladium chondroidum L.W. Hou, L. Cai & Crous,Polyphialocladium fusisporum L.W. Hou, L. Cai & Crous, Sarocladium agarici L.W. Hou, L. Cai & Crous, S. citri L.W. Hou, L. Cai & Crous, S. ferrugineum L.W. Hou, L. Cai & Crous, S. fuscum L.W. Hou, L. Cai & Crous,S. theobromae L.W. Hou, L. Cai & Crous; Valsonectriaceae: Valsonectria crystalligena L.W. Hou, L. Cai & Crous, V. hilaris L.W. Hou, L. Cai & Crous. New combinations: Bionectriaceae: Acremonium purpurascens (Sukapure & Thirum.) L.W. Hou, L. Cai & Crous, Bulbithecium arxii (Malloch) L.W. Hou, L. Cai & Crous, Bu. borodinense (Tad. Ito et al.) L.W. Hou, L. Cai & Crous, Bu. pinkertoniae (W. Gams) L.W. Hou, L. Cai & Crous, Bu. spinosum (Negroni) L.W. Hou, L. Cai & Crous, Emericellopsis exuviara (Sigler et al.) L.W. Hou, L. Cai & Crous, E. fimetaria (Pers.) L.W. Hou, L. Cai & Crous, E. fuci (Summerb. et al.) L.W. Hou, L. Cai & Crous, E. moniliformis (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, E. salmonea (W. Gams & Lodha) L.W. Hou, L. Cai & Crous, E. tubakii (Gams) L.W. Hou, L. Cai & Crous, Fusariella arenula (Berk. & Broome) L.W. Hou, L. Cai & Crous, Hapsidospora chrysogena (Thirum. & Sukapure) L.W. Hou, L. Cai & Crous, H. flava (W. Gams) L.W. Hou, L. Cai & Crous, H. globosa (Malloch & Cain) L.W. Hou, L. Cai & Crous, H. inversa (Malloch & Cain) L.W. Hou, L. Cai & Crous, Hydropisphaera aurantiaca (C.A. Jørg.) L.W. Hou, L. Cai & Crous, Lasionectria atrorubra (Lechat & J. Fourn.) L.W. Hou, L. Cai & Crous, L. bisepta (W. Gams) L.W. Hou, L. Cai & Crous, L. castaneicola (Lechat & Gardiennet) L.W. Hou, L. Cai & Crous, L. cerealis (P. Karst.) L.W. Hou, L. Cai & Crous, L. olida (W. Gams) L.W. Hou, L. Cai & Crous, Lasionectriopsis dentifera (Samuels) L.W. Hou, L. Cai & Crous, Lasionectriella arenuloides (Samuels) L.W. Hou, L. Cai & Crous, La. marigotensis (Lechat & J. Fourn.) L.W. Hou, L. Cai & Crous, Monohydropisphaera fusigera (Berk. & Broome) L.W. Hou, L. Cai & Crous, Musananaesporium tectonae (R.F. Castañeda) L.W. Hou, L. Cai & Crous, Mycocitrus zonatus (Sawada) L.W. Hou, L. Cai & Crous, Nectriopsis microspora (Jaap) L.W. Hou, L. Cai & Crous, Ovicillium asperulatum (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, O. variecolor (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, Paracylindrocarpon multiloculatum (Samuels) L.W. Hou, L. Cai & Crous, Pn. multiseptatum (Samuels)L.W. Hou, L. Cai & Crous, Paragliomastix chiangraiensis (J.F. Li et al.) L.W. Hou, L. Cai & Crous, Px. luzulae (Fuckel) L.W. Hou, L. Cai & Crous, Px. znieffensis (Lechat & J. Fourn.) L.W. Hou, L. Cai & Crous, Protocreopsis rutila (W. Gams) L.W. Hou, L. Cai & Crous, Proxiovicillium blochii (Matr.)L.W. Hou, L. Cai & Crous, Stanjemonium dichromosporum (Gams & Sivasith.) L.W. Hou, L. Cai & Crous, Verruciconidia persicina (Nicot) L.W. Hou, L. Cai & Crous, Ve. verruculosa (W. Gams & Veenb.-Rijks) L.W. Hou, L. Cai & Crous, Waltergamsia citrina (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, W. dimorphospora (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, W. epimycota (Samuels) L.W. Hou, L. Cai & Crous, W. fusidioides (Nicot) L.W. Hou, L. Cai & Crous, W. hennebertii (W. Gams) L.W. Hou, L. Cai & Crous, W. parva (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, W. pilosa (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, W. zeylanica (Petch) L.W. Hou, L. Cai & Crous; Cephalothecaceae: Phialemonium thermophilum (W. Gams & J. Lacey) L.W. Hou, L. Cai & Crous; Clavicipitaceae: Subuliphorum camptosporum (W. Gams) L.W. Hou, L. Cai & Crous; Coniochaetaceae: Coniochaeta psammospora (W. Gams) L.W. Hou, L. Cai & Crous; Nothoacremoniaceae: Nothoacremonium exiguum (W. Gams) L.W. Hou, L. Cai & Crous; Neoacremoniaceae: Neoacremonium minutisporum (Sukapure & Thirum.) L.W. Hou, L. Cai & Crous; Ne. taiwanense (K.L. Pang et al.) L.W. Hou, L. Cai & Crous; Ne. vitellinum (W. Gams) L.W. Hou, L. Cai & Crous; Plectosphaerellaceae: Allomusicillium domschii (W. Gams) L.W. Hou, L. Cai & Crous, Brunneomyces pseudozeylanicus (W. Gams) L.W. Hou, L. Cai & Crous; Pseudoniessliaceae: Pseudoniesslia minutispora (W. Gams et al.) L.W. Hou, L. Cai & Crous; Sarocladiaceae: Chlamydocillium curvulum (W. Gams) L.W. Hou, L. Cai & Crous, Parasarocladium funiculosum (Sukapure & Thirum.) L.W. Hou, L. Cai & Crous; Valsonectriaceae: Valsonectria inflata (C.H. Dickinson) L.W. Hou, L. Cai & Crous, V. roseola (G. Sm.) L.W. Hou, L. Cai & Crous. Epitype (basionym): Sphaeria violacea J.C. Schmidt ex Fr. Neotype (basionym): Mastigocladium blochii Matr.
Citation: Hou LW, Giraldo A, Groenewald JZ, Rämä T, Summerbell RC, Zang P, Cai L, Crous PW (2023). Redisposition of acremonium-like fungi in Hypocreales. Studies in Mycology 105: 23–203. doi: 10.3114/sim.2023.105.02
Keywords: Bionectriaceae, multi-locus, new taxa, phylogeny, taxonomy, Sarocladiaceae, soilborne
INTRODUCTION
The Hypocreales (Pezizomycotina, Ascomycota) is one of the largest orders of the class Sordariomycetes (Hyde et al. 2020b), currently comprising approximately 300 genera in 14 families, with some unresolved genera, such as Bulbithecium, Hapsidospora and Stanjemonium that are regarded as incertae sedis (Lumbsch & Huhndorf 2007, Hyde et al. 2020b). For many years the order Hypocreales was considered to consist of a single family, Hypocreaceae (Rogerson 1970). However, molecular phylogenetic studies suggested that the Clavicipitales should be included within the Hypocreales, and at least three major lineages were recognised, namely the Clavicipitaceae, Hypocreaceae, and Nectriaceae (O’Donnell 1993, Spatafora & Blackwell 1993, 1994, Glenn et al. 1996, Gams et al. 1998). Rossman et al. (1999) examined all available type specimens of the types in 199 genera that were considered to belong in Hypocreales, and two additional families were recognised, i.e. the Bionectriaceae that includes the Bionectria clade distinguished by Rehner & Samuels (1995), and the Niessliaceae for which no molecular data were available at the time. Afterwards, Calcarisporiaceae, Cocoonihabitaceae, Cordycipitaceae, Flammocladiellaceae, Myrotheciomycetaceae, Ophiocordycipitaceae, Stachybotryaceae, Sarocladiaceae and Tilachlidiaceae were added to the order (Sung et al. 2007a, Crous et al. 2014, 2015a, 2018a,b, Lombard et al. 2015, Sun et al. 2017, Zhuang & Zeng 2017).
The Bionectriaceae was once ranked as the largest family in Hypocreales, including 26 perithecial and five cleistothecial genera (Rossman et al. 1999). This family is characterised by having white, pale tan orange or brown, uniloculate, perithecial, rarely cleistothecial ascomata, generally not changing colour in KOH (Rossman et al. 1999, 2001). The most common hyphomycetous asexual morphs of Bionectriaceae are acremonium-like or gliocladium-like, characterised by having phialidic conidiophores, and unicellular, ellipsoid, fusoid or subfusoid, hyaline to greenish conidia (Rossman et al. 1999). Many of the genera now recognised in the Bionectriaceae are based on species initially described in the broadly conceived genus Nectria (Rossman et al. 1999, 2001). With the addition of several recently introduced genera, namely Geonectria, Laniatria, Lasionectriella, Paracylindrocarpon, Periantria, Stromatonectria, Verrucostoma and Xanthonectria (Hirooka et al. 2010, Jaklitsch & Voglmayr 2011, Crous et al. 2016b, Lechat & Fournier 2016b, Lechat et al. 2016a, 2018b, Döbbeler & Davison 2017), and genera that were formerly regarded as incertae sedis (Crous et al. 2016a, Lin et al. 2016), the Bionectriaceae was consolidated in recent studies with 39 genera currently accepted (Wijayawardene et al. 2018).
The genus Acremonium was first described by Link (1809) for a new species that was considered to produce single spores at the ends of its fertile cells. This name consists of “acro-” which means situated at the top, and “mono-” which means single (Link 1809, Summerbell & Scott 2015). Gams (1968) examined Link’s fungarium material and found that the type, A. alternatum, does not produce single conidia, but has conidia arranged in chains from thin, tapering phialides. The genus Acremonium has always been one of the most simply structured fungi of all filamentous asexual fungi. Characteristics of the genus include the production of septate hyphae giving rise to narrow, tapered, mostly lateral phialides with unicellular conidia arranged in mucoid heads or unconnected chains, and differentiated conidiophores with or without verticillate branches may be observed in some species (Gams 1971, 1975, Domsch et al. 2007, Perdomo et al. 2011, Summerbell et al. 2011).
Species of Acremonium sensu lato (s. lat.) are cosmopolitan and distributed throughout a broad range of environments. They are mainly soilborne saprobes or weak to virulent, facultative or obligate pathogens of plants, other fungi, animals or humans (Gams 1971, 1975, Alfaro-García et al. 1996, Novicki et al. 2003, Lin et al. 2004, Zuccaro et al. 2004, Domsch et al. 2007, Perdomo et al. 2011, Mirtalebi et al. 2017, Summerbell et al. 2018, de Hoog et al. 2020, Pérez-Cantero & Guarro 2020, Kim et al. 2021). They are regarded of great importance in agro-forestry, food storage and preservation, industry, clinical mycology and pharmaceutical industries. Acremonium species have proven to be rich sources of novel and bioactive secondary metabolites. To date more than 350 metabolites have been isolated from Acremonium spp. (Tian et al. 2017). Although most of these metabolites are known from saprobic species, an increasing number of interesting metabolites have also been reported from endophytic or marine-derived fungi. For example, Sarocladium strictum (basionym A. strictum) derived from the ocean, is known to produce beta-lactam antibiotic cephalosporins that have been marketed (Burton & Abraham 1951, Gams 1971, Hamilton-Miller 2000). Some species of Acremonium are effective biological control agents for fungal plant pathogens (Auer & Ludwig-Müller 2014, Bobeck & Pearce 2017, Sutton & Mason 2017), or help to protect plants against environmental stress, hence promoting growth and productivity (Bobeck & Pearce 2017, Sutton & Mason 2017). Some Acremonium species are also commonly mentioned as being among the spoiling microorganisms on diverse foods, including fish meals, fruits, noodles, nuts, peas, apples and stored wheat (Gams 1971, Abdel-Hafez 1987, Fernández-Trujillo 1997, Fujikawa 1997, Summerbell & Scott 2015, Summerbell et al. 2018). In addition, species of Acremonium are highly relevant in the clinical field. Patients commonly develop onychomycoses, or following traumatic inoculation of the fungus infections, resulting in fungemia, ocular infections (keratitis), cutaneous and subcutaneous infections and mycetoma (Gupta et al. 2000, Perdomo 2011, Summerbell et al. 2018, Pérez-Cantero & Guarro 2020). Locally invasive infections such as arthritis, osteomyelitis, peritonitis, sinusitis, and less frequently central nervous system infections have also been frequently reported in recent years (Guarro et al. 1997, 2009, Gupta et al. 2000, Das et al. 2010, de Hoog et al. 2020, Pérez-Cantero & Guarro 2020). However, due to the reduced and little-differentiated morphology, the wide ecological distribution and substrate range, as well as the overlapping features among species, species identification in Acremonium is particularly difficult and the names are frequently misapplied.
Although the morphology of the asexual morph is relatively plesiomorphic, Acremonium is perceived to be a heterogeneous taxon because large numbers of morphologically distinct sexual genera of ascomycetes have acremonium-like asexual morphs. Most of the sexually-typified Acremonium members were identified as Nectria species (Gams 1971, Samuels 1973, 1976a, b, Lowen 1995), but the many genera of Hypocreales known from their sexual morphs, such as Emericellopsis, Epichloe, Hydropisphaera, Hypocrea, Hypomyces, Ijuhya, Lasionectria, Mycoarachis, Nectriopsis, Nigrosabulum, Ochronectria, and Pronectria (Malloch & Cain 1970, Gams 1971, Morgan-Jones & Gams 1982, Samuels 1988, Lowen 1995, Rossman et al. 1999), even Chaetomium and Thielavia of Sordariales, are also characterised by the production of acremonium-like asexual morphs (Morgan-Jones & Gams 1982). The main morphotaxonomic groundwork for Acremonium was conceived in the late 20th century by Gams (1971) in his monograph “Cephalosporium-artige Schimmelpilze (Hyphomycetes)”. A total of 82 Acremonium species were studied based on a meticulous morphological observation scheme. In this concept species of the genus Acremonium were classified into three sections based on their morphological characters: sect. Simplex (rarely branched conidiophores that mostly developed as orthophialides); sect. Gliomastix (darkly pigmented conidia or hyaline conidia and the appearance of chondroid hyphae, which make the cultures tough and difficult to cut through); sect. Nectrioidea (conidiophores that are basitonously branched several times, or unbranched but with multiple septa, mostly chromophilic and granular at the base, with small collarette at the tip) (Gams 1971). Later, two additional sections were proposed, sect. Albolanosa (grass endophytes), and sect. Chaetomioidea (short-aculeate to lageniform phialides with sexual morph in Chaetomiaceae) (Morgan-Jones & Gams 1982). Lowen (1995) added the section Lichenoidea, in which most of the lichenicolous species are included. However, due to the little-differentiated morphology and overlapping features among different sections, species classification is particularly difficult, and the classifications proposed by Gams (1971) seems to be artificial and to not reflect the natural evolutionary history of this group.
The first major molecular phylogenetic study of Acremonium was carried out by Glenn et al. (1996), based on partial nuclear ribosomal small subunit sequences (SSU). Results of the preliminary phylogenetic study demonstrated that Acremonium was highly polyphyletic and recognised Acremonium members of the five sections in at least three distinct orders of Sordariomycetes, including Hypocreales, Microascales and Sordariales (Glenn et al. 1996). As a consequence, members of Acremonium were allocated to different families: type of the sections Acremonium, Nectrioidea, and Gliomastix resided in Hypocreaceae of Hypocreales; the clavicipitaceous grass endophytes of sect. Albolanosa were moved to the newly established genus Neotyphodium based on their unique morphology, ecology and obligate parasitism of the Clavicipitaceae; and the section Chaetomioidea which has Thielavia and Chaetomium sexual morphs grouped within Sordariales, and were thus excluded from Acremonium (Glenn et al. 1996). However, the taxonomic status of Acremonium s. lat. remained unresolved.
With the introduction of more taxa to the dataset and additional genetic loci to phylogenetic studies, subsequent research further confirmed that Acremonium is highly polyphyletic. A molecular phylogenetic overview based on SSU and LSU sequences was generated, which grouped over 100 species of Acremonium and related taxa into five groups across different classes of Ascomycota (Coniochaetales, Hypocreales, Microascales, Sordariales and Cephalothecaceae; Summerbell et al. 2011). The bulk of Acremonium species falls into the Hypocreales. Epitypification of the type, A. alternatum, linked Acremonium sensu stricto to the Bionectriaceae (Hypocreales; Summerbell et al. 2011), which also accommodates several other, sexually typified genera with an acremonium-like asexual morph, such as Bulbithecium, Emericellopsis, Hapsidospora, Mycoarachis and Nigrosabulum (Gams 1971, Summerbell et al. 2011). However, the genus Acremonium still proved to be polyphyletic distributed in two major clades with at least 20 smaller clades across Hypocreales, including the Sarocladium, “curvulum-clade”, and “breve-clade”, that led to the proposal that the remaining acremonium-like species should be allocated to other genera or families (Summerbell et al. 2011). Nine of the named Acremonium species in these analyses belong to the Plectosphaerellaceae (Microascales; Summerbell et al. 2011), which were recently revised by Giraldo & Crous (2019). The other species belonging to the Sordariales, Coniochaetales and the Cephalothecaceae had also been revised in subsequent studies (Perdomo et al. 2013). From these results, it became evident that the acremonium-like morphology had evolved multiple times. Therefore, the taxonomy and phylogeny of the acremonium-like taxa urgently required revision.
Multiple gene markers had been used for studies of acremonium-like species, including ITS, SSU, LSU. The rapidly evolving ribosomal internal transcribed spacer (ITS) is difficult to align in Acremonium as the genus is highly heterogeneous and distributed across the ascomycetes (de Hoog et al. 2000). However, ITS makers are alignable and frequently used in restricted Acremonium subgroups (Summerbell et al. 2011). The relatively slowly evolving genes, such as partial nuclear ribosomal small subunit RNA gene (SSU) and the partial nuclear ribosomal large subunit RNA gene (LSU), have the advantage of being alignable among a broad range of Acremonium groups, but always result in unresolved relationships (Summerbell et al. 2011). Combined ITS-LSU or SSU-LSU have also been used in studies of acremonium-like species and related sexual genera (Summerbell et al. 2011, Giraldo et al. 2012, Lechat et al. 2016), but still failed to provide sufficiently clear genera and species boundaries for acremonium-like species. In recent years, protein-coding genes were frequently applied in the phylogenetic analysis for a few acremonium-like groups. The rpb2, tef-1α and tub2 genes were used for studies of Emericellopsis species (Giraldo et al. 2017, Gonçalves et al. 2020) Hagestad et al. 2021, ITS and act1 sequences were used in Parasarocladium and Sarocladium species (Giraldo et al. 2015, Gonçalves et al. 2020), and the combined ITS, LSU, rpb2 and tef-1α were used for the analysis of Acremonium species belonging to the Plectosphaerellaceae (Giraldo & Crous 2019). These results provided a clearer picture of relationships among the Acremonium groups that were imperfectly resolved in LSU and SSU analyses.
Given the importance of the genus Acremonium to agriculture, industry, and medicine, four objectives were defined in the present study: 1) to resolve the phylogenetic and taxonomic placement of species of Acremonium s. lat. across different genera, families and orders within Ascomycota; 2) to clarify the circumscription of Acremonium s. str. by updating and expanding currently available DNA sequence datasets; 3) to assign names to presently undescribed taxa based on morphological observations, and revisit the old species circumscriptions and reconsider their taxonomy based on a re-examination of original material and DNA sequence data; and 4) to construct a phylogenetic overview that delineates the phylogenetic lineages and generic boundaries of the Bionectriaceae via a polyphasic approach.
MATERIALS AND METHODS
Isolates
All the cultures of acremonium-related fungi included in this study were obtained from the culture collection (CBS) of the Westerdijk Fungal Biodiversity Institute (WI) in Utrecht, The Netherlands, the working collection of Pedro W. Crous (CPC), housed at the Westerdijk Institute, the working collection of Teppo Rämä (TR) at UiT the Arctic University of Norway, the CABI Genetic Resource Collection in the UK (IMI), the Canadian Collection of Fungal Cultures (DAOMC) and the BIOTEC Culture Collection in Thailand (BCC). Most of these cultures were identified as species in the genus Acremonium or associated genera according to the morphological study of Gams (1971) and the study of Summerbell et al. (2011) (Supplementary Table S1). Representative cultures of the new species described in this study were deposited in the CBS culture collection.
DNA extraction, PCR amplification and sequencing
Total genomic DNA was extracted from fungal colonies growing on malt extract agar (MEA) or oatmeal agar (OA; Crous et al. 2019) using the Wizard® Genomic DNA purification kit (Promega, Madison, USA), according to the manufacturers’ protocols. Four loci were amplified, including the internal transcribed spacer regions 1 and 2 and 5.8S nuclear ribosomal RNA gene (ITS), partial large subunit nrRNA gene (28S nrDNA; LSU), the protein-coding genes translation elongation factor 1-alpha (tef-1α) and partial DNA-directed RNA polymerase II second largest subunit (rpb2) gene with the primer pairs ITS5/ITS4 (White et al. 1990, de Hoog & Gerrits van den Ende 1998), LR0R/LR5 (Vilgalys & Hester 1990, Vilgalys & Sun 1994), EF-983F/EF-2218R (Rehner & Buckley 2005), and RPB2-5F2/RPB2-7cR (Liu et al. 1999, Sung et al. 2007b), respectively. The PCR amplifications were performed in a total volume of 25 μL containing 2.5 μL 10× EasyTaq Buffer (Bioline, Luckenwalde, Germany), 50 μM dNTPs, 0.1 μM of each primer, 0.75 U Taq DNA polymerase and 1–10 ng genomic DNA. The PCR amplifications of ITS, LSU and tef-1α were set as follows: an initial denaturation at 95 °C for 5 min, followed by 35 cycles of denaturation, annealing and extension, and a final extension step at 72 °C for 10 min. For the LSU amplification, the 35 cycles consisted of 45 s at 95 °C, 45 s at 48 °C and 2 min at 72 °C; for the ITS 30 s at 95 °C, 30 s at 48 °C and 80 s at 72 °C; and for the tef-1α region 30 s at 95 °C, 30 s at 52 °C and 80 s at 72 °C. The procedures for amplifying and sequencing the rpb2 were performed as described in Hou et al. (2020). PCR products for four loci were purified and sequenced in both directions using an Applied Biosystems 3730xl DNA Analyzer (Thermo Fisher Scientific) as explained in Crous et al. (2013). The consensus sequences of each culture were assembled from forward and reverse sequences using Seqman Pro v. 10.0.1 (DNASTAR, Madison, USA). Novel sequences generated in this study were deposited in GenBank (http://www.ncbi.nlm.nih.gov, Supplementary Table S1).
Phylogenetic analyses
Sequence alignments of the four individual loci (LSU, ITS, rpb2, tef-1α) were generated with MAFFT v. 7 using the default settings (http://mafft.cbrc.jp/alignment/server/index.html) and were then manually edited in MEGA v. 7.0.21 (Kumar et al. 2015).
Both Maximum Likelihood (ML) and Bayesian analysis (BA) were used for phylogenetic inferences of individual sequence alignments, followed by the concatenated alignments. The best substitution model of evolution for each of the four data partitions were estimated using jModeltest v. 2.1.4 (Darriba 2012) before the Bayesian analyses. Bayesian analyses were performed using MrBayes v. 3.2.6 (Ronquist et al. 2012) as described by Hou et al. (2020). Markov Chain Monte Carlo sampling (MCMC) analyses of four chains were started in parallel from a random tree topology. Four simultaneous Markov chains were run for 10 M generations with a sampling frequency set to the 1 000th generation (resulting in 10 000 total trees per parallel run) or until the run was stopped automatically when the average standard deviation of split frequencies fell below 0.01. The burn-in fraction was set to 0.25, and the remaining trees were used to calculate posterior probabilities (Chen et al. 2015). Maximum Likelihood analyses include 1 000 bootstrap replicates and were conducted using RAxML v. 7.2.6 (Stamatakis 2014). A general time reversible (GTR) model was applied with a gamma-distributed rate variation. The resulting trees were viewed using FigTree v. 1.4.2 (http://tree.bio.ed.ac.uk/software/figtree). Alignments and the phylogenetic trees derived from this study were uploaded to figshare (doi: 10.6084/m9.figshare.22258765).
Morphology
Cultures were cultivated on fresh oatmeal agar (OA), malt extract agar (MEA), potato dextrose agar (PDA) and synthetic nutrient-poor agar (SNA; Crous et al. 2019) and incubated at 25 °C in the dark for 4 wk. Colony diameter and characters were measured after 14 d of incubation. Colony colours (upper surface and reverse) were rated following the colour charts of Rayner (1970). Micromorphological observations of reproductive structures were carried out from cultures grown on OA after 14 d of incubation. Clear lactic acid was used as mounting medium for the observation of micromorphological structures of mature ascomata/conidiomata, ascospores/conidia and asci/conidiophores (Gams 1971). Observations were performed with a Nikon Eclipse 80i compound microscope with differential interference contrast (DIC) optics, and a Nikon AZ100 dissecting microscope. Photomicrographs and measurements were taken with a Nikon DS-Ri2 high-definition colour digital camera using the NIS-elements D software v. 4.50. At least 30 measurements were made for all morphologically informative features.
Descriptions of novelties and taxonomic recombinations were deposited in MycoBank, and the fungarium specimens for the novel taxa represented by dried sporulating cultures were deposited in the CBS Fungarium, Utrecht, The Netherlands.
RESULTS
Phylogeny
A total of 922 isolates resembling Acremonium spp. were included in this study. An overview phylogeny based on ITS, LSU and rpb2 sequences was conducted in order to position the isolates in the treated order, family and genera (Dataset 1). In order to portray more precise phylogenetic relationships of Acremonium and its related species, more inclusive analyses based on DNA sequence data from four loci (ITS, LSU, rpb2 and tef-1α) were carried out for three focused families from the overview tree separately (datasets 2–4), including all available cultures belonging to these families. Datasets 2–4 were analysed with the same phylogenetic methods applied to Dataset 1.
Dataset 1 consisted of a concatenated alignment of three loci (ITS, LSU, and rpb2) that contained 392 taxa to represent several families belonging to the Hypocreales and its related orders, which was used to infer delimitation at the family and order levels. Saccharata proteae (CBS 115206; Saccharataceae, Botryosphaeriales, Dothideomycetes) was used as outgroup (Fig. 1). The final alignment of ITS, LSU and rpb2 contained a total length of 2 770 characters including alignment gaps (gene boundaries ITS: 1–1 071, 1 071 bp; LSU: 1 072–1 889, 818 bp; rpb2: 1 890–2 770, 881 bp). Among those, 858 characters were conserved sites and 1 794 were variable sites, including 287 characters that were parsimony-uninformative and 1 507 characters that were parsimony informative. According to the result of jModeltest, a GTR+I+G model was proposed for Bayesian analysis of ITS, LSU and rpb2. The Bayesian analysis of the concatenated three-locus alignment lasted for 9 885 000 generations and a total of 14 852 trees were generated after the BI analysis reached the stop value of 0.01, with the first 25 % of trees discarded as the burn-in phase. The posterior probabilities (PP) were calculated from the remaining trees. The ML tree confirmed the same tree topology and the clades as those presented in the Bayesian phylogeny. Therefore, only the ML tree based on the combined dataset was presented here with the bootstrap support values of the ML analysis (MLBS) and relevant Bayesian posterior probabilities (BPP) shown at the nodes (Fig. 1; MLBS > 50 %, BPP > 0.90 shown).
Fig. 1.
Phylogenetic tree inferred from a Maximum Likelihood analysis based on a concatenated alignment of LSU, ITS and rpb2 sequences of 392 strains representing Hypocreales and related orders (Cephalothecales, Coniochaetales, Glomerellales). The RAxML bootstrap support values (MLBS) above 50 % and Bayesian posterior probabilities (BPP) above 0.90 are given at the nodes (BPP/MLBS). Some of the basal branches were shortened to facilitate layout. The scale bar represents the expected number of changes per site. Families are delimited in coloured boxes, with the family name indicated to the right. Strains with special status are indicated with a superscript letter after the accession number (T: ex-type). New species are printed in red font and new combinations in blue font. The tree is rooted to Saccharata proteae CBS 115206.
Five main supported clades were represented in the tree (Fig. 1), corresponding to four known orders: Cephalothecales, Coniochaetales, Glomerellales, Hypocreales and the outgroup clade. A total of 21 subclades were recognised in the strongly supported clade representing the Hypocreales (clades A–V), of which 16 represented existing families, and five are proposed here as new, Nothoacremoniaceae (clade I), Neoacremoniaceae (clade K), Chrysonectriaceae (clade L), Pseudoniessliaceae (clade M), and Valsonectriaceae (clade U).
The Clade A (BPP < 0.9, MLBS = 90 %), representing those species classified in Clavicipitaceae, contains a new genus Subuliphorum proposed for a species with curved conidia, S. camptosporum (basionym: Acremonium camptosporum). The Clade G & Clade H comprised species of the genera Acremoniopsis, Collarina, Cylindromonium, Eucasphaeria, Myrtacremonium, Neoeucasphaeria, Niesslia, Phialoseptomonium, Rosasphaeria, Trichonectria, and Trichosphaerella representing the family Niessliaceae. Several strains previously recognised as Acremonium and Cephalosporium species also clustered in Niessliaceae, including ex-type strain of A. guillematii (CBS 766.69), A. cavaraeanum (CBS 101149), A. incrustatum (CBS 159.70), A. nigrosclerotium (CBS 154.72) and Cephalosporium ballagii (CBS 134.33). However, phylogeny of Niessliaceae was not well resolved and several genera remain polyphyletic, i.e. Niesslia, Cylindromonium, Trichonectria. Therefore, because of the unresolved phylogeny of Niessliaceae, the taxonomy of these Acremonium and Cephalosporium taxa has not been resolved. The new family Nothoacremoniaceae (Clade I; BPP = 1, MLBS = 100 %) grouped in one clade, which corresponds to the type genus Nothoacremonium. This clade encompassed the type species No. exiguum (basionym: A. exiguum) and two new species with acremonium-like asexual morphs, No. subcylindricum and No. vesiculophorum. The clade K (BPP = 1, MLBS = 95 %), which consists of several isolates previously recognised in Acremonium, is proposed as the new family Neoacremoniaceae. The type genus Neoacremonium is proposed for Ne. flavum, Ne. minutisporum (basionym: Cephalosporium minutisporum), Ne. taiwanense (basionym: Sedecimiella taiwanensis), Ne. vitellinum (basionym: A. vitellinum), and the type species Ne. distortum. The ex-type strain of Parapyrenis maritima (CBS 538.93) together with the strain CBS 795.69 that previously recognised as “A. minutisporum”, formed a subclade in Neoacremonium. The taxonomy and phylogeny of Parapyrenis maritima awaits further confirmation. The new family Chrysonectriaceae (clade L; BPP = 1, MLBS = 100 %) comprises the type species of Chrysonectria (C. finisterensis) and a novel species C. crystallifera.
The new family Pseudoniessliaceae (clade M; BPP = 1, MLBS = 100 %) is introduced to accommodate the new genus Pseudoniesslia proposed for Ps. minutispora (basionym: Niesslia. minutispora). The strain CBS 122798, previously recognised as “Hydropisphaera suffulta”, formed a lineage basal to the clade M. Considering the low support value, the phylogenetic position and taxonomic status of this species remains unresolved pending more cultures and DNA sequence data. The Clade O (BPP = 1, MLBS = 74 %) comprises species of Bionectriaceae. A separate tree with additional taxa of Bionectriaceae is shown in Fig. 2. Clade P (BPP < 0.9, MLBS = 89 %) contains type species of the monospecific genera Xanthonectria (X. pseudopeziza) and Bullanockia (B. australis) which was originally proposed as members of Bionectriaceae. Clade Q (BPP = 1, MLBS = 100 %) comprises Flammocladiella aceris, F. anomiae and F. decora, representing the family Flammocladiellaceae. The proposed new family Valsonectriaceae (clade U; BPP = 1, MLBS = 100 %) comprises three known Valsonectria species (V. portsmouthensis, V. pulchella and V. simpsonii), three novel species (V. crystalligena, V. hilaris and V. soli) and two species previously recognised as Acremonium (V. roseola and V. inflata). Clade V (BPP = 0.95, MLBS = 100 %), representing those species classified in Sarocladiaceae, is strongly supported and includes four subclades. A separate tree with additional taxa of Sarocladiaceae is shown in Fig. 3. The outgroup taxon Saccharata proteae (CBS 115206) and three monophyletic groups representing families in three orders (Cephalothecales, Coniochaetales and Glomerellales) clustered basal in the three-locus tree (Fig. 1). Cephalothecales (clade X; BPP = 1, MLBS = 100 %) comprises the genus Phialemonium, containing Ph. atrogriseum, Ph. obovatum, and Ph. thermophilum (basionym: A. thermophilum); Coniochaetales (clade Y; BPP = 1, MLBS = 100 %) is represented by Coniochaetaceae, containing the ex-type strain of Acremonium psammosporum (CBS 590.63); Glomerellales (clade W; BPP = 1, MLBS = 100 %) is represented by the family Plectosphaerellaceae, and also presented in a separate tree (Fig. 4).
Fig. 2.
Phylogenetic tree inferred from a Maximum Likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tef-1α sequences of 419 strains representing Bionectriaceae and outgroups. The RAxML bootstrap support values (MLBS) above 50 % and Bayesian posterior probabilities (BPP) above 0.90 are given at the nodes (BPP/MLBS). Some of the basal branches were shortened to facilitate layout. The scale bar represents the expected number of changes per site. Genera are delimited in coloured boxes, with the generic name indicated to the right. Strains with special status are indicated with a superscript letter after the accession number (T: ex-type). New species are printed in red font and new combinations in blue font. The tree is rooted to Flammocladiella aceris CBS 138906, F. decora CBS 142776, F. anomiae CLL 16017, Tilachlidium brachiatum CBS 363.97 and T. brachiatum CBS 505.67.
Fig. 3.
Phylogenetic tree inferred from a Maximum Likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tef-1α sequences of 77 strains representing Sarocladiaceae and outgroups. The RAxML bootstrap support values (MLBS) above 50 % and Bayesian posterior probabilities (BPP) above 0.90 are given at the nodes (BPP/MLBS). The scale bar represents the expected number of changes per site. Genera are delimited in coloured boxes, with the genus name indicated to the right. Strains with special status are indicated with a superscript letter after the accession number (T: ex-type). New species are printed in red font and new combinations in blue font. The tree is rooted to Acremonium egyptiacum CBS 124.42, A. alternatum CBS 407.66, and Paracremonium binnewijzendii CBS 698.
Fig. 4.
Phylogenetic tree inferred from a Maximum Likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tef-1α sequences of 90 strains representing Plectosphaerellaceae and outgroups. The RAxML bootstrap support values (MLBS) above 50 % and Bayesian posterior probabilities (BPP) above 0.90 are given at the nodes (BPP/MLBS). The scale bar represents the expected number of changes per site. Genera are delimited in coloured boxes, with the genus name indicated to the right. Strains with special status are indicated with a superscript letter after the accession number (T: ex-type). The new species are printed in red font and new combinations in blue font. The tree is rooted to Monilochaetes infuscans CBS 869.66 and CBS 379.77.
Other clades such as Hypocreaceae (clade B; BPP = 0.85, MLBS = 93 %), Cocoonihabitaceae (clade C; BPP = 1, MLBS = 100 %), Ophiocordycipitaceae (clade D; MLBS = 83 %), Cordycipitaceae (clade E; BPP = 1, MLBS = 96 %), Calcarisporiaceae (clade F; BPP = 1, MLBS = 100 %), Nectriaceae (clade J; BPP = 1, MLBS = 92 %), Stachybotryaceae (clade N; BPP = 0.98, MLBS = 78 %), Tilachlidiaceae (clade R; BPP = 1, MLBS = 94 %), Stromatonectriaceae (clade S; BPP = 1, MLBS = 100 %), and Myrotheciomycetaceae (clade T; BPP = 1, MLBS = 100 %) were recognised in Hypocreales (Fig. 1).
Dataset 2 consists of 419 ingroup isolates that formed a well-supported clade representing Bionectriaceae, with Flammocladiella aceris (CBS 138906), F. anomiae (CBS 142775), F. decora (CBS 142776), Tilachlidium brachiatum (CBS 363.97 and CBS 505.67) serving as outgroup (Fig. 2). The final alignment consists of 3 107 characters, including alignment gaps (gene boundaries ITS: 1–690, 690 bp; LSU: 691–1 478, 788 bp; rpb2: 1 479–2 280, 802 bp; tef-1α: 2 281–3 107, 827 bp). Among those, 1 494 characters were conserved sites and 1 555 were variable sites, including 196 characters that were parsimony-uninformative and 1 359 characters that were parsimony-informative. The jModeltest results recommended that the Bayesian analysis should use Dirichlet base frequencies for all data partitions. The GTR+I+G model was proposed for ITS, LSU, rpb2 and tef-1α. The sequence dataset did not show conflict in the tree topologies for the 70 % reciprocal bootstrap trees, which allowed us to combine the four genes for the multi-locus analysis. The Bayesian analysis lasted for 9 590 000 generations and a total of 14 388 trees were generated after the analysis reached the stop value of 0.01. The first of 25 % trees were discarded as the burn-in phase, and posterior probabilities (PP) were calculated from the remaining trees. The topology of the BI tree was confirmed by ML analysis and the posterior probabilities (BPP) from the BI inference were mapped with MLBS at the tree nodes on the ML tree (Fig. 2; MLBS > 50 %, BPP > 0.90 shown).
The phylogenetic trees based on dataset 2 (Fig. 2), which were generated with Bayesian and Maximum Likelihood methods, distributed the phylogenetic species into 39 well-supported genera (Clades O1–O39) in Bionectriaceae. Clade O1 (BPP = 1, MLBS = 100 %) accommodated type species of the genus Gliomastix (G. murorum) and five other species with melanised conidia (G. masseei, G. musae, G. polychroma, G. roseogrisea and G. tumulicola). Clade O2 (BPP = 1, MLBS = 100 %) comprised six Paracylindrocarpon species with cylindrocarpon-like conidia and ascospores with three or more septa, Paracylindrocarpon aloicola, Pn. multiloculatum (basionym: Nectria multiloculata), Pn. multiseptatum (basionym: Nectria multiseptata), Pn. nabanheensis, Pn. pandanicola, Pn. xishuangbannaensis and two novel species, Pn. aurantiacum and Pn. foliicola. Clade O3 (BPP = 1, MLBS = 100 %) included four species of the genus Fusariella, F. atrovirens, F. concinna, F. curvata, F. hughesii, and another species of Hydropisphaera which was recombined into this genus, F. arenula (syn.: H. arenula). Clades O4, O5 and O7 each comprised a single strain, representing Selinia, Roumegueriella, Synnemellisia, respectively. Clade O6 (BPP = 1, MLBS = 100 %) encompassed the ex-type strains of the two species of Verrucostoma, V. freycinetiae and V. martinicense. The proposed new monotypic genera Musananaesporium (Clade O8) and Gossypinidium (Clade O9) included one strain of the type, respectively, M. tectonae (basionym: Acremonium tectonae), and G. sporodochiale. The Monohydropisphaera clade (Clade O11) was recognised as a novel monophyletic genus, containing the type species of this genus, M. fusigera (basionym: Monotospora fusigera). Clade 12 (BPP = 1, MLBS = 93 %) comprised five species of Hydropisphaera, H. cyatheae, H. fungicola, H. peziza, H. suffulta, and another species of Heleococcum which was recombined into this genus, H. aurantiaca (basionym: Heleococcum aurantiacum; syn.: Heleococcum japonense). Clades O13, O15 and O16 each comprised a single strain, representing Geonectria (G. subalpina), Septofusidium (S. berolinense) and Pseudoacremonium (Pm. sacchari), respectively. The new genus Paragliomastix (clade O14; BPP = 1, MLBS = 99 %) included Px. chiangraiensis (basionym: Acremonium chiangraiense), Px. luzulae (basionym: Torula luzulae), Px. znieffensis, and a novel species Px. rosea. Clade O17 (BPP = 1, MLBS = 100 %) included five known species of the genus Lasionectria (L. antillana, L. boothii, L. krabiense, L. mantuana, and L. sylvana), and another five species were recombined into this genus, L. atrorubra (basionym: Nectriella atrorubra), L. bisepta (basionym: A. biseptum), L. castaneicola (basionym: Hydropisphaera castaneicola), L. cerealis (basionym: Coniosporium cerealis), and L. olida (basionym: A. olidum). Clade O18 (BPP = 1, MLBS = 100 %) comprised seven species accommodated in a novel genus Verruciconidia, i.e. Ve. persicina (syn.: A. persicinum), Ve. verruculosa (basionym: A. verruculosum) and five novel species with verrucose conidia, Ve. erythroxyli, Ve. infuscata, Ve. quercina, Ve. siccicapita and Ve. unguis. Clade O19 (BPP = 1, MLBS = 100 %) accommodated the genus Lasionectriopsis with its type species, L. germanica, and two other species, L. pteridii and L. dentifera (basionym: Nectria dentifera). Clade O20 (BPP = 1, MLBS = 100 %) contained four isolates of Ochronectria, including the generic type, O. calami. Clade O21 (BPP = 1, MLBS = 100 %) accommodated the genus Lasionectriella, including the generic type, La. rubioi. Clade O22 (BPP = 1, MLBS = 100 %) contained three novel species that were accommodated in a new genus proposed below, Ramosiphorum, namely R. echinoporiae, R. polyporicola and R. thailandicum. Clade O23 accommodated the genus Protocreopsis, including five known species, one novel species Pt. finnmarkica, and an Acremonium species that was recombined to the genus as Pt. rutila (basionym: A. rutilum). The genus Nectriopsis (clade O24; BPP = 1, MLBS = 100 %) comprised eight species, including the generic type N. violacea. Clades O25 to O26 encompassed two genera: Bionectria/ Clonostachys (clade O25; BPP = 1, MLBS = 100 %) and Stephanonectria (clade O26; BPP = 1, MLBS = 100 %). Clade O27 (BPP = 1, MLBS = 100 %) comprised three species, Mycocitrus odorus, M. phyllostachydis, and M. zonatus (basionym: A. zonatum). Emericellopsis (Clade O28; BPP = 1, MLBS = 100 %) was represented by 15 previously described species (including the type species E. terricola), the new species E. brunneiguttula, and another six species, E. exuviara (basionym: A. exuviarum), E. fimetaria (syn. Stilbella fimetaria), E. fuci (basionym: A. fuci), E. moniliformis (basionym: A. moniliforme), E. salmonea (basionym: A. salmoneum), and E. tubakii (basionym: A. tubakii). Clade O29 (BPP = 1, MLBS = 100 %) comprised the type species, Stanjemonium grisellum, and three other species, S. dichromosporum (basionym: A. dichromosporum), S. fuscescens and S. ochroroseum. Clade O30 (BPP = 1, MLBS = 100 %) comprised four isolates previously received as Acremonium and characterised by producing abundant phialides with apical percurrent proliferation, representing one species, which belong to a newly introduced genus Proliferophialis, namely Pro. apiculata. Clade O31 (BPP = 1, MLBS = 100 %) accommodated the genus Acremonium s. str., which is represented by eight previously described species (including the generic type species A. alternatum), the 21 isolates that were previously identified as Acremonium spp., representing 10 novel species, namely A. aerium, A. brunneisporum, A. chlamydosporium, A. ellipsoideum, A. gamsianum, A. longiphialidicum, A. subulatum, A. synnematoferum, A. multiramosum, and A. mycoparasiticum. One Cephalosporium species clustered within the Acremonium clade, phylogenetically close to A. aerium, and was thus recombined to this genus, as A. purpurascens (basionym: C. purpurascens). The majority of isolates that clustered in clade O32 (BPP = 1, MLBS = 100 %) were identified as “Acremonium” spp., and a new generic name Waltergamsia is introduced for this clade, which comprised 12 accepted species, four novel species, W. alkalina, W. catenata, W. moroccensis, W. obpyriformis, and eight species that were received as Acremonium, Cephalosporium or Nectriopsis species including W. citrina (basionym: A. citrinum), W. dimorphospora (basionym: A. dimorphosporum), W. epimycota (basionym: N. epimycota), W. fusidioides (syn. A. fusidioides), W. hennebertii (basionym: A. hennebertii), W. parva (basionym: A. parvum), W. pilosa (basionym: A. pilosum), and W. zeylanica (basionym: C. zeylanicum). Clade O33 (BPP = 1, MLBS = 100 %) encompassed the ex-type strains of three species of Geosmithia, G. lavendula, G. microcorthyli and G. pallidum. Bulbithecium formed a well-supported clade (clade O34; BPP = 1, MLBS = 100 %) and included the generic type species B. hyalosporum, four species that were received as Acremonium spp. or Leucosphaerina spp., namely B. arxii (basionym: L. arxii), B. borodinense (basionym: A. borodinense), B. pinkertoniae (basionym: A. pinkertoniae), B. spinosum (basionym: Cephalosporium spinosum) and three novel species B. ammophilae, B. ellipsoideum and B. truncatum. Clade O35 (BPP = 1, MLBS = 100 %) comprised six isolates of Ovicillium, representing five species, including its type species, O. attenuatum, and two species previously assigned to other genera, O. variecolor (basionym: A. variecolor) and O. asperulatum (basionym: A. asperulatum). The new genus Proxiovicillium (clade O36; BPP = 1, MLBS = 100 %) comprised Pr. blochii (basionym: Mastigocladium blochii, syn. Acremonium blochii), the type species, and the new species Pr. lepidopterorum. Clade O37 (BPP = 1, MLBS = 100 %) comprised seven species which belong to Hapsidospora. This clade contains two species initially classified as Acremonium and Cephalosporium species; and two species from the two genera from the same family with Hapsidospora, which were recombined as H. globosa (basionym: Nigrosabulum globosum) and H. inversa (basionym: Mycoarachis inversa). Clade O38 (BPP = 1, MLBS = 100 %) comprised two isolates representing two species, which belong to a new genus introduced here, Alloacremonium, namely Al. ferrugineum and Al. humicola. The clade corresponding to Stilbocrea (clade O39; BPP = 1, MLBS = 100 %), included two species, S. macrostoma and S. walteri.
Dataset 3 consisted of 77 strains belonging to Sarocladiaceae, with Acremonium egyptiacum (CBS 124.42), A. alternatum (CBS 407.66), and Paracremonium binnewijzendii CBS 698.86 as outgroup (Fig. 3). The final alignment consisted of 3 087 characters including gaps (gene boundaries ITS: 1–593, 593 bp; LSU: 594–1 382, 789 bp; rpb2: 1 383–2 156, 774 bp; tef-1α: 2 157–3 087, 931 bp). Among those, 1 694 characters were conserved sites and 1 235 were variable sites, including 190 characters that were parsimony-uninformative and 1 045 characters that were parsimony-informative. The jModeltest results recommended that the Bayesian analysis should use Dirichlet base frequencies for all data partitions. The GTR+I+G model was proposed for ITS, LSU, rpb2 and tef-1α. The sequence dataset did not show conflict in the tree topologies for the 70 % reciprocal bootstrap trees, which allowed us to combine the four genes for the multi-locus analysis. The BA lasted for 5 550 000 generations and a total of 8 287 trees were generated after the BI analysis reached the stop value of 0.01. The first 25 % trees were discarded as the burn-in phase, and posterior probabilities (PP) were calculated from the remaining 6 215 trees. The topology of the BI tree was confirmed by ML analysis and therefore, the posterior probabilities (PP) from the BI inference were mapped with MLBS at the tree nodes on the ML tree (Fig. 3; MLBS > 50 %, BPP > 0.90 shown).
The families that belong to Sarocladiaceae, represented by the species grouping in clades V1–V4, clustered in a strongly supported clade (BPP = 1, MLBS = 100 %). Clade V1 was represented by 21 accepted species of Sarocladium (BPP = 1; MLBS = 100 %), and six strains that were labelled as Acremonium spp. were placed in five independent and well-supported clades/lineages, representing five novel species, S. agarici, S. citri, S. ferrugineum, S. fuscum, and S. theobromae. Clade V2 (BPP = 1, MLBS = 89 %) contained seven accepted species of Parasarocladium and two undescribed lineages, representing two species, Par. funiculosum (basionym: Cephalosporium acremonium var. funiculosum) and Par. chondroidum. Clade V3 (BPP = 1, MLBS = 100 %) encompassed one subclade representing Chlamydocillium cyanophilum, and seven subclades comprising seven cultures that were labelled as A. curvulum, including the ex-type culture of A. curvulum. Clade V1 (BPP = 1, MLBS = 100 %) was basal to Clades V1–V3, containing two “A. alternatum” cultures and forming an independent lineage, which represented the novel genus Polyphialocladium.
Dataset 4 consisted of ITS, LSU, rpb2 and tef-1α sequences of 90 strains representing members of Plectosphaerellaceae, with Monilochaetes infuscans (CBS 869.66 and CBS 379.77) as outgroups (Fig. 4). The final alignment consisted of 3 036 characters including gaps (gene boundaries ITS: 1–614, 614 bp; LSU: 615–1448, 834 bp; rpb2: 1449–2268, 820 bp; tef-1α: 2 269–3 036, 768 bp). Among those, 1 855 characters were conserved sites and 1 134 were variable sites, including 1 009 characters that were parsimony-informative. The jModeltest results recommended that the Bayesian analysis should use Dirichlet base frequencies for all data partitions. The GTR+I+G model was proposed for ITS, LSU, rpb2 and tef-1α. The sequence dataset did not show conflict in the tree topologies for the 70 % reciprocal bootstrap trees, which allowed us to combine the four genes for the multi-locus analysis. The BA lasted for 295 000 generations and a total of 439 trees were generated after the BI analysis reached the stop value of 0.01, with the first 25 % trees discarded as the burn-in phase. The posterior probabilities (PP) were calculated from the remaining 330 trees. The topology of the BI tree was confirmed by ML analysis and therefore, the posterior probabilities (PP) from the BI inference were mapped with MLBS at the tree nodes on the ML tree (Fig. 4; MLBS > 50 %, BPP > 0.90 shown).
A total of 23 well-supported clades represent 23 known genera in the phylogenetic tree based on dataset 4: Acremoniisimulans (BPP < 0.90; MLBS = 97 %), Acrostalagmus (BPP = 1; MLBS = 100 %), Brunneochlamydosporium (BPP = 1; MLBS = 100 %), Brunneomyces (BPP = 1; MLBS = 100 %), Chlamydosporiella (BPP = 1; MLBS = 100 %), Chordomyces (BPP = 1; MLBS = 100 %), Furcasterigmium (BPP = 1; MLBS = 100 %), Fuscohypha (BPP = 1; MLBS = 100 %), Gibellulopsis (BPP = 1; MLBS = 100 %), Lectera (BPP = 1; MLBS = 100 %), Musicillium (BPP = 1; MLBS = 100 %), Musidium (BPP = 1; MLBS = 100 %), Nigrocephalum (BPP = 1; MLBS = 100 %), Paramusicillium (BPP = 1; MLBS = 100 %), Paragibellulopsis (BPP = 1; MLBS = 100 %), Phialoparvum, Plectosphaerella (BPP = 1; MLBS = 100 %), Sayamraella (BPP = 1; MLBS = 74 %), Sodiomyces (BPP = 1; MLBS = 100 %), Stachylidium (BPP = 1; MLBS = 97 %), Summerbellia (BPP = 1; MLBS = 100 %), Theobromium (BPP = 1; MLBS = 93 %), and Verticillium (BPP = 1; MLBS = 99 %). Two clades with species identified as Acremonium s. lat. were reassigned to new genera: Allomusicillium (BPP = 1; MLBS = 96 %), and Parafuscohypha (BPP = 1; MLBS = 78 %), respectively.
Taxonomy
Based on multi-locus phylogenetic inference, supported by morphological observations, habitat information and geographical comparisons, a total of 633 acremonium-like strains were examined in this study. Strains were shown to represent 327 taxa belonging to Bionectriaceae, Clavicipitaceae, Cordycipitaceae, Myrotheciomycetaceae, Nectriaceae, Niessliaceae, Sarocladiaceae (Hypocreales), Cephalothecaceae (Cephalothecales) and Plectosphaerellaceae (Glomerellales). Among these, five new families, 17 new genera and 63 new combinations are proposed, with descriptions of 65 new species. Furthermore, one epitypification and one neotypification are proposed, two species resurrected and eight species reduced to synonymy. Four new species that proved to be sterile are described based on DNA sequence data, following the approach of Gomes et al. (2013) and Lombard et al. (2016). Families and genera are arranged according to their position on the phylogenetic tree following the clade number (Figs 1–4). Species in the separate trees of Bionectriaceae, Plectosphaerellaceae and Sarocladiaceae are alphabetically arranged (Figs 2–4).
Clade A
Clavicipitaceae Rogerson, Mycologia 62: 900. 1970.
Classification: Hypocreales, Sordariomycetes.
Subuliphorum L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845792.
Etymology: Named after the subulate phialides produced by the type species.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores mostly aggregated, erect, straight or curved, arising directly from aerial or substratal mycelium, unbranched or basitonously branched, or repeatedly verticillate towards the apex and form sporodochia. Conidiogenous cells enteroblastic, monophialidic, lateral or terminal, arising laterally from hyphae or in terminal pairs, or verticils of three, or small monopodially branched tufts of up to four from conidiophores, subulate, hyaline, thin-, smooth-walled, commonly with inconspicuous periclinal thickening. Conidia aseptate, short cylindrical or lunate, hyaline, thin-, smooth-walled, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed (revised from Gams 1971).
Type: Subuliphorum camptosporum (W. Gams) L.W. Hou, L. Cai & Crous
Notes: This monotypic genus is established to accommodate a species of Acremonium s. lat., A. camptosporum, since it is not congeneric with Acremonium s. str. in Bionectriaceae (Figs 1, 2) based on A. alternatum, but forms a basal lineage in the family Clavicipitaceae (Fig. 1). Subuliphorum can be distinguished by the repeatedly verticillately or basitonously branched conidiophores, and the thin, long phialides.
Subuliphorum camptosporum (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845801. Fig. 5.
Fig. 5.

Subuliphorum camptosporum (ex-type culture CBS 756.69). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Basionym: Acremonium camptosporum W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 57. 1971.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, abundantly gracile and long mycelial ropes formed, 1–2.5 μm wide. Conidiophores mostly aggregated, rarely solitary, erect, straight or curved, arising directly from aerial or substratal mycelium, or from rope formed by mycelium, unbranched or basitonously branched, or repeatedly verticillate towards the apex, bearing 1–3 whorls of 1–5 phialides, forming sporodochia in older cultures, with upper branching reminiscent of Verticillium, up to 84 μm long, with 1–2 septate, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, terminal, arising laterally from hyphae or in terminal pairs, or verticils of three, or small monopodially branched tufts of up to five from conidiophores, subulate, hyaline, thin-, smooth-walled, 14.9–69.5 μm long, 0.9–2.2 μm wide at base, commonly with inconspicuous periclinal thickening and collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, short cylindrical or lunate, curved, hyaline, thin-, smooth-walled, eguttulate, 2.5–4 × 1.1–1.5 μm, arranged in slimy heads. Crystal present. Chlamydospores and sexual morph not observed (revised from Gams 1971).
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 30–35 diam, flat, granulose, dusty, white, margin entire, reverse olivaceous buff at centre, buff at periphery; On MEA reaching 30–35 mm diam, raised, radially folded, felty, white, margin crenate, reverse light saffron, with radially white lines; On PDA reaching 33 mm diam, flat, felty, creamy white, with luteous crystals, margin entire, reverse luteous with buff margin; On SNA reaching 30–35 mm diam, flat, dusty, white, margin entire, reverse concolourous.
Typus: Germany, Kiel-Kitzeberg, aerial contaminant, unknown collection date, isol. 1965, coll. and isol. by W. Gams, No. 517, CBS H-6602, CBS H-8108, CBS H-8109, CBS H-8110, CBS H-8110 & CBS H-8111 (holotype CBS 756.69 preserved as metabolically inactive culture, ex-type culture CBS 756.69).
Additional materials examined: Cuba, Habana, insect, 17 Jan. 1991, R.F. Castañeda, culture CBS 835.91 = INIFAT C91/63-1. Germany, Kiel, soil, parasitic on nematodes (Panagrellus redivivus), unknown collection date and collector, isol. M. Hashem, CBS H-5053, culture CBS 890.85. South Africa, Kwa-Zulu-Natal Province, Kosi Bay, soil, unknown collection date and collector, isol. 1974 by W.J. Jooste, No. 74/16, culture CBS 677.74. Unknown, unknown substrate, collection date and collector, isol. M.C. Papendorf, No. 337, culture CBS 757.69.
Notes: The name Subuliphorum camptosporum (basionym: Acremonium camptosporum) refers to the curved conidia of this species (Gams 1971). Morphological characters of the cultures used in this study agree well with the description of A. camptosporum from literature (Gams 1971), except the longer phialides observed in the present study (14.9–69.5 μm vs 16–40 μm). This species is morphologically similar to Xenoacremonium recifei (previously Acremonium recifei) (Gams 1971). However, according to our phylogenetic analysis, S. camptosporum clusters basally in a clade adjacent to the Clavicipitaceae, distant from X. recifei that was placed in Nectriaceae (Fig. 1). The phylogenetic result agrees well with the previous result of Summerbell et al. (2011), placing this species in a unique lineage. It is hereby accommodated in the new genus Subuliphorum.
Clade E
Cordycipitaceae Kreisel ex G.H. Sung et al., Stud. Mycol. 57: 48. 2007.
Classification: Hypocreales, Sordariomycetes.
Simplicillium W. Gams & Zare, Nova Hedwigia 73: 38. 2001.
Type: Simplicillium lanosoniveum (J.F.H. Beyma) Zare & W. Gams.
Other accepted species with available sequences: S. lamellicola (F.E.V. Sm.) Zare & W. Gams
Simplicillium lanosoniveum (J.F.H. Beyma) Zare & W. Gams, Nova Hedwigia 73: 39. 2001.
Basionym: Cephalosporium lanosoniveum J.F.H. Beyma, Antonie van Leeuwenhoek 8: 121. 1942.
Synonyms: Cephalosporium salviniae R.T. Jones & Frederick, Mycopathol. Mycol. Appl. 43: 195. 1971.
Acremonium byssoides W. Gams & T.M. Lim, Trans. Brit. Mycol. Soc. 64: 391. 1975.
Descriptions: van Beyma (1942), Jones & Frederick (1971), Zare & Gams (2001).
Typus: Netherlands, from hair of Cibotium schiedei (Dicksoniaceae), unknown collection date and collector, isol. Jun. 1942 by Habekotté, Lab. Techn. Botanie, Delft (neotype of Cephalosporium lanosoniveum CBS H-7277, ex-neotype culture CBS 123.42).
Additional materials examined: Malaysia, Kuala Lumpur, from Oidium heveae (Erysiphaceae) on Hevea brasiliensis (Euphorbiaceae), unknown collection date, Kim, isol. T.M. Lim (holotype of Acremonium byssoides CBS H-6642, isotype CBS H-6643, ex-type culture CBS 321.72 = ATCC 32204 = IMI 185371); Kuala Lumpur, from Oidium heveae (Erysiphaceae) on Hevea brasiliensis (Euphorbiaceae), unknown collection date and collector, dep. T.M. Lim, culture CBS 322.72. USA, Georgia, Atlanta, from Salvinia rotundifolia (Salviniaceae) in aquarium, unknown collection date and collector, dep. L. Frederick, ex-type culture of Cephalosporium salviniae CBS 531.72 = ATCC 22503 = AU 1147.
Notes: The ex-type cultures of Acremonium byssoides (CBS 321.72) and Cephalosporium salviniae (CBS 531.72) are phylogenetically identical to the ex-neotype culture of S. lanosoniveum (CBS 123.42; Fig. 1). Therefore, A. byssoides and C. salviniae are synonymised under S. lanosoniveum based on morphology and their identical sequences.
Clade I
Nothoacremoniaceae L.W. Hou, L. Cai & Crous, fam. nov. MycoBank MB 845802.
Classification: Hypocreales, Sordariomycetes.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores erect, straight or irregularly bent at base, unbranched or with irregularly basitonous side branches, with 1–2 septa at base, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Conidiogenous cells enteroblastic, monophialidic or polyphialidic, lateral or terminal, unbranched or basitonously branched, cylindrical, acicular, or subulate, hyaline, thick-, smooth-walled, with inconspicuous periclinal thickening and collarette at conidiogenous locus; with short sterile outgrowths; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, ellipsoidal, cylindrical or fusoid, straight, hyaline, thin- and thick-, smooth-walled, eguttulate or with small guttules, arranged in slimy heads or long chains. Chlamydospores and sexual morph not observed.
Type genus: Nothoacremonium L.W. Hou, L. Cai & Crous
Nothoacremonium L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845803.
Etymology: Referring to its similarity with Acremonium. Notho = nothus in Greek, fake, close but different; Acremonium = acremonium-like morphology.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores erect, straight or irregularly bent at base, unbranched or with irregularly basitonous side branches, with 1–2 septa at base, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Conidiogenous cells enteroblastic, monophialidic or polyphialidic, lateral or terminal, unbranched or basitonously branched, cylindrical, acicular, or subulate, hyaline, thick-, smooth-walled, with inconspicuous periclinal thickening and collarette at conidiogenous loci; with short sterile outgrowths; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, ellipsoidal, cylindrical or fusoid, straight, hyaline, thick-, smooth-walled, eguttulate or with small guttules, arranged in slimy heads or long chains. Chlamydospores and sexual morph not observed.
Type: Nothoacremonium exiguum (W. Gams) L.W. Hou, L. Cai & Crous
Other accepted species with available sequences: No. subcylindricum L.W. Hou, L. Cai & Crous, No. vesiculophorum L.W. Hou, L. Cai & Crous
Notes: In our study the ex-type culture of Acremonium exiguum (CBS 587.73) clustered in a separate clade containing two new species, clearly distant from Acremonium s. str. in the Bionectriaceae (Fig. 1). Therefore, a new genus, Nothoacremonium, is proposed here to accommodate these taxa. This genus accommodates three species, No. exiguum (basionym: A. exiguum), No. subcylindricum and No. vesiculophorum. Nothoacremonium is morphologically similar to Acremonium but can be distinguished mainly by the results of phylogenetic analysis.
Nothoacremonium exiguum (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845804. Fig. 6.
Fig. 6.

Nothoacremonium exiguum (ex-type culture CBS 587.73). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads on mycelial ropes. E–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Basionym: Acremonium exiguum W. Gams, Trans. Brit. Mycol. Soc. 64: 390. 1975.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–1.7 μm wide. Sporulation moderate, phalacrogenous nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, irregularly wavy at base, or straight, arising directly from submerged or superficial hyphae, or ropes formed by mycelium, unbranched, poorly branched, 14.2–52.5(–80.5) μm long, 1–2-septate at base or lower part, hyaline, smooth-, thin-walled, occasionally rough-walled at lower part, cell walls usually thicker than those of vegetative hyphae. Phialides lateral, cylindrical or subulate, tapering at top, hyaline, thick-, smooth-walled, 13.5–39 μm long, 0.9–1.9 μm wide at base, with inconspicuous periclinal thickening and collarette at conidiogenous loci; with short sterile outgrowths; polyphialides with two conidiogenous loci are occasionally present. Conidia aseptate, cylindrical or ellipsoidal, with both ends rounded, straight, hyaline, thin-, smooth-walled, with several small guttules, 2.5–5.3 × 1–1.5 μm, arranged in slimy heads. Chlamydospores and sexual morph not observed (revised from Gams 1975).
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 21 mm diam, flat, membranous without aerial mycelium or dusty, white, margin entire; reverse concolourous; On MEA reaching 20–21 mm diam, flat, radially folded, membranous without aerial mycelium, pale rosy buff, with white and crenate margin; reverse umber, with buff radial lines; On PDA reaching 24 mm diam, flat, radially folded at centre, membranous without aerial mycelium, creamy white, buff at periphery, margin fimbriate; reverse buff; On SNA reaching 16 mm diam, flat, membranous without aerial mycelium, white, margin entire; reverse whitish. Lacking odour on all media.
Typus: Sri Lanka, Hakgala Garden, from Tubulicium dussii (Hydnodontaceae) on Dicksonia antarctica (Dicksoniaceae), Jan. 1973, W. Gams, CBS H-6609 (holotype CBS 587.73 preserved as metabolically inactive culture, ex-type culture CBS 587.73 = ATCC 32205 = IMI 185370).
Notes: Nothoacremonium exiguum (basionym: Acremonium exiguum) is a fungicolous species isolated from Tubulicium dussii in Sri Lanka (Gams 1975). It is a typical acremonium-like species and is difficult to identify without the use of DNA sequence data. According to the phylogenetic analyses this species clusters in a well-supported lineage in the genus Nothoacremonium and is closely related to No. vesiculophorum (Fig. 1). Morphologically, characters of the ex-type culture are similar to the description available in the literature (Gams 1975), except for the production of slightly longer conidia [2.5–5.3 × 1–1.5 μm vs 2–2.7(–3.5) × 1–1.5 μm], which could result from different media used for cultures. For a morphological comparison with the other species of this genus, see notes under No. vesiculophorum.
Nothoacremonium subcylindricum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845805. Fig. 7.
Fig. 7.

Nothoacremonium subcylindricum (ex-type culture CBS 416.68). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, G–J. Branched or unbranched conidiophores. E. Conidiophores radiating out from coils formed by the mycelium. F. Conidial heads. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the subcylindrical shape of its conidia.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.5–2.6 μm wide, mycelial ropes formed, radially arranged and parallel to the colony surface. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight or irregularly bent at base, arising directly from submerged or superficial hyphae, or ropes and coils formed by mycelium, mostly with 1–2 irregularly basitonous side branches, or unbranched, (17–)37–135 μm long, with a single septum at base, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate, hyaline, thick-, smooth-walled, (10–)15–27.5 μm long, 1–2 μm wide at base, with inconspicuous periclinal thickening and collarette at conidiogenous loci; with short sterile outgrowths; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, subcylindrical, ellipsoidal, both ends rounded, hyaline, thin-, smooth-walled, eguttulate, 2.6–5.7 × 1.3–1.9 μm, arranged in slimy heads, confluent and salmon with age. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 25–28 mm diam, flat, with sparse aerial mycelium, dusty, salmon at centre, with a white belt in middle, dirty white at periphery, margin entire, reverse buff at centre, dirty white at periphery; On MEA reaching 16 mm diam, raised, aerial mycelium abundant, moist, short hairy, dirty white, margin entire, reverse saffron; On PDA reaching 26–28 mm diam, flat, aerial mycelium sparse, dusty, salmon at centre, creamy white at periphery, margin filiform, reverse buff; On SNA reaching 28 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse concolourous.
Typus: Netherlands, Overijssel Province, Enschede, from human skin and nail, 15 May 1968, R. Braakenburg van Backum, No. 9224 (holotype CBS H-8336, ex-type culture CBS 416.68).
Additional materials examined: Germany, Kiel, Hortus Botanicus, on decaying wood in heated greenhouse, 1965, W. Gams No. 656, CBS H-8330, culture CBS 190.70; unknown substrate, collection date and collector, isol. H.I. Nirenberg, No. 1.9/1V2(1), culture CBS 611.95. USA, Florida, from deformed toenail, unknown collection date and collector, isol. 1968 by N. Zaias, No. 1485, CBS H-8328, culture CBS 781.69.
Notes: Cultures representing Nothoacremonium subcylindricum were initially identified as Acremonium potronii. There are no ex-type cultures of A. potronii that have survived. Although type materials of A. potronii were not examined, observation of the conidiogenous structures in culture revealed No. subcylindricum to have conidiophores with basitonous side branches, longer phialides and conidia without a tapered base, which differ from the description of A. potronii (Gams 1971). This species clade is fully supported by the phylogenetic analysis (Fig. 1; BPP/MLBS = 1/100 %).
Nothoacremonium vesiculophorum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845806. Fig. 8.
Fig. 8.

Nothoacremonium vesiculophorum (ex-type culture CBS 397.70B). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–H, K. Conidiophores. I, J. Phialides extending into vesicular expansion at apex (arrows). L. Conidia. Scale bars = 10 μm.
Etymology: Referring to the production of phialides with a vesicular structure at the apex.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–1.5 μm wide, mycelial coils formed. Conidiophores solitary, erect, straight or curved, arising directly from submerged or superficial hyphae, or radiating out from mycelial coils, unbranched or poorly branched, proliferating sympodially, showing conidiogenous cells as short lateral, cylindrical, asymmetrical projections, or bearing 1–2 phialides per node, up to 67 μm long, 1-septa at base, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral, acicular, hyaline, thick-, smooth-walled, (14.3–)20.7–57 μm long, 1–2.3 μm wide at base, occasionally extending into a 1.3–1.7 μm wide vesicular expansion at apex, with inconspicuous periclinal thickening and collarette at conidiogenous loci, commonly with a percurrent proliferation; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, fusoid, straight, with thickened, truncate base (hilum) at both ends, hyaline, thick-, smooth-walled, 3.4–5.2 × 1.3–1.8 μm, eguttulate, arranged in long chains, soon collapsing into conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 15–16 mm diam, flat, sparse aerial mycelium, dusty, white to pale salmon, margin entire, reverse rosy buff; On MEA reaching 16–18 mm diam, flat, with moderate aerial mycelium, moist, hairy, white, margin crenate, reverse saffron, with buff radial lines; On PDA reaching 17–18 mm diam, flat, sparse aerial mycelium, dusty, dirty white or pale salmon, margin entire, reverse pale saffron; On SNA reaching 15–18 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse white. Lacking odour on all media.
Typus: Netherlands, North Holland Province, De Vuntus near Loosdrecht, on dying leaf of Cladium mariscus (Cyperaceae), unknown collection date and collector, isol. Jun. 1968 by W. Gams, No. 1406 (holotype CBS H-24637, ex-type culture CBS 397.70B).
Notes: The culture CBS 397.70B was labelled as “Acremonium implicatum (currently Sarocladium implicatum)”. However, initial molecular work based on LSU/SSU sequences indicated that it is not a Sarocladium but a member of the “A. exiguum” clade (Summerbell et al. 2011). In our present study, the culture clusters in Nothoacremonium according to the phylogenetic analysis based on the combined four genes and represents a monocillium-like species producing vesicular as well as acicular phialides. However, it is not congeneric with Monocillium or Niesslia (Fig. 1). This is the only monocillium-like morphology observed in Nothoacremonium. Morphologically, it differs from the other two species in the genus Nothoacremonium in the production of vesicular structure at the apex on phialides and conidia arranged in long chains, while No. exiguum and No. subcylindricum have cylindrical or subulate phialides and conidia arranged in slimy heads.
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hit using the LSU sequence of No. vesiculophorum is a sequence from the ex-isotype culture of No. subcylindricum CBS 416.68 [Genbank HQ232097.1; Identity = 769/778 (99 %), no gaps]. The closest hit using the ITS sequence is CBS 587.73 [GenBank NR_159619.1; Identity = 444/493 (90 %), 11 gaps (2 %)], a sequence from the ex-type culture of A. exiguum (currently No. exiguum).
Clade J
Nectriaceae Tul. & C. Tul. [as ‘Nectriei’], Select. Fung. Carpol. (Paris) 3: 3. 1865.
Classification: Hypocreales, Sordariomycetes.
Type genus: Nectria (Fr.) Fr.
Xenoacremonium L. Lombard & Crous, Stud. Mycol. 80: 234. 2015.
Stromata erumpent, orange to brown, pseudoparenchymatous, cells forming textura angularis, intergrading with ascomatal wall. Ascomata superficial, aggregated in groups of 2–3, brown, subglobose to globose. Peridium 50–90 μm thick, comprises two regions, outer region intergrading with stroma, cells forming textura angularis, inner region elongate, thin-walled, hyaline cells, forming textura prismatica. Paraphyses 2–3 μm wide, persistent, aseptate, not branched, tapering towards the apex. Asci 8-spored, unitunicate, mainly uniseriate to partly biseriate, with inconspicuous ring at apex, clavate. Ascospores ellipsoidal to fusoid with rounded ends, straight or slightly curved, aseptate, hyaline, smooth-walled. Mycelium consisting of hyaline, septate, branched hyphae. Conidiophores either as lateral phialidic pegs or arising laterally from somatic hyphae, erect, cylindrical to subcylindrical, unbranched, branched, or repeatedly verticillate towards the apex, aseptate or septate, smooth, hyaline to pale brown. Conidiogenous cells lateral or terminal, monophialidic, hyaline, smooth, elongate-ampulliform or subcylindrical, tapering towards the apex, with periclinal thickening and inconspicuous collarette, with short sterile outgrowths in some species. Conidia aseptate, allantoid, fusoid to ellipsoidal to cylindrical, slightly or strongly curved, forming slimy heads on the conidiophore (emended from Lombard et al. 2015).
Type: Xenoacremonium recifei (Leão & Lôbo) L. Lombard & Crous
Other accepted species with available sequences: Xenoacremonium allantoideum L.W. Hou, L. Cai & Crous, X. brunneosporum Dayar., E.B.G. Jones & K.D. Hyde, X. falcatum L. Lombard & Crous
Xenoacremonium allantoideum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845808. Fig. 9.
Fig. 9.

Xenoacremonium allantoideum (ex-type culture CBS 505.94). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads. E. Mycelial ropes with conidiophores. F–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Etymology: Referring to the allantoid shape of its conidia.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.5–3 μm wide, mycelial ropes formed. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores mostly aggregated, erect, straight, arising directly from aerial or substratal mycelium, or from ropes and coils formed by mycelium, unbranched, basitonously branched, or repeatedly verticillate towards the apex, bearing 1–4 levels with 2–3 phialides per node, occasionally chondroid at base, 30.5–98.5 μm long, with 1–2 septa, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides solitary and in divergent whorls of 2–3, lateral, rarely terminal, subcylindrical, hyaline, thick-, smooth-walled, 17.5–67.5 μm long, 1.4–2.5 μm wide at base, commonly with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci, with short sterile outgrowths; polyphialides not observed. Conidia aseptate, allantoid, with rounded ends, curved, hyaline, thin-, smooth-walled, eguttulate, 3.6–6 × 1.3–2 μm, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 50–53 mm diam, flat, membranous at centre, thinly felty at periphery, dirty white, margin entire, pale salmon in old cultures, reverse buff; On MEA reaching 48 mm diam, raised, with abundant aerial mycelium, felty, or short hairy, white, with ochreous edge, margin entire, reverse apricot; On PDA reaching 48–49 mm diam, flat, with moderate aerial mycelium, floccose at centre, thinly felty at periphery, white with dirty white and entire margin, reverse dirty white; On SNA reaching 53–55 mm diam, flat, with sparse aerial mycelium, dusty, white, margin entire, reverse concolourous. Lacking odour on all media.
Typus: Unknown country, substrate, collection date and collector, dep. G. Seehann (holotype CBS H-24635, ex-type culture CBS 505.94).
Notes: The ex-type culture of Xenoacremonium allantoideum was previously labelled as Acremonium cf. curvulum, which is probably based on its curved conidia. Previous phylogenetic analysis demonstrated that CBS 505.94 clustered with the ex-type of Acremonium recifei (currently Xenoacremonium recifei), distant from ex-type of A. curvulum (Summerbell et al. 2011). In this study, the phylogenetic results agreed with the previous results of Lombard et al. (2015), which placed culture CBS 505.94 in a separate lineage related to X. recifei (Fig. 1). Morphologically, culture CBS 505.94 differs from X. recifei in producing longer phialides (17.5–67.5 μm long vs 15–55 μm long) and allantoid conidia, while X. recifei has strongly curved club-shaped conidia. In addition, CBS 505.94 differs from X. recifei by the lack of chlamydospores (Lombard et al. 2015) and can be distinguished from X. falcatum in its repeatedly basitonously or verticillately branched conidiophores, and the phialides with conspicuous periclinal thickening and cylindrical flared collarette at the conidiogenous loci (Lombard et al. 2015).
Clade K
Neoacremoniaceae L.W. Hou, L. Cai & Crous, fam. nov. MycoBank MB 845809.
Classification: Hypocreales, Sordariomycetes.
Mycelium consisting of branched, septate, smooth-, thin-walled hyphae. Conidiophores solitary or aggregated, erect, arising directly from submerged or superficial hyphae, or ropes formed by mycelium, occasionally basitonously branched or unbranched, proliferating sympodially, hyaline, septate, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Conidiogenous cells enteroblastic, mono- or polyphialidic, lageniform, subcylindrical, subulate, hyaline, thin- or thick-, smooth-walled, with conspicuous or inconspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides with 2–3 conidiogenous loci occasionally present. Conidia aseptate, cylindrical, ellipsoid, ovoid, wide fusoid, spindle-shaped, straight, hyaline, thin-, smooth-walled, eguttulate, arranged in chains or slimy heads. Chlamydospores laterally on short stalks, single, globose to sub-globose, hyaline, smooth-, thick-walled. Sexual morph not observed.
Type: Neoacremonium L.W. Hou, L. Cai & Crous
Notes: The family Neoacremoniaceae presently only includes Neoacremonium, based on Ne. distortum. Five acremonium-like species together with Parapyrenis maritima formed a fully supported clade basal to the Nectriaceae, separated from all known families in the Hypocreales (Fig. 1).
Neoacremonium L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845810.
Etymology: Referring to its morphological similarity to the genus Acremonium.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores solitary or aggregated, erect, arising directly from submerged or superficial hyphae, or ropes formed by mycelium, with 1–2 irregularly basitonous side branches, or unbranched, proliferating sympodially, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Conidiogenous cells enteroblastic, mono- or polyphialidic, lateral or terminal, lageniform, subcylindrical, subulate, hyaline, thin- or thick-, smooth-walled, with conspicuous or inconspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides with 2–3 conidiogenous loci occasionally present; adelophialides present in some species. Conidia aseptate, often variable in shape and size, cylindrical, ellipsoid, ovoid, wide fusoid, spindle-shaped, straight, hyaline, thin-, smooth-walled, eguttulate, arranged in chains or slimy heads. Chlamydospores laterally on short stalks, single, globose to sub-globose, hyaline, smooth-, thick-walled. Sexual morph not observed.
Type species: Neoacremonium distortum L.W. Hou, L. Cai & Crous
Other accepted species with available sequences: Neoacremonium flavum L.W. Hou, L. Cai & Crous, Ne. minutisporum (Sukapure & Thirum.) L.W. Hou, L. Cai & Crous, Ne. taiwanense (K.L. Pang, Alias & E.B.G. Jones) L.W. Hou, L. Cai & Crous, Ne. vitellinum (W. Gams) L.W. Hou, L. Cai & Crous
Notes: Neoacremonium currently accommodates five species of Acremonium s. lat. However, they are not congeneric with the type of Acremonium s. str. based on the phylogenetic analysis, which shows that Neoacremonium forms an independent lineage with high support values in Hypocreales (Fig. 1). Although species in this genus are morphologically variable and share few characters, we prefer not to introduce more genera but retain them in one genus, until more materials are available to clarify and stabilise the taxonomy of these taxa.
Neoacremonium distortum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845811. Fig. 10.
Fig. 10.

Neoacremonium distortum (ex-type culture CBS 314.72). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Unbranched conidiophores. F–I. Branched conidiophores with mono- or polyphialides. J. Conidia. Scale bars = 10 μm.
Etymology: Named after the distorted phialides produced by this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, becoming rough- and thick-walled with age, 1.6–3.2 μm wide. Conidiophores solitary or aggregated, erect, arising directly from submerged or superficial hyphae, or from ropes formed by mycelium, mostly with 1–2 irregularly basitonous side branches, bearing 1–3 levels with 1–2 phialides per node, often proliferating sympodially, showing conidiogenous cells as short lateral and cylindrical, asymmetrical projections, or unbranched, 16–57 μm long, 1–2-septate at base and upper part, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate, distorted, hyaline, thick-, smooth-walled, 10.5–35.5 μm long, 1.5–2.5 μm wide at base, with conspicuous periclinal thickening and cylindrical collarette at the conidiogenous loci, commonly with a percurrent subterminal proliferation; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, ellipsoid, broad fusoid, apiculate at both ends, hyaline, thin-, smooth-walled, eguttulate, 2.8–4.7 × 1.5–2.6 μm, arranged in chains, collapse into heads with age. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 35–38 mm diam, dusty, white, with buff pigment, margin entire, reverse olivaceous buff; On MEA reaching 45–47 mm diam, flat, dusty, slightly hairy at centre, mycelium arranged in radially lines, white at centre, colourless at periphery, margin entire, reverse luteous; On PDA reaching 65 mm diam, flat, dusty, white, with creamy white and entire margin, reverse dirty white, with buff radial lines.
Typus: Netherlands, Flevoland Province, Zuidelijk Flevoland, from soil under Phragmites australis (Poaceae), unknown collection date and collector, isol. J.W. Veenbaas-Rijks, No. 711123/4 (holotype CBS H-6647, ex-type culture CBS 314.72 = IPO 1110).
Additional materials examined: Canada, Manitoba, Winnipeg, unknown substrate, collection date and collector, isol. J. Reid, No. UM 185, culture CBS 706.73. Netherlands, Baarn, Cantonspark, from leaf sheath of Musa sp., 11 Oct. 1967, W. Gams, CBS H-8468, culture CBS 291.70A; Flevoland Province, Zuidelijk Flevoland, from soil, unknown collection date and collector, isol. J.W. Veenbaas-Rijks, No. 711013/933, culture CBS 146.72; Flevoland Province, Zuidelijk Flevoland, from soil, unknown collection date and collector, isol. J.W. Veenbaas-Rijks, No. 720111/361, culture CBS 315.72 = IPO 1115; Gelderland Province, Wageningen, from air, unknown collection date and collector, isol. J. van der Spek, culture CBS 291.70B; Utrecht Province, Baarn, aerial contaminant, unknown collection date and collector, isol. 1975 by W. Gams, culture CBS 665.75; Utrecht Province, Maartensdijk, greenhouse, from dead leaf sheath of Musa sapientum (Musaceae), unknown collection date, isol. Jul. 1973, coll. and isol. by W. Gams, culture CBS 710.73.
Notes: Eight cultures labelled Acremonium distortum and A. vitellinum were examined, including the culture CBS 314.72, which was recorded as ex-type culture of A. distortum in the database of the CBS culture collection. However, the name A. distortum had never been published. According to the phylogenetic inference in the present study, all cultures form a fully supported and independent clade that is distant from the ex-type culture of A. vitellinum (CBS 792.69) and other species in Neoacremonium (Fig. 1). Thus, a new species is described here as Ne. distortum. Morphologically, Ne. distortum differs from Ne. flavum in its longer conidiophores with asymmetrical projections (16–57 μm vs up to 36.3 μm), and longer conidia (2.8–4.7 μm vs 2.2–3.3 μm).
Neoacremonium flavum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845947. Fig. 11.
Fig. 11.

Neoacremonium flavum (ex-type culture CBS 398.70). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial chains. F–L. Conidiophores. M. Conidia. Scale bars = 10 μm.
Etymology: From Latin flavus, yellow, due to the yellow colony of this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.5–2.3 μm wide. Sporulation abundant, phalacrogenous, nematogenous. Conidiophores aggregated or solitary, (sub-)erect, mostly curved, irregular bend at base, arising directly from submerged and superficial hyphae, densely arranged, forming sporodochia-like structure in old cultures, mostly with 1–2 irregularly basitonous side branches, or unbranched, up to 36.3 μm long, 1–2(–4)-septate at base and middle, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate or lageniform, hyaline, thick-, smooth-walled, 5.7–24.6 μm long, 1.1–2.5 μm wide at base, with conspicuous periclinal thickening and minute collarette at conidiogenous loci; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, ellipsoid or ovoid, hyaline, thin-, smooth-walled, eguttulate, 2.2–3.3 × 1.9–2.5 μm, arranged in long chains. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA flat, spreading, felty and sulphur yellow at centre, membranous without aerial mycelium and greenish yellow at periphery, margin entire, abundant yellow pigment, reverse sulphur yellow; On MEA reaching 25–29 mm diam, flat, felty, granulose, slightly hairy at centre, white, with dirty white and entire margin, reverse umber; On PDA reaching 38–39 mm diam, flat, felty, slightly short hairy at centre, dirty white, margin entire, reverse pale olivaceous buff at centre, buff at periphery; On SNA reaching 32 mm diam, flat, sparse aerial mycelium, dusty, white, margin entire, reverse white. With strong geosmin odour on all media.
Typus: Netherlands, Utrecht Province, Baarn, Cantonspark, from dead petiole of Chamaerops humilis (Arecaceae), unknown collection date and collector, isol. W. Gams, No. 1269 (holotype CBS H-8470, ex-type culture CBS 398.70).
Additional material examined: Netherlands, Utrecht Province, Baarn, Cantonspark, from stem of Musa sp. (Musaceae), unknown collection date and collector, isol. Oct. 1967 by W. Gams, No. 1257, CBS H-8469, culture CBS 452.70.
Notes: The two cultures representing Neoacremonium flavum were formerly identified as Acremonium vitellinum (currently Ne. vitellinum). However, they fall in a separate branch in Neoacremonium, phylogenetically distant from the branch bearing the ex-type culture of Ne. vitellinum, which also shows low sequence similarity (97 % on ITS, 98 % on LSU, 85 % on rpb2) (Fig. 1). Neoacremonium flavum can be easily distinguished from Ne. vitellinum based on its shorter phialides (5.7–24.6 μm) and conidia arranged in long chains, while Ne. vitellinum has longer phialides (32–80 μm) and conidia arranged in slimy heads (this study). Besides, Ne. flavum has greenish yellow colonies on OA, while those of Ne. vitellinum are dirty white to salmon.
Neoacremonium minutisporum (Sukapure & Thirum.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845948. Fig. 12.
Fig. 12.

Neoacremonium minutisporum (ex-type culture CBS 147.62). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads. E–G. Monophialidic conidiophores. H, I. Adelophialide. J. Polyphialides. K, L. Chlamydospores. M. Conidia. Scale bars = 10 μm.
Basionym: Cephalosporium minutisporum Sukapure & Thirum., Mycologia 55: 566. 1963.
Synonym: Acremonium minutisporum (Sukapure & Thirum.) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 51. 1971.
Mycelium consisting of branched, septate, smooth, hyaline, thin-walled hyphae, up to 2.7 μm wide. Conidiophores solitary, erect, straight, arising from submerged or superficial hyphae, unbranched or branched at lower part, up to ca. 38.6 μm long, 1.2–3 μm wide at base, normally with 1–2-septa at base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, subcylindrical or lageniform, hyaline, thin-, smooth-walled, often borne on short cylindrical subtending cells, 9.3–35.5 μm long, 1–3 μm wide at base, with inconspicuous minute collarette and periclinal thickening at conidiogenous loci; adelophialides present, 10–17 × 1.5–2.5 μm; polyphialides with up to three conidiogenous loci occasionally present. Conidia aseptate, short cylindrical, ovoid, both ends rounded, hyaline, thin-, smooth-walled, 2.5–4.3 × 1.2–1.7 μm, arranged in slimy heads. Chlamydospores laterally on short stalks, single, globose to sub-globose, hyaline, smooth-, thick-walled, 2.5–3.7 × 2.5–3.4. Sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 65–70 mm diam, flat, membranous with sparse aerial mycelium, dirty white, margin entire, reverse concolourous; On MEA reaching 75–80 mm diam, flat, radially folded, hairy, dirty white, margin fimbriate, reverse ochreous, with radial lines; On PDA reaching 73–80 mm diam, flat, membranous with sparse aerial mycelium, slightly hairy at centre, dirty white, margin fimbriate, reverse concolourous, with buff radial lines; On SNA reaching 75–80 mm diam, flat, membranous without aerial mycelium, colourless, margin fimbriate, reverse colourless. With geosmin odour on all media.
Typus: India, Bombay, Sewri, from salt-marsh soil, 11 Jul. 1958, M.J. Thirumalachar, CBS H-6794 (holotype of Cephalosporium minutisporum HACC 108, ex-type cultures CBS 147.62 = ATCC 14612 = IMI 091576).
Notes: Cephalosporium minutisporum was isolated from a soil sample collected in a salt marsh at the sea coast, an area which turns to clay in the summer, with few fungi surviving in this habitat (Sukapure & Thirumalachar 1963). This species was later transferred to the genus Acremonium as one of the species in Acremonium section Simplex (Gams 1971). According to the phylogenetic analysis, the ex-type of C. minutisporum (CBS 147.62) clusters with the new genus Neoacremonium (Fig. 1), and it is therefore recombined as Ne. minutisporum. This species is morphologically characterised by the production of single chlamydospores laterally borne on short stalks, a feature that distinguishes it from other species in this genus. Morphologically, characters of the ex-type culture are similar to the description available in the literature (Gams 1971).
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hit using the LSU sequence of N. minutisporum is a sequence from the ex-isotype culture of Sedecimiella taiwanensis [currently Ne. taiwanense, CY5100; GenBank HM451496.1; Identity = 775/777 (99.74 %), no gaps]. However, only LSU sequences of the culture CY5100 were available for comparison, and there is only 2 bp difference between the LSU sequences of the ex-type cultures of Ne. minutisporum and Ne. taiwanense. Morphological comparison is difficult as Ne. taiwanense was only described based on a sexual morph (Pang et al. 2010), while Ne. minutisporum was only observed as an asexual morph in culture. Therefore, whether they are conspecific still awaits to be confirmed with more molecular data and morphological comparisons. Furthermore, if the two species proved to be conspecific, the Ne. minutisporum should be adopted as this species was published earlier than S. taiwanensis.
Neoacremonium taiwanense (K.L. Pang et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 848117.
Basionym: Sedecimiella taiwanensis K.L. Pang et al., Bot. Mar. 53: 495. 2010.
Description and illustration: Pang et al. (2010).
Typus: China, Taiwan, Chunan, on a twig of Kandelia obovata (Rhizophoraceae), 12 Aug. 2008, K.L. Pang (holotype BBH26390, BIOTEC Bangkok Herbarium, dried wood).
Additional material examined: China, Shenzhen, Futian Nature Reserve, on a twig of unidentified mangrove wood, 14 Mar. 2006, K.L. Pang, ex-isotype culture CY5100-CY5101.
Notes: This species was described based on a sexual morph that is characterised by producing 16 globose ascospores in asci; its asexual morph is unknown (Pang et al. 2010). Only LSU (HM451496) and SSU (HM451495) sequences are available, and the phylogenetic tree based on limited taxa in the original article shows this species is close to Niesslia exilis (CBS 357.70), on which basis it was placed in Niessliaceae (Pang et al. 2010). However, its LSU sequence is 99 % similar to CBS 147.62 (ex-type culture of Cephalosporium minutisporum, currently Neoacremonium minutisporum) based on the blastn search. Phylogenetically, Ne. taiwanense has a close phylogenetic affinity to Ne. minutisporum and distant from Niesslia exilis (Fig. 1). The habitat of the two species shares some similarities: Ne. taiwanense was described from a twig of a mangrove plant (Kandelia obovata) that grows in coastal areas, while Ne. minutisporum was from a soil sample collected in salt marsh areas on the sea coast (Sukapure & Thirumalachar 1963). Therefore, Ne. taiwanense is possibly the first species of Neoacremoniaceae with a known sexual morph. However, considering the close phylogenetic affinity, whether Ne. minutisporum and Ne. taiwanense are conspecific awaits to be clarified with more sequences or cultures.
Neoacremonium vitellinum (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845949. Fig. 13.
Fig. 13.

Neoacremonium vitellinum (ex-type culture CBS 792.69). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial heads. F. Unbranched conidiophore. G, H. Conidiophores with sterile basal outgrowths. I–K. Branched conidiophores. L. Conidia. Scale bars = 10 μm.
Basionym: Acremonium vitellinum W. Gams, Cephalosporium-artige Schimmelpilze: (Stuttgart): 65. 1971.
Mycelium consisting of branched, septate, smooth, hyaline, thin-walled hyphae, up to 2 μm wide. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, arising from submerged or superficial hyphae, straight or slightly curved, unbranched or branched, often with short sterile basal outgrowths, up to 82 μm long, 1–3 μm wide at base, normally with 1–2 septa at base, sometimes up to 4 septa, inflated at base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal, lateral, cylindrical, hyaline, thick-, smooth-walled, often borne on short cylindrical subtending cells, 32–80 μm long, 1–2.2 μm wide at base, with cylindrical collarette and inconspicuous periclinal thickening at conidiogenous loci. Conidia aseptate, broad ellipsoid, ovoid, spindle-shaped, often with an apiculate basal end and obtuse apices,, hyaline, thin-, smooth-walled, 2.8–4.9 × 1.6–2.5 μm, arranged in slimy heads. Crystals are usually abundantly formed. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 18 mm diam, flat, dusty, salmon at centre, white at periphery, margin entire, reverse pale saffron at centre, dirty white at periphery; On MEA reaching 22 mm diam, flat, abundant aerial mycelium, hairy, dirty white, buff at centre, margin entire, reverse orange at centre, saffron at periphery; On PDA reaching 22 mm diam, flat, abundant aerial mycelium, hairy, white, margin fimbriate, reverse pale orange at centre, dirty white at periphery; On SNA reaching 16 mm diam, flat, membranous without aerial mycelium, white, margin fimbriate, reverse white. Lacking odour on all media.
Typus: Netherlands, Utrecht Province, Baarn, Cantonspark, greenhouse, from dead leaf of Phoenix dactylifera (Arecaceae), unknown collection date and collector, isol. Oct. 1967 by W. Gams, No. 1273, CBS H-6673 (holotype CBS 792.69 preserved as metabolically inactive culture, ex-type culture CBS 792.69).
Additional material examined: Netherlands, Utrecht Province, Baarn, Cantonspark, greenhouse, from old petiole of Phoenix dactylifera (Arecaceae), unknown collection date and collector, isol. Oct. 1967 by W. Gams, No. 1274, CBS H-8472, culture CBS 793.69.
Notes: Gams (1971) reported conidia of Acremonium vitellinum to be arranged in chains, and tends to form sporodochium based on culture CBS 452.70, which proved to be a different species (Fig. 1). However, in the present study, the ex-type culture of A. vitellinum (CBS 792.69) was observed to produce longer phialides [32–80 μm vs 15–25(–30) μm] and conidia arranged in slimy heads. In addition, adelophialides were not observed as described in Gams (1971).
Parapyrenis maritima Aptroot, Nova Hedwigia 60: 354. 1995.
Description and illustration: Aptroot (1995).
Typus: Papua New Guinea, Madang Province, Laing Island in Hansa Bay near Bogia, alt. 1 m, from wood in coastal forest on coral island, 20 Jul. 1992, A. Aptroot (holotype CBS H-5923, ex-holotype culture CBS 538.93 = Aptroot No. 30166).
Additional material examined: Japan, Wakayama, from seashore sand, unknown collection date, K. Tubaki (No. V-I), CBS H-8246, culture CBS 795.69.
Notes: Parapyrenis maritima is a member of Parapyrenis originally placed in Requienellaceae, Xylariales. It is characterised by the production of globose ascomata that are immersed in a stroma, ellipsoid, medium brown ascospores, 1-septate, without spinulose ornamentation, and with a thick endosporium leaving a cordate to sexangular lumen. The asexual morph of this species is unknown (Aptroot 1995). The ex-type culture CBS 538.93, examined in the present study, clustered within the Neoacremonium clade, with an acremonium-like species, Ne. vitellinum. Morphologically, culture CBS 538.93 was only observed as an acremonium-like asexual morph, and therefore cannot be compared with the protologue. However, it is unknown whether Pa. maritima truly had the acremonium-like asexual morph or the culture was swapped or contaminated by an acremonium-like species at some point before or after it was deposited. DNA sequences derived from the holotype material of Pa. maritima (CBS H-5923; if possible) would help resolve this problem.
Clade L
Chrysonectriaceae L.W. Hou, L. Cai & Crous, fam. nov. MycoBank MB 845950.
Classification: Hypocreales, Sordariomycetes.
Sexual morph: ascomata superficial, non-stromatic, sub-globose, not collapsing upon drying, pale orange, changing colour in 3 % KOH and lactic acid, overlain by golden yellow hyphal elements; asci 8-spored, cylindrical to fusoid, with a refractive apical apparatus; ascospores subfusoid to narrowly clavate, two-celled, smooth. Asexual morph: mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores solitary or aggregated, (sub-)erect, mostly curved, irregularly wavy, arising directly from submerged or superficial hyphae, verticillately branched, bearing 1–3 whorls of 1–3 phialides per node, rarely unbranched and reduced to single phialides, septate at base and middle, hyaline, thick- and smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, cylindrical or subulate, straight or curved at base, hyaline, thick-, smooth-walled, with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, subglobose, broadly ellipsoid, straight, hyaline, thick-, smooth-walled, eguttulate, arranged in slimy heads. Abundant yellow crystals present. Chlamydospores not observed.
Type genus: Chrysonectria Lechat & J. Fourn.
Notes: According to the phylogenetic analysis based on the combined ITS-LSU sequences and ITS, LSU and rpb2 sequences, species of Chrysonectria form an independent branch that is different from all known families (Fig. 1, Supplementary Fig. S1). Morphological and phylogenetical divergences of Chrysonectria confirm the distinctiveness within the known nectriaceous families of Hypocreales.
Chrysonectria Lechat & J. Fourn., Ascomycete.org 10: 122. 2018.
Sexual morph: ascomata superficial, non-stromatic, sub-globose, not collapsing upon drying, pale orange, changing colour in 3 % KOH and lactic acid, overlain by golden yellow hyphal elements; asci 8-spored, cylindrical to fusoid, with a refractive apical apparatus; ascospores subfusoid to narrowly clavate, two-celled, smooth. Asexual morph: mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores solitary or aggregated, (sub-)erect, mostly curved, irregularly wavy, arising directly from submerged or superficial hyphae, unbranched and reduced to single phialides, or verticillately branched, bearing 1–3 whorls with 1–3 phialides per node, 1–4-septate at base and middle, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, cylindrical or subulate, straight or curved at base, hyaline, thick-, smooth-walled, with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, subglobose, broadly ellipsoid, straight, hyaline, thick-, smooth-walled, eguttulate, arranged in slimy heads. Abundant yellow crystals present. Chlamydospores not observed (adapted from Lechat et al. 2018a).
Type: Chrysonectria finisterrensis Lechat, J. Fourn. & Priou
Other accepted species with available sequences: Chrysonectria crystallifera L.W. Hou, L. Cai & Crous
Notes: Chrysonectria was described to accommodate C. finisterrensis (Lechat et al. 2018a). When it was originally introduced, the phylogenetic analysis based on the single LSU sequences and limited genera placed this genus in Nectriaceae, which is characterised by having soft-textured and brightly coloured ascomata which change colour in 3 % KOH or lactic acid (Lechat et al. 2018a). However, the morphology of the type is unique and different from Nectriaceae and its closest phylogenetic neighbours, Neoacremoniaceae and Pseudoniessliaceae, by having pale orange ascomata diffusing abundant, orange-yellow pigment in 3 % KOH, or bright yellow in lactic acid, overlain by golden-yellow hyphal elements.
Chrysonectria crystallifera L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845951. Fig. 14.
Fig. 14.

Chrysonectria crystallifera (ex-type culture CBS 102567). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–H. Branched conidiophores. I. Crystals. J. Conidia. Scale bars = 10 μm.
Etymology: Named after the crystals produced by this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–2 μm wide. Conidiophores solitary or aggregated, (sub-)erect, mostly curved, irregularly wavy at base, arising directly from submerged or superficial hyphae, mostly repeatedly verticillately branched, bearing up to 1–3 whorls with 1–3 phialides per node, or unbranched and reduced to single phialides, 37–124.5 μm long, 1.2–2.9 μm wide at base, 1–4-septate at base and middle, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, cylindrical or subulate, straight or curved at base, hyaline, thick-, smooth-walled, 10–36 μm long, 1.2–2.4 μm wide at base, with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, subglobose, broadly ellipsoidal, straight, hyaline, thick-, smooth-walled, eguttulate, 2.7–4.5 × 1.9–2.7 μm, arranged in slimy heads. Abundant yellow crystals present. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 7 mm diam, flat, felty, pale luteous to orange, with orange pigment around the margin, margin crenate, reverse pale rust; On MEA reaching 6 mm diam, raised, felty, flesh, later become pale scarlet, margin crenate, reverse sienna, with caramel odour; On PDA reaching 6 mm diam, raised, felty, luteous, later become orange, margin dentate, reverse pale rust, with buff margin; On SNA reaching 4 mm diam, flat, with sparse aerial mycelium, dusty, luteous, margin dentate, reverse concolourous. Lacking odour on PDA, OA and SNA.
Typus: Japan, Ohgato, Botanical Garden, from bark of Monstera sp. (Araceae), Mar. 1996, T. Okuda (holotype CBS H-24707, ex-type culture CBS 102567 = BS 4561 = F 7404).
Notes: The distinctive morphological features of Chrysonectria crystallifera, especially the repeatedly verticillately branched conidiophore and abundantly produced brown crystals, plus the phylogenetic distance from the other genera and families (Fig. 1, Supplementary Fig. S1), led to the recognition of the new species.
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hits using the ITS sequence had highest similarity to ex-type culture of C. finisterrensis [culture JPP17021, GenBank MF611678.1; Identities = 453/492 (92 %), 9 gaps (1 %)]; Closest hits using the LSU sequence is ex-type culture of C. finisterrensis [culture JPP17021, GenBank MF611689.1; Identities = 770/777 (99 %), no gaps]; Closest hits using the rpb2 sequence is Xenogliocladiopsis eucalyptorum [culture CPC 16271, GenBank KM232331.1; Identities = 593/743 (80 %), 10 gaps (1 %)].
Clade M
Pseudoniessliaceae L.W. Hou, L. Cai & Crous, fam. nov. MycoBank MB 845952.
Classification: Hypocreales, Sordariomycetes.
Mycelium consisting of hyaline, thin-walled hyphae. Crystals absent. Sporulation abundant, phalacrogenous sporodochia can be present, with conidia adhering in slimy droplets, often coalescent. Phialides often aggregated in whorls on long stalk cells, slightly thick-walled throughout, aculeate. Conidia aseptate, subglobose, with hardly differentiated base, smooth-, thin-walled. Chlamydospores absent.
Type genus: Pseudoniesslia L.W. Hou, L. Cai & Crous
Notes: Based on morphological characteristics, phylogenetic analysis and comparison with known genera and families in the Hypocreales, Pseudoniessliaceae is proposed as a new family with the type genus Pseudoniesslia and type species Ps. minutispora.
Pseudoniesslia L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845953.
Etymology: Referring to the genus Niesslia, to which it is morphologically similar.
Mycelium consisting of hyaline, thin-walled hyphae. Crystals absent. Sporulation abundant, phalacrogenous, sporodochia can be present, with conidia adhering in slimy droplets, often coalescent. Phialides often aggregated in whorls on long stalk cells, slightly thick-walled throughout, aculeate. Conidia aseptate, subglobose, with hardly differentiated base, smooth-, thin-walled. Chlamydospores absent.
Type: Pseudoniesslia minutispora (W. Gams et al.) L.W. Hou, L. Cai & Crous
Notes: The monotypic genus Pseudoniesslia is introduced to accommodate a species that was originally described as Niesslia minutispora (Gams et al. 2019). This species is represented by five strains in the phylogenetic analysis, forming a separate clade different from all known genera and families (Fig. 1). Morphologically it is characterised by the sporodochial conidiomata and conidiophores with a terminal whorl of relatively long phialides.
Pseudoniesslia minutispora (W. Gams et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845954. Fig. 15.
Fig. 15.

Pseudoniesslia minutispora (ex-type culture CBS 246.82). A–C. Colonies on OA, PDA and SNA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial heads. F–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Basionym: Niesslia minutispora W. Gams et al., Mycol. Progr. 18: 70. 2019.
Description and illustration: Gams et al. (2019).
Typus: Netherlands, Flevoland Province, Noordoostpolder, Nagele, from agricultural soil, unknown collection date, isol. Feb. 1982, coll. and isol. H. Nielander, No. 377, CBS H-24638 (holotype CBS H-1649, ex-type culture CBS 246.82).
Materials examined: Belgium, Heinsch, near Arlon, Road to Neufchâteau, on bark of Salix cinerea (Salicaceae), together with Claussenomyces sp. (Tympanidaceae), 8 Apr. 1989, G. Marson, No. 3914, isol. W. Gams, No. 3914, culture CBS 267.89. Germany, Schleswig-Holstein, Kiel-Kitzeberg, from dead twig of Fraxinus excelsior (Oleaceae), unknown collection date and collector, isol. 28 Jul. 1965 by G.L. Hennebert, CBS H-24709, culture CBS 735.69 = MUCL 21824 = MUCL 6976. Netherlands, Utrecht Province, Utrecht, Oud-Amelisweerd, from decaying wood of Fagus sylvatica, unknown collection date and collector, isol. 1968 by W. Gams, No. 1410, CBS H-8340, culture CBS 148.70; North Holland Province, Vogelenzang, Amsterdamse Waterleidingduinen, from Auricularia auricula-judae (=Hirneola auricula-judae; Auriculariaceae), unknown collection date and collector, isol. Nov. 1968 by A.C.M. Hoozemans, CBS H-24708, culture CBS 392.70.
Notes: This species was originally introduced as Niesslia minutispora (Gams et al. 2019). However, the sporodochial conidiomata and conidiophores with terminal whorls of relatively long phialides are unusual for Niesslia. In the present study, the ex-type culture of N. minutispora together with other four strains from diverse plant formed an independent branch, representing a new genus. In addition, a strain that was identified as “Hydropisphaera suffulta” (CBS 122798) was placed in a sister branch to Pseudoniesslia minutispora (Fig. 1). Considering the relatively low support, it possibly represents a novel genus. The phylogenetic position and taxonomy status of this species remains unresolved pending more cultures and DNA sequence data.
Clade O
Bionectriaceae Samuels & Rossman, Stud. Mycol. 42: 15. 1999.
Classification: Hypocreales, Sordariomycetes.
Type genus: Bionectria Speg.
Notes: Bionectriaceae was originally introduced for 26 genera having white, pale tan orange or brown, uniloculate, perithecial, rarely cleistothecial ascomata, generally not changing colour in KOH (Rossman et al. 1999, 2001). Diverse asexual morphs were discovered in this family, including acremonium-like, clonostachys-like, verticillium-like and sesquicillium-like (Rossman et al. 1999, Summerbell et al. 2011). The obtained phylogenetic results in this study confirmed the conclusion of Rossman et al. (1999) that Bionectriaceae was classified in Hypocreales. Species of Bionectriaceae clustered in a moderate supported clade in Hypocreales (Fig. 1). Thirty-nine well-supported monophyletic genera were recognised in the separate phylogenetic tree of Bionectriaceae, among which, ten were introduced as novel genera (Fig. 2).
Clade O1
Gliomastix Guég., Bull. Soc. Mycol. France 21: 240. 1905.
Synonyms: Torulina Sacc. & D. Sacc., Syll. Fung. (Abellini) 18: 566. 1906.
Basitorula G. Arnaud, Bull. Trimestriel Soc. Mycol. France. 69: 276. 1954 (1953) (nom. inval.).
Haplotrichella G. Arnaud, Bull. Trimestriel Soc. Mycol. France. 69: 282. 1954 (1953) (nom. inval.).
Nematomyces Faurel & Schotter, Rev. Mycol. (Paris). 30: 344. 1966 (1965).
Colonies on natural substrata green, brown or black, usually with sparse floccose aerial mycelium and abundant sporulation. In culture many species form mycelial ropes which are usually white contrasting sharply with the dark-pigmented spore masses. Phialides are simple, hyaline or dark, with a single apical pore which may or may not bear a collarette. They are formed directly on the vegetative hyphae or on hyphal branches which may be slightly modified in being wider and/or darker than normal. Phialide proliferation occurs rarely in some species. Phialospores are formed in basipetal succession, dry and forming chains or slimy and aggregating in balls at the phialide apex, non-septate, globose, ovoid or ellipsoid, green, brown or black but not hyaline, with their pigment being especially noticeable when viewed in mass (Dickinson 1968).
Type: Gliomastix murorum (Corda) S. Hughes, Canad. J. Bot. 36: 769. 1958.
Other accepted species with available sequences: Gliomastix masseei (Sacc.) Matsush., G. musae L.W. Hou, L. Cai & Crous, G. polychroma (J.F.H. Beyma) Matsush., G. roseogrisea (S.B. Saksena) Summerb., G. tumulicola (Kiyuna, K.D. An, R. Kigawa & Sugiy.) Summerb.
Notes: Gliomastix is typified by G. murorum (syn. G. chartarum), and is distinguished by “chondroid hyphae”, darkly pigmented ameroconidia arranged in slimy heads or dry chains, and the absence of chlamydospores (Guéguen 1905, Hughes 1958, Gams 1971). The genus Gliomastix was once considered a section of Acremonium introduced by Gams (1971) (as Acremonium sect. Gliomastix). However, it was recently resurrected based on five species, namely G. masseei, G. murorum, G. polychroma, G. roseogrisea and G. tumulicola (Summerbell et al. 2011). Sexual morphs had been linked to Gliomastix. Despite a previous study demonstrating the connection of G. fusigera with a sexual morph in Hydropisphaera (Lechat et al. 2010), G. fusigera (currently Monohydropisphaera fusigera) is phylogenetically distant from the Gliomastix s. str. in the present study (Fig. 2) and is therefore excluded from Gliomastix. However, an Hydropisphaera species, H. spinulosa (Zeng & Zhuang 2016), originally described based on the sexual morph clustered within the Gliomastix clade, closely related to G. masseei (Fig. S2). This is consistent with the prediction of Gams (1978) that Gliomastix could be connected to a sphaeriaceous sexual morph. Species in Gliomastix are commonly distributed in soil, and may also be associated with plants or isolated as airborne fungi.
Gliomastix musae L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845812. Fig. 16.
Fig. 16.

Gliomastix musae (ex-type culture CBS 617.94). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–I. Monophialides. J, K. Collarettes of phialides. L. Conidia. Scale bars = 10 μm.
Etymology: Referring to Musa, the host genus from which the ex-type culture of this fungus was isolated.
Mycelium consisting of septate, hyaline, smooth-, thin-walled hyphae, up to 2.5 μm wide when young, becoming thick-walled, up to 3.5 μm wide in old cultures. Conidiophores erect, straight or slightly flexuose at base, arising directly from submerged or superficial hyphae, mostly unbranched, poorly branched, up to 60 μm long, 2.3–3.7 μm wide at base, 1–2-septate at base, hyaline, smooth-walled. Phialides lateral, terminal, subulate, 28–49.5 μm long, 1.5–3 μm wide at base, hyaline at first, brown in old cultures, thick-, smooth-walled, with conspicuous periclinal thickening and dark brown cylindrical to flared collarettes; polyphialides not observed. Conidia aseptate, ovoid to ellipsoid, hyaline, thin-walled at beginning, brown to olivaceous brown, thick-, smooth-walled in old cultures, 4.7–6.7 × 2.5–3 μm, eguttulate, arranged in long dry chains. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 45–50 mm diam, flat, dusty or granulose at centre, thinly felty at periphery, olivaceous, dirty white at periphery, margin entire, reverse buff. Colonies on MEA reaching 35–45 mm diam, raised, irregularly rugose, aerial mycelium abundant, floccose and granulose, salmon, dirty white at periphery, margin undulate, reverse sienna, luteous at periphery. Colonies on PDA reaching 65–66 mm diam, flat, felty to granulose, olivaceous, with concentric rings, dirty white at centre and at periphery, margin entire, reverse olivaceous black at centre, dirty white with concentric rings at periphery. Colonies on SNA reaching 45–50 mm diam, flat, sparse aerial mycelium, dusty with concentric rings, dirty white, margin entire, reverse white.
Typus: Colombia, from fruit of Musa sp. (Musaceae), unknown collection date, L. Verbruggen (holotype CBS H-24692, ex-type culture CBS 617.94 = No. 94019).
Additional materials examined: Colombia, from Musa sapientum (Musaceae), unknown collection date and collector, isol. 1994 by L. Verbruggen, culture CBS 678.94. Cuba, air, unknown collection date, isol. 4 Feb. 1993, coll. and isol. R.F. Castañeda, culture CBS 561.93 = INIFAT C93/17. Suriname, from air, unknown collection date, J.H. van Emden, CBS H-8318, culture CBS 296.70A. USA, Florida, Camp Indian Bay, from cot straps, unknown collection date and collector, CBS H-8321, culture CBS 296.70E = QM 710.
Notes: In this study, 17 cultures labelled as Gliomastix polychroma were examined. They are phylogenetically heterogeneous and distributed in different clades on the tree (Fig. 2). Five of them formed a fully supported clade sister to the G. polychroma clade that is represented by 12 cultures, including the ex-type culture CBS 181.27. Strains in this clade are both morphologically and phylogenetically different from G. polychroma. Morphologically, they differ from G. polychroma in their faster growth rate (3.1–3.5 mm/d vs 2–2.8 mm/d), longer phialides (28–49.5 μm vs 20–30 μm) and conidia (4.7–6.7 μm vs 3.8–4.9 μm), presence of collarettes on phialides and the absence of granular conidia (Gams 1971). Therefore, a new species is introduced here as G. musae.
Gliomastix musicola (syn. Coniosporium musicola) was originally isolated from Musa sapientum, the same host genus as for G. musae in Argentina (Spegazzini 1911), and was then placed in Acremonium subg. Gliomastix as A. musicola. However, G. musae clearly differs from G. musicola in morphology: phialides of G. musae are smooth-walled and longer (28–49.5 μm), while those of G. musicola are very warty and shorter (15–18 μm) (Gams 1971).
Clade O2
Paracylindrocarpon Crous et al., Persoonia 36: 367. 2016.
Mycelium consisting of hyaline, smooth, branched, septate hyphae, forming hyphal coils. Ascomata scattered to gregarious, globose, subglobose or tympaniform, superficial, flat at the base, conspicuous on the host surface, easy to remove, orange, pale yellow, dull, collapsing cupulate or not when dry, solitary, uniloculate, with or without papilla, with central ostiole or lacking ostiole. Peridium composed of several layers of thick-walled, hyaline to pale brown, pale orange cells of textura angularis. Hamathecium comprising ellipsoid, cylindrical, filamentous, unbranched, guttulate, septate paraphyses. Asci (6–)8-spored, unitunicate, cylindrical, clavate or cylindrical-clavate, with short pedicel, with J-apical ring. Ascospores fusiform, conical towards both ends, straight or slightly curved, 3–6(–10)-septate, occasionally up to 15–20 septate in some species, not constricted at septa, or slightly constricted at the median septum, hyaline to subhyaline, guttulate without mucilaginous sheath, smooth-walled or verrucose. Conidiophores solitary, hyaline, smooth, erect, straight to geniculate-sinuous, arising from superficial hyphae, unbranched or branched, septate. Conidiogenous cells phialidic, hyaline, smooth, cylindrical, subcylindrical with slight apical taper, unflared at tip, straight to slightly irregularly curved, terminal or lateral on conidiophores, apex with minute periclinal thickening. Conidia hyaline, smooth, granular, cylindrical, filiform, apex obtuse, base truncate, occasionally with a protuberant, basal, abscission scar, (0–)1–3(–7)-septate, arranged in conidial heads. (emended from Crous et al. 2016b, Tibpromma et al. 2018).
Type: Paracylindrocarpon aloicola Crous et al.
Other accepted species with available sequences: Paracylindrocarpon aurantiacum L.W. Hou, L. Cai & Crous, Pn. foliicola Lechat & J. Fourn., Pn. multiloculatum (Samuels) L.W. Hou, L. Cai & Crous, Pn. multiseptatum (Samuels) L.W. Hou, L. Cai & Crous, Pn. nabanheensis Tibpromma & K.D. Hyde, Pn. pandanicola Tibpromma & K.D. Hyde, Pn. xishuangbannaensis Tibpromma & K.D. Hyde
Notes: Paracylindrocarpon was introduced by Crous et al. (2016b) based on Pn. aloicola isolated from leaves and twigs of Aloe sp. in South Africa. Paracylindrocarpon is morphologically similar to other cylindrocarpon-like species, having hyaline, granular, cylindrical septate conidia each with an obtuse apex and truncate base (Crous et al. 2016b). Phylogenetic analyses showed that Paracylindrocarpon forms a fully supported clade distantly related to Hydropisphaera (Crous et al. 2016b). At present, four species of Paracylindrocarpon are listed in Index Fungorum. Three of them were only observed as a sexual morph (Pn. aloicola, Pn. nabanheensis, Pn. xishuangbannaensis), while Pn. pandanicola was only observed as an asexual morph (Crous et al. 2016b, Tibpromma et al. 2018). Previous studies illustrate that some Hydropisphaera species were suggested to be better accommodated in Paracylindrocarpon (Crous et al. 2016b). In the present study, two Hydropisphaera species, H. multiseptata and H. multioculata are transferred to Paracylindrocarpon based on the phylogenetic analysis (Fig. 2) and morphological features. Morphologically, these species originally placed in Hydropisphaera produce cylindrocarpon-like conidia and ascospores with three or more septa, while Hydropisphaera s. str. typified by H. peziza possesses an acremonium-like asexual morph and 1-septate ascospores.
Paracylindrocarpon aloicola Crous et al., Persoonia 36: 367. 2016.
Description and illustration: Crous et al. (2016b).
Typus: South Africa, Western Cape Province, Robben Island, on leaves and twigs of Aloe sp. (Xanthorrhoeaceae), May 2015, P.W. Crous & F. Roets (holotype CBS H-22613, ex-type culture CBS 141300 = CPC 27362).
Additional material examined: New Zealand, Auckland, Waiwera, Wenderholm Regional Park, from Rhopalostylis sapida (Arecaceae), 26 Sep. 1973, J.M. Dingley, G.J. Samuels, S. Haydon & J.D. Fletcher, G.J.S. 73-205, PDD 32658, culture CBS 135907 = ATCC 36091; Auckland Province, Waitemata County, Waitakere Ranges, vic. Piha, Kitekite Track, from leaf of Cordyline banksia (Asparagaceae), 17 Dec. 1974, J.M. Dingley, G.J. Samuels & S. Francis, G.J.S. 74-137, PDD 34939, culture CBS 335.77
Notes: When Paracylindrocarpon aloicola was originally described, both single ITS and LSU sequences placed it in an independent lineage in Bionectriaceae (Crous et al. 2016b). This species was noted for the production of a cylindrocarpon-like asexual morph (Crous et al. 2016b). In our present study, the ex-type strain (CBS 141300) of Pn. aloicola grouped with CBS 335.77 that was received as Hydropisphaera erubescens (Rossman et al. 1999; Fig. 2), which was reidentified as Pn. aloicola.
Paracylindrocarpon aurantiacum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845813. Fig. 17.
Fig. 17.

Paracylindrocarpon aurantiacum (ex-type culture CBS 135909). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Ascomata on pine needle. E. Ascoma. F–H. Asci. I. Ascospores. Scale bars: E = 100 μm; F–I = 10 μm.
Etymology: From Latin, aurantiaca, meaning orange. Referring to the production of orange ascomata.
Perithecia superficial, solitary, scattered, globose to sub-globose, hyaline or pale yellow at first, becoming orange, dull, smooth, uniloculate, lacking ostiole, with cylindrical, septate, unbranched hyphae with rounded tips, with depressed and rounded ornamentations, 210–305 × 210–290 μm. Perithecial wall 15–40 um wide, composed of several layers of thick-walled, hyaline to pale brown cells of textura angularis. Paraphyses not observed. Asci 8-spored, unitunicate, apex simple, cylindrical-clavate, (53–)59–81(–83) × 8–11.5 μm. Ascospores biseriate, hyaline, smooth-walled, fusoid, curved, conical at both ends, inconspicuous 3-septate, not constricted at septa, guttulate, without mucilaginous sheath, 19–26.5 × 3.5–5 um. Asexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 35–37 mm diam, flat, dusty, dirty white, with concentric rings, margin fimbriate, reverse concolourous; On MEA reaching 26–28 mm diam, raised, floccose, dirty white, with pale apricot zones, with concentric rings, margin fimbriate, reverse umber with orange margin; On PDA reaching 45–46 mm diam, flat, felty, pale fawn at centre, dirty white at periphery, with concentric rings, margin fimbriate, reverse rust at centre, buff at periphery; On SNA reaching 14–15 mm diam, flat, membranous without aerial mycelium, dirty white, margin filiform, reverse concolourous. Strong geosmin odour in all media.
Typus: France, Messigny et Vantoux (21) Marais de Jouvence, from Lamium galeobdolon (Lamiaceae), unknown collection date, A. Gardiennet, isol. C. Lechat (holotype CBS H-24630, ex-type culture CBS 135909 = AG13111).
Notes: The only strain representing Paracylindrocarpon aurantiacum was formerly identified as Hydropisphaera erubescens in the CBS culture collection. However, it clustered within the Paracylindrocarpon clade and is phylogenetically closely related to Pn. foliicola (Fig. 2). Morphologically, Pn. aurantiacum differs from H. erubescens in producing pale yellow to orange and non-ostiolate ascomata, and differs from Pn. foliicola in its larger asci [(53–)59–81(–83) × 8–11.5 μm vs 50–60 × 8–10 μm], and larger ascospores with 3 septa (19–26.5 × 3.5–5 μm, 3-septate vs (14–)15–17 (–18) × 4–4.7(–5) μm 1–3-septate) (Rossman et al. 1999, Lechat & Fournier 2017).
Paracylindrocarpon foliicola Lechat & J. Fourn., sp. nov. MycoBank MB 845815.
Basionym: Hydropisphaera foliicola Lechat & J. Fourn., Ascomycete.org 9: 6. 2017. (nom. inval., Art. F.5.1 (Shenzhen); the identifier cited in the protologue, MB 815589, was not issued for that name).
Etymology: “foliicola” referring to its habitat on a dead leaf.
Description and illustration: Lechat & Fournier (2017).
Typus: France, Martinique, Fort-de-France, forêt de Colson, Plateau-Perdrix, on dead leaf of Pouteria pallida (Sapotaceae), 20 Aug. 2015, C. Lechat (holotype CLLM15128 in LIP, ex-type culture CBS 140758).
Notes: Hydropisphaera foliicola was described based on the culture from Pouteria pallida (Sapotaceae) of France (Lechat & Fournier 2017). However, when this species was published, the identifier cited in the protologue was not issued for that name. Thus, the epithet is invalid based on the International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code). Here, this species is validated by describing it as a new species of Paracylindrocarpon. Phylogenetically, the ex-type strain CBS 140758 clustered within Paracylindrocarpon and is closely related to Pn. aurantiacum (Fig. 2). Despite the similarity with Hydropisphaera species in the production of perithecia that are collapsing and cupulate when dry, its 1–3-septate ascospores match better with the generic characters of Paracylindrocarpon, while species in Hydropisphaera s. str. produce 1-septate ascospores.
Paracylindrocarpon multiloculatum (Samuels) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845816.
Basionym: Nectria multiloculata Samuels, New Zealand J. Bot. 16: 78. 1978.
Synonym: Hydropisphaera multiloculata (Samuels) Rossman & Samuels, Stud. Mycol. 42: 31. 1999.
Description and illustration: Samuels (1978).
Typus: New Zealand, Auckland, Waitemata County, Waitakere Ranges, Lucy Cranwell Track, vic. Kitakiki Stream, on dead leaf of Astelia sp. (Asteliaceae), 30 May 1973, J.M. Dingley, G.J. Samuels & S. Haydon, G.J.S. 73-98 (holotype PDD 31786, ex-type culture CBS 339.77).
Additional materials examined: New Zealand, Little Barrier Island, track to summit of Mt. Hauturu, from dead leaf of Astelia sp. (Asteliaceae), 22 Feb. 1976, G.J. Samuels, G.J.S. 76-8, PDD 34940, culture CBS 340.77.
Notes: Paracylindrocarpon multiloculatum was originally described as Nectria multiloculata by Samuels (1978) from a dead leaf of Astelia sp. in New Zealand. It was subsequently transferred to Hydropisphaera (Rossman 1999). The original materials examined by Samuels (1978), i.e., CBS 339.77 and CBS 340.77 are included in the present study. According to our phylogenetic inference, the ex-type of N. multiloculata CBS 339.77 falls in a fully supported clade representing the genus Paracylindrocarpon.
Paracylindrocarpon multiseptatum (Samuels) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845817.
Basionym: Nectria multiseptata Samuels, New Zealand J. Bot. 16: 77. 1978.
Synonym: Hydropisphaera multiseptata (Samuels) Rossman & Samuels, Stud. Mycol. 42: 31. 1999.
Description and illustration: Samuels (1978).
Typus: New Zealand, Auckland, Waitakere Range, vic. Piha, Marowhara Loop Track, from leaf of Phormium tenax (Asphodelaceae), 17 Dec. 1974, J.M. Dingley, G.J. Samuels & S. Francis, G.J.S. 74-136, PDD 34937, culture CBS 337.77.
Additional materials examined: New Zealand, Auckland, Waitakere Range, vic. Piha, Marowhara Loop Track, from leaf of Phormium tenax, 19 Jul. 1973, G.J. Samuels, G.J.S. 73-141, PDD 32423, culture CBS 336.77; Wenderholm Scenic Reserve, from midrib of Rhopalostylis sapida (Arecaceae), 26 Sep.1973, J.M. Dingley et al., G.J.S. 73-205, PDD 32658, culture CBS 333.77.
Clade O3
Fusariella Sacc., Atti Ist. Veneto Sci. Lett. Arti., Sér. 6 2: 463. 1884.
Colonies compact or effuse, greyish green, blackish green or black. Mycelium superficial and immersed. Stroma none. Setae and hyphopodia absent. Conidiophores semi-macronematous, mononematous, branched irregularly or sometimes dichotomously or trichotomousIy, flexuous, colourless or pale brown, smooth or verruculose. Conidiogenous cells monophialidic, integrated and terminal, or discrete, determinate, often curved, cylindrical, subulate or lageniform, with collarettes. Conidia catenate, acrogenous, semi-endogenous, developing in basipetal succession and frequently hanging together in slipped chains, the tip of each conidium except the apical one being deflected laterally, simple, straight, bent or flexuous, often fusiform pointed at the apex blunt at the base but sometimes cylindrical, dumb-bell-shaped, clavate or obclavate, pale to mid olive brown, olive green or greyish green, pale greyish green, blackish green or black in mass, usually smooth, 1–3-septate (Ellis 1971).
Type: Fusariella atrovirens (Berk.) Sacc.
Other accepted species with available sequences: Fusariella arenula (Berk. & Broome) L.W. Hou, L. Cai & Crous, F. concinna (Syd.) S. Hughes, F. curvata C.G. Lin, Yong Wang bis & K.D. Hyde, F. hughesii Chab.-Frydm.
Notes: The genus Fusariella is characterised by semi- to macronematous, mononematous conidiophores, with cylindrical, subulate or lageniform phialidic conidiogenous cells, which produce catenate, septate, curved to straight, subhyaline to brown conidia (Hughes 1949, Chabelska-Frydman 1964, Roy & Rai 1968, Ellis 1971, 1976, Seifert et al. 2011, Lin et al. 2016). Fusariella has been known for more than 130 years since it was established by Saccardo (1884), and its classification remained uncertain because of the lack of molecular data and comprehensive taxonomic treatment (Lin et al. 2016). Recently, a phylogenetic analysis based on SSU, LSU, tef1-α and rpb2 sequence data indicated that the genus Fusariella belongs to the family Bionectriaceae (Lin et al. 2016). Our study agrees with this conclusion that Fusariella clusters in Bionectriaceae, closely related to Paracylindrocarpon (Fig. 2). In addition, two Hydropisphaera species, H. cirsii and H. arenula, clustered within the Fusariella clade (Fig. 2, Supplementary Fig. S1). Both species have fusoid or subfusoid ascospores and are finely spinulose (Samuels 1978, Lechat & Fournier 2020), while most species of Hydropisphaera s. str. have ellipsoid, smooth-walled ascospores (Rossman et al. 1999). However, H. cirsii only had ITS and LSU sequences data available (Supplementary Fig. S2), and therefore the phylogenetic position of this species could not be confirmed.
Fusariella arenula (Berk. & Broome) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845818.
Basionym: Sphaeria arenula Berk. & Broome, Ann. Mag. Nat. Hist., Ser. 2, 9: 320. 1852.
Synonyms: Nectria arenula (Berk. & Broome) Berk., Outl. Brit. Fung. (London): 394. 1860.
Dialonectria arenula (Berk. & Broome) Cooke, Grevillea 12(no. 64): 110. 1884.
Cucurbitaria arenula (Berk. & Broome) Kuntze, Revis. Gen. Pl. (Leipzig) 3: 460. 1898.
Hydropisphaera arenula (Berk. & Broome) Rossman & Samuels, Stud. Mycol. 42: 30. 1999.
Description and illustration: Samuels (1978).
Materials examined: New Zealand, Auckland, Waitemata County, Riverhead State Forest, 2 m NE of Riverhead, from leaf of Phormium tenax (Asphodelaceae), 23 Jun. 1973, G.J. Samuels, C.S. Samuels & D.R.W. Watson, G.J.S. 73-125, PDD 31886, culture CBS 329.77; idem. G.J.S. 73-131, PDD 31887, culture CBS 330.77.
Notes: This species was originally introduced as Sphaeria arenula (Berkeley & Broome 1852). The taxonomic history of this species is complex and has been addressed by multiple authors (Berkeley 1860, Cooke 1884, Booth 1959, Samuels 1978). Booth (1959) examined the co-type specimen and described the ascospores as fusoid to ellipsoid, occasionally slightly curved, and hyaline with faint longitudinal striations when mature, measuring 15–18 × 3–5 μm. Samuels (1978) recombined Sphaeria arenula as Nectria arenula, but did not examine the holotype specimen. Later it was transferred to Hydropisphaera based on morphology (Rossman et al. 1999), and the recent phylogenetic analysis based on LSU sequences supported the result of Rossman et al. (1999), showing that this species clustered in Hydropisphaera (Lechat & Fournier 2020). Although most Hydropisphaera species were included in the study of Lechat & Fournier (2020), a limited number of strains/species of its related genera, especially Acremonium, Fusariella and Paracylindrocarpon, were included in the phylogenetic analysis (Lechat & Fournier 2020). In the present study, this species appears phylogenetically distant from Hydropisphaera s. str. as circumscribed in this study (Fig. 2), clustering within the Fusariella clade. Morphologically, its curved and multi-septate conidia are in agreement with the generic characters of Fusariella, and it differs from other species in its conidia that are not constricted at their septa, while conidia of most Fusariella species are commonly constricted at their conidial septa.
Fusariella atrovirens (Berk.) Sacc., Atti Reale Ist. Veneto Sci. Lett. Arti ser. 6, 2: 463 (1884)
Basionym: Fusisporium atrovirens Berk., Engl. Fl., Fungi 5(2): 351. 1836.
Synonym: Fusarium atrovirens (Berk.) Mussat, Syll. Fung. (Abellini) 15: 144. 1901.
Material examined: Algeria, Western Sahara, near Béni-Abbès (Saoura), from desert soil, Oct. 1972, unknown collector, isol. J. Nicot & J. Mouchacca, culture CBS 311.73 = IMI 171130 = LCP 2177.
Fusariella hughesii Chab.-Frydm., Canad. J. Bot. 42: 1485. 1964.
Description and illustration: Chabelska-Frydman (1964).
Material examined: Netherlands, Flevoland Province, Oostelijk Flevoland, from agricultural soil, under permanent potato cultivation, unknown collection date and collector, isol. Oct. 1969 by J.W. Veenbaas-Rijks, culture CBS 435.70.
Fusariella sp.
Material examined: China, Hubei, Shennongjia, alt. 1 800 m, rotten twig, 17 Sep. 2006, W.Y. Zhuang & N. Ye, 5805, culture CBS 128364.
Notes: The culture was isolated from the rotten twig collected from Shennongjia in China and was originally labelled as Hydropisphaera erubescens (Nong & Zhuang 2005). However, the phylogeny based on sequence obtained from the ITS, LSU, rpb2 and tef-1α place this strain in a sister branch to Fusariella hughesii (Fig. 2). Unfortunately, the culture was sterile and morphological comparison with other Fusariella species was impossible. This is probably a novel species and awaits further study.
Clade O8
Musananaesporium L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845821.
Etymology: Name derived from the banana-shaped (Musa nana) conidia.
Mycelium consisting of branched, septate, (sub-)hyaline, thin-, smooth-walled hyphae, brown and thick-walled in old cultures. Conidiophores arising from submerged mycelium or ropes formed by the mycelium, erect, with multiple septa, unbranched or branched, commonly repeatedly proliferating sympodially. Conidiogenous cells enteroblastic, mono- to polyphialidic, solitary, lateral, cylindrical or subulate, hyaline to (sub-)hyaline, thick-, smooth-walled, with inconspicuous collarette and periclinal wall thickening at conidiogenous loci, with percurrent or subterminal proliferations. Polyphialides with up to four conidiogenous loci occasionally present. Conidia aseptate, becoming 1–4-septate with age, cylindrical, obclavate, fusoid, with a protuberant and curved, basal abscission scar, straight to curved, thick-, smooth-walled, hyaline, produced in slimy heads. Typical chlamydospores absent, but hyphae disintegrate into arthrospore-like fragments in old cultures. Sexual morph not observed.
Type: Musananaesporium tectonae (R.F. Castañeda) L.W. Hou, L. Cai & Crous
Notes: This monotypic genus, Musananaesporium, is established to accommodate Acremonium tectonae, since it is not congeneric with Acremonium s. str. based on A. alternatum. Musananaesporium tectonae is represented by a single lineage in the phylogenetic analysis (Fig. 2). The morphology of this species is unique in producing branched, repeatedly sympodially proliferating conidiophores and phialides, and cylindrical, obclavate, fusoid conidia, with a protuberant, curved basal abscission scar, rendering it different from other known genera in Bionectriaceae (Fig. 2).
Musananaesporium tectonae (R.F. Castañeda) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845822. Fig. 18.
Fig. 18.

Musananaesporium tectonae (culture CBS 725.87) A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidia arranged in slimy heads. F. Unbranched conidiophore. G, H. Percurrently proliferated conidiophores. I. Polyphialide. J. Branched conidiophore. K. Septate mycelium. L. Conidia. Scale bars = 10 μm.
Basionym: Acremonium tectonae R.F. Castañeda [as ‘Acromoniun’], Fungi Cubenses II (La Habana): 2. 1987.
Mycelium consisting of branched, septate, (sub)hyaline, thin-, smooth-walled hyphae, brown and thick-walled in old cultures, 3–4 μm wide. Sporulation phalacrogenous, plectonematogenous, and nematogenous. Conidiophores (sub-erect, straight, arising from submerged hyphae or ropes of hyphae, unbranched or basitonously branched, bearing 1–2 phialides per node, often repeatedly proliferating sympodially, up to 268 μm long, 2.7–6.2 μm wide at base, with 1–7 septa at basal, middle and apical part, with cell walls usually thicker than those of vegetative hyphae. Phialides solitary, lateral, cylindrical or subulate, hyaline to (sub-)hyaline, thick-, smooth-walled, (15–) 25–76(–120) long, 1.7–6.2 μm wide at base, with inconspicuous collarette and periclinal wall thickening at conidiogenous loci; polyphialides terminally and subterminally proliferating, with up to four conidiogenous loci occasionally present. Conidia aseptate, becoming 1–4-septate with age, ellipsoidal, cylindrical, obclavate and fusoid, straight, curved at base, with a protuberant and curved basal abscission scar, thick-, smooth-walled, hyaline, (9–)11.5–25.5(–28.5) × 2.5–4.5 μm, produced in slimy heads. Typical chlamydospores absent, but catenate hyphae disintegrate into arthroconidium-like fragments in old cultures, hyaline, thick-, smooth-walled, 4.2–9.5 long, 3–4 μm diam.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 20 mm diam, flat, with sparse aerial mycelium, dusty, becoming felty at some zones with age, pale saffron, margin entire, with brown pigment produced on old culture, reverse concolourous; On MEA reaching 11–14 mm diam, raised, with sparse aerial mycelium, cerebriform, peach, margin dendritic, reverse apricot; On PDA reaching 10–13 mm diam, raised, cerebriform, with sparse aerial mycelium, dusty, pale saffron, margin crenate, reverse ochreous, with slightly pale brown pigment; On SNA reaching 15–17 mm diam, flat, membranous without aerial mycelium, colourless, margin entire, reverse colourless.
Typus: Cuba, Matanzas Province, San Miguel de Los Baños, from living leaf of Tectona grandis (Lamiaceae), isol. 23 Jan. 1987, R.F. Castañeda & G. Arnold, CBS H-24609 (holotype INIFAT C87/32, ex-type culture CBS 725.87).
Notes: Musananaesporium tectonae was originally described as A. tectonae by Castañeda (1987) from a living leaf of Tectona grandis in Cuba. The ex-type culture was examined in this study, and morphologically matched the original description except for a few characters. In its original description, the conidiophores were described as macronematous and mononematous, phialides were monophialidic, terminal or lateral, conidia were unicellular, and no mention was made of the production of moniliform hyphae and the septate conidia. In the present study, the septate and thick-walled conidia are commonly produced in old cultures, becoming up to 4-septate. In addition, arthroconidium-like hyphae are present, which are much thicker than normal hyphae. This species differs from the other acremonium-like taxa by producing longer, septate conidia and abundant macronematous conidiophores. According to the phylogenetic inference, the ex-type culture CBS 725.87 falls in a separate lineage, representative of a novel genus in Bionectriaceae (Fig. 2).
Clade O9
Gossypinidium L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845819.
Etymology: From Latin, gossypina (= cottony), referring to the cottony sporodochia produced by the type of this genus.
Mycelium consisting of branched, septate, hyaline at first, pale brown in old cultures, slightly warted and thick-walled hyphae. Crystals absent. Sporulation abundant from sporodochia, rarely from conidiophores formed directly on substratal or aerial mycelium. Conidiophores in aerial mycelium erect, straight, slightly curved, repeatedly verticillate towards the apex, bearing 1–3(–4) whorls of 1–3 phialides, rarely unbranched, septate, hyaline, smooth-walled. Conidiogenous cell from solitary conidiophores enteroblastic, monophialidic, mostly lateral, sub-cylindrical to subulate, hyaline, thick-, smooth-walled, with inconspicuous minute collarette and periclinal thickening at conidiogenous loci. Conidiophores in pale pink or orange cottony sporodochia mostly branched, hyaline, smooth-walled. Sporodochial phialides enteroblastic, monophialidic, mostly lateral, aculeate or subulate, tapering at top, hyaline, thick-, smooth-walled, with minute collarette and inconspicuous conspicuous periclinal thickening at conidiogenous loci. Conidia from solitary conidiophores and sporodochial conidiophores not significantly different, aseptate, short ellipsoid, hyaline, thin-, smooth-walled, arranged in chains. Chlamydospores and sexual morph not observed.
Type: Gossypinidium sporodochiale L.W. Hou, L. Cai & Crous
Notes: Gossypinidium presents a distinct lineage on the 4-locus (ITS-LSU-rpb2-tef-1α) phylogenetic tree (Fig. 2). It produces abundant cottony sporodochia with abundant conidia arranged in chains, a character that clearly distinguishes it from other genera of Bionectriaceae.
Gossypinidium sporodochiale L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845820. Fig. 19.
Fig. 19.

Gossypinidium sporodochiale (ex-type culture CBS 101694). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Sporodochia on SNA. E. Sporodochia. F. Unbranched conidiophore. G–I. Branched conidiophores. J. Conidia. Scale bars = 10 μm.
Etymology: Named after the abundant sporodochia produced in culture by this species.
Mycelium consisting of branched, septate, hyaline at first, pale brown in old cultures, slightly warted, thick-walled hyphae, up to 4 μm wide. Crystals absent. Sporulation abundant from sporodochia, rarely from conidiophores formed directly on the aerial or substratal mycelium. Conidiophores from aerial mycelium erect, straight or slightly curved, repeatedly verticillate towards the apex, bearing up to 4 whorls of 1–3 phialides, or unbranched, up to 60 μm long, 1–2.4 μm wide at base, with 1–3 septa, hyaline, smooth-walled. Phialides from solitary conidiophores mostly lateral, sub-cylindrical to subulate, hyaline, thick-, smooth-walled, 10–25 μm long, 1.3–2.3 μm wide at base, with minute collarette and inconspicuous periclinal thickening at conidiogenous loci. Conidiophores in pale pink or orange cottony sporodochia mostly branched, bearing multiple levels with 1–3 phialides per node, hyaline, smooth-walled. Sporodochial phialides mostly lateral, aculeate or subulate, tapering at top, hyaline, thick-, smooth-walled, 10–15 μm long, 1.5–2 μm wide at base, with minute collarette and inconspicuous conspicuous periclinal thickening at conidiogenous loci. Conidia from solitary conidiophores and sporodochial conidiophores not significantly different, aseptate, short ellipsoid, hyaline, thin-, smooth-walled, 3–4.6 × 2–2.8 μm, eguttulate, arranged in chains. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 46–47 mm diam, flat, floccose, granulose at centre, salmon at centre, white at periphery, margin crenate, reverse buff at centre, dirty white at periphery; On MEA reaching 30–40 mm diam, raised, felty or woolly, rosy buff at centre, buff at periphery, margin lobulated, reverse umber, pale umber at periphery; On PDA reaching 75 mm diam, flat, felty, white, buff or creamy white at periphery, margin fimbriate, reverse pale brown at centre, buff at periphery.
Typus: Puerto Rico, Luquillo National Forest, El Yunque Trail, from dead rachis of Praestoea montana (Arecaceae), Jun. 1998, W. Gams (holotype CBS H-24608, ex-type culture CBS 101694).
Notes: The strain CBS 101694 formed a distinct branch and was originally identified as A. persicinum based on the LSU and SSU phylogenetic analysis for the majority of Acremonium (Summerbell et al. 2011), although phylogenetically different from other A. persicinum strains, including the ex-type CBS 310.59 (Summerbell et al. 2011). Phylogenetic analysis based on more loci (ITS-LSU-rpb2-tef-1α) shows that CBS 101694 forms a distinct branch in Bionectriaceae (Fig. 2). It differs from closely related genera by producing repeatedly verticillate conidiophores and cottony sporodochia with abundant ellipsoid conidia arranged in long chains.
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hits using the ITS sequence are Fusariella curvata from a leaf of decaying Quercus sp. in Thailand [culture MFLUCC 15-0844; GenBank KX025152; Identity = 473/522 (91 %), 11 gaps (2 %)] and F. atrovirens [culture CBS 311.73; GenBank MH860688.1; Identity = 472/522 (90 %), 11 gaps (2 %); Lin et al. 2016]; the closest hit using the LSU sequence is Acremonium persicinum [culture CBS 469.67; GenBank MH870741.1; Identity = 760/778 (98 %), 3 gaps (0 %)]; the closest hit using the rpb2 sequence is Hydropisphaera peziza [culture CBS 102038; GenBank DQ522444.1; Identity = 664/756 (98 %), no gaps]; the closest hit using the tef-1α sequence is Fusariella curvata [culture MFLUCC 15-0844; GenBank KX025155.1; Identity = 734/771 (95 %), 2 gaps (0 %)].
Clade O11
Monohydropisphaera L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845823.
Etymology: Referring to its similarity with Hydropisphaera.
Mycelium with hyphae branching, septate, hyaline to pale brown, smooth. Perithecia solitary or crowded in groups of 2–10, superficial, subglobose, reddish brown, collapsing cupulate when dry, not changing colour in 3 % KOH or lactic acid. Perithecial apex with short, acute papilla, margin with fasciculate, thick-walled hairs, arising from cells of ascomatal wall. Hairs brownish orange, cylindrical, slightly flexuous, thick-walled, rounded at tips, septate. Perithecial wall composed of two regions: outer region of globose to ellipsoid, thick-walled cells, inner region of elongate, flattened, thin-walled cells. Asci unitunicate, clavate, apices rounded, without ring, with eight biseriate ascospores. Ascospores aseptate, fusoid, hyaline, striate with striations finely verrucose. Asexual morph: conidiophores borne on aerial hyphae, macronematous, mononematous, unbranched, elongate, erect, straight to flexuous, hyaline to light brown, surface smooth to very faintly roughened. Conidiogenous cells integrated, monophialidic, terminal, subulate towards apex, at times with a minutely flared collarette. Conidia solitary or catenulate in short chains, obpyriform to fusiform with apices rounded to somewhat acute and bases having a prominent, almost apiculate hilum, aseptate, walls smooth to verrucose, hyaline, becoming dark brown, paler and dark brown to black in mass (emended from Lechat et al. 2010).
Type: Monohydropisphaera fusigera (Berk. & Broome) L.W. Hou, L. Cai & Crous
Notes: Monohydropisphaera is proposed to accommodate the single species M. fusigera, which was originally received as Hydropisphaera fusigera (Lechat et al. 2010), but distinct from Hydropisphaera s. str. in the multi-locus phylogenetic tree in this study with more strains and loci included for phylogenetic inferences (Fig. 2). Morphologically, the ascospores of Monohydropisphaera are aseptate and coarsely striate with striae somewhat wavy, which are distinct characters that differentiate it from other genera in Bionectriaceae.
Monohydropisphaera fusigera (Berk. & Broome) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845824.
Basionym: Monotospora fusigera Berk. & Broome, J. Linn. Soc., Bot. 14(no. 74): 99. 1873 (1875).
Synonyms: Gliomastix fusigera (Berk. & Broome) C.H. Dickinson, Mycol. Pap. 115: 7. 1968.
Acremonium fusigerum (Berk. & Broome) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 94. 1971.
Hydropisphaera bambusicola Lechat, Mycotaxon 111: 96. 2010.
Hydropisphaera fusigera (Berk. & Broome) Rossman et al., Stud. Mycol. 80: 242 (2015).
Description and illustration: Lechat et al. (2010).
Material examined: French West Indies, Martinique, Prêcheur, Anse Couleuvre, sentier de la cascade Couleuvre, in dead tops of Bambusa vulgaris (Poaceae), 25 Aug. 2008, C. Lechat (holotype of Hydropisphaera bambusicola CLL8323 in LIP, ex-type culture CBS 124147).
Notes: This species was originally linked to the sexual morph H. bambusicola that characterised by the production of ascomata that often collapse upon drying to form cupulate perithecia (Lechat et al. 2010), and was transferred to Hydropisphaera, as H. fusigera (Lombard et al. 2015). However, species in Hydropisphaera s. lat. had proved to be highly polyphyletic (Rossman et al. 2001). In the present study, H. fusigera forms a separate lineage that is distinct from the Hydropisphaera s. str. clade (Fig. 2), and also remote from any known genera in Bionectriaceae. Morphologically, H. fusigera produces aseptate ascospores that are coarsely striate with somewhat wavy striae, which is rare in Hydropisphaera (Lechat et al. 2010). And although H. fusigera resembles some species of Protocreopsis, it differs from the latter by lacking white to tan hyphae that envelop the ascomatal wall (Doi 1977). The asexual morph of H. fusigera was reported to belong to the genus Gliomastix as G. fusigera (Lechat et al. 2010), which is characterised by producing dark brown conidia in chains occurring on members of the Arecaceae and Poaceae throughout the tropics (Lechat et al. 2010). However, it is distinct from other Gliomastix species in having much larger conidia that are longer than 12 μm which is rare in Gliomastix, and therefore further supports it as a different genus.
Clade O12
Hydropisphaera Dumort., Comment. Bot. (Tournay): 89. 1822.
Synonyms: Nectria subgen. Hyphonectria Sacc., Syll. Fung. (Abellini) 2: 501. 1883.
Neohenningsia Koord., Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk., sect. 2, 13: 164. 1907.
Perrotiella Naumov, Zap. Ural’sk. Obshch. Lyubit. Estestv. 35(11–12, Champ. Oural.): 25. 1916.
Heleococcum C.A. Jørg., Bot. Tidsskr. 37: 417. 1922.
Neuronectria Munk, Dansk bot. Ark. 17(1): 56 (1957).
Hyphonectria (Sacc.) Petch, J. Bot. 75: 220 (1937).
Ascomata superficial, perithecial, rarely cleistothecial, non-stromatic, pale yellow, orange or umber, KOH-, globose to subglobose or doliiform, usually collapsed and deeply cupulate, smooth or with fasciculate hairs. Ascomatal wall generally over 25 μm thick, of two regions; outer region of thin-walled, globose cells. Asci clavate. Ascospores ellipsoid, 1-septate, rarely multiseptate, hyaline, generally finely to coarsely striate, rarely smooth or spinulose (emended from Rossman et al. 1999).
Type: Hydropisphaera peziza (Tode) Dumort.
Other accepted species with available sequences: H. aurantiaca (C.A. Jørg.) L.W. Hou, L. Cai & Crous, H. cyatheae (Dingley) Rossman & Samuels, H. fungicola Rossman et al., H. suffulta (Berk. & M.A. Curtis) Rossman & Samuels
Notes: The genus Hydropisphaera was established by Dumortier based on H. peziza (Dumortier 1822). This genus comprised a number of species with nectria-like sexual morphs and had previously been placed in the Nectria peziza group (Booth 1959, Samuels 1976b, Rossman 1983). Hydropisphaera was long considered a synonym of the genus Nectria until Rossman et al. (1999) resurrected it as a distinct genus in Bionectriaceae (Rossman et al. 1999, Lechat et al. 2010). Hydropisphaera s. lat. is characterised by producing ascomata with walls that are generally over 25 μm thick, deeply collapsed, cupulate perithecia upon drying, and one- to multi-septate ascospores that are often finely to coarsely striate, spinulose or smooth (Rossman et al. 1999). The asexual morph of Hydropisphaera has been recognised and placed in diverse genera including acremonium-like, cylindrocarpon-like, Cephalosporium or Gliomastix (Samuels 1978, Rossman et al. 2008, Lechat et al. 2010, Lechat & Fournier 2016a, 2017). Although more than 30 species have been described in Hydropisphaera, this genus has been revealed to be highly polyphyletic (Rossman et al. 2001). In our present phylogenetic analysis based on combined ITS, LSU, rpb2 and tef-1α genes, Hydropisphaera species were distributed in at least eight clades that could be differentiated from each other based on their phylogenetic and morphological characters (Fig. 2). Hydropisphaera is here restricted to those taxa related to the type H. peziza; they produce only an acremonium-like asexual morph, and have thick-walled ascomata with 1-septate, finely to coarsely striate ascospores. Furthermore, the cleistothecial genus Heleococcum is synonymised under Hydropisphaera based on the phylogenetic placement of the type, He. aurantiacum.
Hydropisphaera aurantiaca (C.A. Jørg.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845825.
Basionym: Heleococcum aurantiacum C.A. Jørg., Bot. Tidsskr. 37: 417. 1922.
Synonym: Heleococcum japonense Tubaki, Trans. Mycol. Soc. Japan 8: 5. 1967.
Description and illustration: Jørgensen (1922).
Typus: Denmark, Botanical Garden of the University of Copenhagen, in the door, on the moist soil, 1921, L.K. Rosenvinge (C; NY, slides of holotype). Unknown, unknown substrate, unknown collection date and collector, BPI 691939 (recorded in BPI as type).
Additional materials examined: Japan, Hokkaido, from wood panel of Abies firma (Pinaceae) in seawater, Oct. 1966, K. Tubaki (holotype of Heleococcum japonense CBS H-13245, ex-type culture CBS 397.67 = ATCC 18157 = IFO 8643). Unknown, from mushroom compost, unknown collection date and collector, dep. F.C. Wood, culture CBS 201.35.
Notes: Although Heleococcum has cleistothecial ascomata and lacks a nectrioid centrum, its fleshy, bright-coloured ascomata, 1-septate, hyaline ascospores and acremonium-like asexual morph suggest that Heleococcum fits well in Hypocreales and it was therefore placed in Bionectriaceae (Rossman et al. 1999). In the present study, the reference culture of He. aurantiacum (CBS 201.35) and the ex-type culture He. japonense (CBS 397.67) had identical sequences on four genes and grouped in a fully supported clade in the Hydropisphaera clade, closely related to Hy. peziza (Fig. 2), based on which we concluded that they are conspecific, with He. aurantiacum having priority. Although the morphological features of the two species are not always constant, the discrepancies could be influenced by external factors.
Hydropisphaera cirsii Lechat & J. Fourn., Ascomycete.org 12: 39. 2020.
Description and illustration: Lechat & Fournier (2020).
Typus: Germany, North Rhine-Westphalia, MTB 4506/441 Duisburg, city-forest, Uhlenhorstweg, S of cultural monument ‘Steinbruch’, 51°41’27” N, 6°80’41” E, on Cirsium arvense (Asteraceae), 17 Feb. 2013, leg. K. Müller, comm. K. Siepe (holotype CLL 13012 in LIP, ex-type culture CBS 135615).
Notes: When this species was initially described, the only available LSU sequences placed it within the genus Hydropisphaera, which formed a long branch with low support values (Lechat & Fournier 2020). Hydropisphaera cirsii was observed to be different from known species of Hydropisphaera in having erect, glassy hairs scattered on its lateral ascomatal wall, finely spinulose ascospores and its occurrence on Cirsium arvense (Lechat & Fournier 2020). Based on the phylogenetic analysis performed on the four-gene alignment and more available cultures, this species forms a single lineage in Fusariella (Supplementary Fig. S2). The phylogenetic position of this species remains unresolved pending more cultures and DNA sequence data.
Hydropisphaera cyatheae (Dingley) Rossman & Samuels, Stud. Mycol. 42: 30. 1999.
Basionym: Nectria cyatheae Dingley [as ‘cyathea’], Trans. Roy. Soc. New Zealand. 83: 652. 1956.
Illustration: Samuels (1976b).
Typus: New Zealand, Auckland, on stipes of Cyathea medullaris (Cyatheaceae), Apr. 1984, J.M. Dingley (holotype PDD 6201).
Material examined: New Zealand, Auckland, Waitakere Ranges, Rangemore Track, on rachis of Cyathea medullaris (Cyatheaceae), 10 Jul. 1974, G.J. Samuels, G.J.S. 74-103, PDD 32563, culture CBS 575.76.
Hydropisphaera erubescens (Roberge ex Desm.) Rossman & Samuels, Stud. Mycol. 42: 30. 1999.
Basionym: Sphaeria erubescens Roberge ex Desm., Ann. Sci. Nat., Bot., sér. 3, 6: 72. 1846.
Synonyms: Calonectria erubescens (Roberge ex Desm.) Sacc., Michelia 1 (no. 3): 309. 1878.
Nectria erubescens (Roberge ex Desm.) W. Phillips & Plowr., Grevillea 10 (no. 54): 70. 1881.
Dialonectria erubescens (Roberge ex Desm.) Cooke, Grevillea 12: 111. 1884.
Calonectria umbelliferarum Seaver, Mem. New York Bot. Gard. 6: 507. 1916.
Amphinectria erubescens (Roberge ex Desm.) Sacc. ex Speg., Bol. Acad. Nac. Ci. Córdoba 26(2–4): 347. 1921.
Calonectria venezuelensis Syd., Ann. Mycol. 33: 88. 1935.
Calonectria crescentiae Seaver & Waterston, Mycologia 32: 404. 1940.
Dimerosporiella guarapiensis (Speg.) Rossman & Samuels, Stud. Mycol. 42: 23. 1999.
Descriptions: Rossman (1983), Samuels (1978).
Typus: France, on old leaves of Ilex aquifolium (Aquifoliaceae), 1846, J.B.H.J. Desmazières, lectotype of Sphaeria erubescens, NY No.1766.
Notes: This species was originally described by Desmazières (1846) as Sphaeria erubescens from old leaves of Ilex aquifolium in France based on the sexual morph. Our present study includes three cultures (CBS 333.77 = PDD 32658, CBS 334.77 = PDD 32478, CBS 335.77 = PDD 34939) that were examined and demonstrated to have identical morphological characters to type specimens of Sphaeria erubescens (currently H. erubescens; Samuels 1978). Based on the phylogenetic analysis, the three strains are genetically heterogeneous. Sequences obtained from CBS 334.77 are identical to the ex-type strain of Acremonium strictum (currently Sarocladium strictum, CBS 346.70; Fig. 3), suggesting that CBS 334.77 was swapped or contaminated by A. strictum at some point before or after it was deposited, since the morphology does not match what was described and illustrated as protologue of S. erubescens (Samuels 1978). The sequences obtained from the culture CBS 333.77 were identical to H. multiseptata (currently Paracylindrocarpon multiseptatum, CBS 337.77; Fig. 2), possibly due to the inaccurate identification. The third culture CBS 335.77 has identical sequences with the ex-type culture of Pn. aloicola (CBS 141300; Fig. 2). Unfortunately, culture CBS 335.77 proved to be sterile, and we were unable to compare its morphology with that of the type description.
Hydropisphaera fungicola Rossman et al., Fungal Planet 24: 2. 2008.
Description and illustration: Rossman et al. (2008).
Typus: USA, Idaho, Lapwai Canyon, in the riparian community of Lapwai Creek, on Ulocladium atrum (Pleosporaceae) associated with Melampsora rust on decaying leaves of Populus trichocarpa (Salicaceae), unknown collection date, G. Newcombe (holotype BPI 878275, ex-type culture AR 4170 = CBS 122304).
Hydropisphaera peziza (Tode) Dumort., Comment. Bot. (Tournay): 90. 1822.
Basionym: Sphaeria peziza Tode, Fung. Mecklenb. Sel. (Lüneburg) 2: 46. 1791.
Synonyms: Nectria peziza (Tode) Fr., Summa veg. Scand., Sectio Post. (Stockholm) 2: 388. 1849.
Dialonectria peziza (Tode) Cooke, Grevillea 12: 110. 1884.
Cucurbitaria peziza (Tode) Kuntze, Revis. Gen. Pl. (Leipzig) 3: 461. 1898.
Neuronectria peziza (Tode) Munk, Dansk Bot. Ark. 17: 58. 1957 [nom. inval., Art. 41.5 (Melbourne)].
Illustrations: Booth (1959, fig. 32, as Nectria peziza); Rossman et al. (1999).
Typus: Sweden, on rotten wood, Sclerom. Suec. 24 no. 235, 1882 [(BPI, in Sbarbaro collections in bound volumes 1–3), lectotype designated by Rossman et al. (1999)].
Materials examined: Austria, from Juglans sp. (Juglandaceae), unknown collection date, W. Jaklitsch, culture CLL 13025 = CBS 135908. France, Ile de Ré, Saint-Martin, from dead wood, 11 Oct. 2014, isol. 14 Oct. 2014, coll. and isol. C. Lechat, culture CLL 14063 = CBS 139487. UK, Yorkshire, Mulgrave Woods, from Polyporus squamosus (Polyporaceae), unknown collection date and collector, isol. 1953 by C. Booth, CBS H-15060, culture CBS 296.65 = IMI 053559.
Hydropisphaera suffulta (Berk. & M.A. Curtis) Rossman & Samuels, Stud. Mycol. 42: 32. 1999.
Basionym: Nectria suffulta Berk. & M.A. Curtis, J. Linn. Soc., Bot. 10(no. 46): 378. 1868 (1869).
Synonyms: Lasionectria suffulta (Berk. & M.A. Curtis) Cooke, Grevillea 12(64): 112. 1884.
Cucurbitaria suffulta (Berk. & M.A. Curtis) Kuntze, Revis. Gen. Pl. (Leipzig) 3(3): 461. 1898.
Neohenningsia suffulta (Berk. & M.A. Curtis) Höhn. ex Petch, Trans. Brit. Mycol. Soc. 21: 268. 1938.
Description and illustration: Samuels et al. (1976a).
Typus: Cuba, on palm leaves, unknown date, C. Wright (ex herb. M.J. Berkeley) (holotype of Nectria suffulta Berk. & M.A. Curtis 1868, K(M) 36225).
Material examined: Indonesia, Sulavesi, Eastern Dumoga-Bone Nat. Park, Komangaan, Limestone Caves, alt. 400 m, on inflorescence of Cocos nucifera (Arecaceae), 24 Oct. 1985, G.J. Samuels, G.J.S. 85-194, representative culture CBS 122.87 = G.J.S. 2363.
Clade O14
Paragliomastix L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845826.
Etymology: Morphologically resembling the genus Gliomastix, but phylogenetically distinct.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores solitary or aggregated, erect, arising directly from vegetative hyphae or ropes formed by the mycelium, usually reduced to single phialides, unbranched or poorly branched, hyaline or pale brown, smooth-, or roughen- and finely spinulose-walled, septate at base, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, terminal, subulate, hyaline at first, becoming pale brown with age, thick-, rough-walled, with conspicuous or inconspicuous cylindrical collarette and periclinal thickening at conidiogenous loci; polyphialides with up to two conidiogenous loci occasionally present. Conidia aseptate, ovoid, fusiform, with symmetrically apiculate base, having prominent, darker, truncate hilum at one end or both ends, hyaline at beginning, becoming olivaceous green, dark brown with age, or with darker equatorial belt, thick-walled, smooth, spinulose or wrinkled, arranged in dry, long chains. Chlamydospores and sexual morph not observed.
Type: Paragliomastix luzulae (Fuckel) L.W. Hou, L. Cai & Crous
Other accepted species with available sequences: Paragliomastix chiangraiensis (J.F. Li et al.) L.W. Hou, L. Cai & Crous, Px. rosea L.W. Hou, L. Cai & Crous, Px. znieffensis (Lechat & J. Fourn.) L.W. Hou, L. Cai & Crous
Notes: Paragliomastix has a close morphological similarity with Gliomastix by having darkly pigmented ameroconidia. However, Paragliomastix produces spinulose, rough-walled phialides and conidia arranged in long, dry chains, while most Gliomastix species produce smooth-walled phialides and conidia arranged in slimy heads or chains. The phylogenetic analysis of the combined ITS, LSU, rpb2 and tef-1α dataset revealed that species of Paragliomastix clustered distant from Gliomastix in Bionectriaceae (Fig. 2).
Paragliomastix chiangraiensis (J.F. Li et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845827.
Basionym: Acremonium chiangraiense J.F. Li et al., Fungal Diversity 100: 197. 2020.
Description and illustration: Hyde et al. (2020a).
Typus: Thailand, Chiang Rai Province, Khun Korn Waterfall, on dead moistened leaf of palm, 9 Jan. 2014, J.F. Li, H-10b (holotype MFLU 14-0202, isotype KUN-HKAS, ex-type culture MFLUCC 14-0397).
Material examined: Colombia, Cundinamarca, near Fómeque, from dead leaf of Zea mays (Poaceae), 5 Dec. 1979, W. Gams, Col 161c, culture CBS 277.80B.
Notes: Acremonium chiangraiense was described by Hyde et al. (2020a), which was collected from Khun Korn Waterfall in Thailand and was isolated from a dead, moist palm leaf. This fungus is characterised by producing ampulliform conidiophores with phialidic conidiogenous cells and bearing hyaline to green-yellow conidia in chains (Hyde et al. 2020a), which morphologically correlated with the generic characteristics of Paragliomastix. Phylogenetically, it falls in a well-supported lineage closed to the type of Paragliomastix, Px. luzulae (Fig. 2).
Paragliomastix luzulae (Fuckel) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845828. Fig. 20.
Fig. 20.

Paragliomastix luzulae (culture CBS 494.67). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial chains on pine needle. E–H. Conidiophores. I, J. Conidia. Scale bars = 10 μm.
Basionym: Torula luzulae Fuckel, Fungi Rhenani Exsiccati, Supplementi Fasc. 2: no. 1624. 1866.
Synonyms: Gliomastix luzulae (Fuckel) E.W. Mason, Nat. Hist. Scarborough Distr. 1: 154. 1953. [nom. inval., Art. 41.5 (Melbourne)].
Gliomastix luzulae (Fuckel) E.W. Mason ex S. Hughes, Canad. J. Bot. 36: 769. 1958.
Acremonium luzulae (Fuckel) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 92. 1971.
Sagrahamala luzulae (Fuckel) Subram., Curr. Sci. 41: 48. 1972.
Fusidium viride Grove, J. Bot., Lond. 23: 164. 1885.
Description based on culture CBS 494.67: Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–2.9 μm wide. Conidiophores solitary or aggregated, erect, straight, arising directly from vegetative hyphae or from a granule formed by mycelium, usually reduced to single phialides, unbranched or poorly branched, up to 44 μm long, 1.8–3.5 μm wide at base, hyaline at beginning, turning pale brown with age, roughened, septate at base, with warts or spinulate at the upper part, rarely pigmented at the tip, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, terminal, subulate, hyaline at first, becoming pale brown with age, thick-, rough-walled, 22–35 μm long, 2–3.5 μm wide at base, with inconspicuous collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, ovoid or fusoid, with symmetrically truncate base at both ends, having prominent, darker, apiculate hilum at one end or both ends, hyaline at beginning, becoming olivaceous green with age, mostly with a distinct darker equatorial belt, sometimes with distinct darker zones at one side of conidia, and darker zones enlarged, irregularly encrusted with pigment, thick-, rough-walled, 4.9–7.5 × 1.9–2.8 μm, arranged in chains. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 12–14 mm diam, raised, felty, granular at centre, olivaceous black at centre, white at periphery, margin crenate, brown pigment, reverse pale olivaceous; On MEA reaching 16 mm diam, raised, felty, granular at centre, olivaceous with abundant liquid exudate at centre, olivaceous black at periphery, margin crenate, reverse olivaceous, with buff edge; On PDA reaching 21–22 mm diam, flat, felty, moist with abundant liquid exudate, olivaceous black with buff edge, with fawn pigment at agar, margin lobate, reverse concolourous with fawn pigment; On SNA reaching 16–17 mm diam, flat, dusty, greenish olivaceous, white at periphery, margin lobate, reverse concolourous. Lacking odour on OA, MEA, SNA media; with strong geosmin odour on PDA.
Materials examined: Germany, Kr. Rendsburg, Gut Schierensee, from decaying wood of Picea sp. (Pinaceae), together with many other fungi, unknown collection date, W. Gams, CBS H-8241 & CBS H-24595, culture CBS 494.67 = IAM 14654; Spessart, Lochmühle near Bieber, from decaying wood of Fagus sylvatica (Fagaceae), Sep. 1969, W. Gams, No. 1614, CBS H-8243, culture CBS 935.69. Russia, Novgorod, Saint Sophia Cathedral, work of art, unknown collection date and collector, isol. D. Kudritsina, dep. L.A. Belyakova, CBS H-8030, culture CBS 495.67 = ATCC 18665 = IMI 133983 = VKM F-1168.
Notes: This species was originally described as Torula luzulae from leaves of Luzula maxima (Fuckel 1866), and later transferred to Gliomastix (Hughes 1958). However, Gams (1971) demonstrated that it belonged to Acremonium in the Luzulae-series because of its pigmented conidia. According to our multi-locus phylogenetic analyses, this species is not congeneric with Gliomastix s. str. nor Acremonium s. str., but clustered with a separate clade representing the novel genus Paragliomastix in Bionectriaceae (Fig. 2). Morphologically, Px. luzulae is characterised by its conidial shape, having truncate ends, and with a dark belt in the middle of conidia, based on which it is differentiated from other species in Paragliomastix.
Paragliomastix rosea L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845829. Fig. 21.
Fig. 21.

Paragliomastix rosea (ex-type culture CBS 277.80A). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores arising from mycelial ropes. F–H. Conidiophores. I. Conidia. J. Conidial chains. Scale bars = 10 μm.
Etymology: Epithet derived from the colony colour on PDA.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.5–2.8 μm wide. Conidiophores solitary or aggregated, erect, straight, arising directly from vegetative hyphae or ropes formed by the mycelium, usually reduced to single phialides, unbranched or poorly branched, up to 34.5 μm long, 2–3.4 μm wide at base, with 1–2 septa at base and middle part, initially hyaline, becoming pale brown with age, roughened, warty or spinulate at the upper part, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, terminal, subulate, hyaline at first, becoming pale brown with age, thick-, rough-walled, 19–31.5 μm long, 2–3.5 μm wide at base, with cylindrical collarette and inconspicuous periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, narrow fusiform, with symmetrically truncate base at both ends, having prominent, darker, apiculate hila at one end or both ends, hyaline at beginning, becoming olivaceous green with age, sometimes with a distinct darker equatorial belt, thick- and rough-walled, becoming wrinkled on surface with age, (5–)5.8–7.5(–8) × (1.8–)2–2.5 μm, arranged in chains. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 23–25 mm diam, flat, sparse aerial mycelium, greenish olivaceous and hairy at centre, white and membranous without mycelium at periphery, with olivaceous mycelial ropes at centre, margin entire, reverse concolourous; On MEA reaching 24–25 mm diam, raised, rugose, felty, olivaceous black at centre, grey or dirty white at periphery, margin entire, reverse black with brown edge, with abundant dark vinaceous pigment; On PDA reaching 21 mm diam, flat, radially folded at centre, sparse aerial mycelium, thinly felty, concentric circles of different colours, centre olivaceous black, rosy buff to grey olivaceous at periphery, margin dendritic, reverse olivaceous black at centre, pale sienna at periphery; On SNA reaching 10 mm diam, flat, membranous without aerial mycelium, white, margin undulate, reverse concolourous.
Typus: India, Kurukshetra, unknown substrate, collection date and collector, dep. R.S. Mehrotra (holotype CBS H-24596, ex-type culture CBS 277.80A).
Notes: Culture CBS 277.80A was received as Acremonium luzulae (currently Px. luzulae). In this study, it nestled in the Paragliomastix clade closely related to Px. chiangraiensis and Px. luzulae, but forms a separate lineage (Fig. 2). Paragliomastix rosea is morphologically similar to Px. luzulae in producing comparably shaped and sized conidia, but can be distinguished by lacking swollen dark zones on its conidia that has wrinkled stripes on the conidial surface, and by olivaceous black, rosy buff to grey olivaceous colour on PDA. Paragliomastix chiangraiensis differs from Px. rosea in producing pale white colonies, and having larger conidiophores (71.2–83 μm vs up to 34.5 μm) and conidia [11.9–15.3 × 2.9–4.5 μm vs (5–)5.8–7.5(–8) × (1.8–)2–2.5 μm)] (Hyde et al. 2020a).
Paragliomastix znieffensis (Lechat & J. Fourn.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845830. Fig. 22.
Fig. 22.

Paragliomastix znieffensis (ex-type culture CBS 140584). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at ca. 25°C. D. Conidiophores with conidial chains arising from mycelial ropes. E, G, H. Conidiophores and monophialidic conidiogenous cells. F. Polyphialidic conidiogenous cells. I. Conidia. Scale bars = 10 μm.
Basionym: Hydropisphaera znieffensis Lechat & J. Fourn., Ascomycete.org 8: 55. 2016.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.8–3 μm wide. Sporulation abundant, phalacrogenous, nematogenous or plectonematogenous. Conidiophores solitary or aggregated, erect, straight to flexuous, arising directly from vegetative hyphae or ropes formed by the mycelium, usually reduced to single phialides and unbranched, occasionally with short sterile outgrowths, or basitonously branched and then bearing two phialides per node, up to 74 μm long, with 1–3(–4) septa at the lower part, hyaline at beginning, turning pale brown with age, spinulate in old cultures, thicken and rough-walled at base, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, terminal, subulate, hyaline at first, becoming pale brown with age, thick-, rough-walled, 25.5–46 μm long, 2.5–4.2 μm wide at base, with cylindrical collarette and conspicuous periclinal thickening at conidiogenous loci; polyphialides with up to two conidiogenous loci occasionally present. Conidia aseptate, fusiform, with truncate ends, having prominent, darker, apiculate hilum at one end or both ends, hyaline and smooth-walled at beginning, dark brown and thick-, rough-walled in old cultures, strongly verrucose, 6.3–8.3 × 1.8–3.2 μm, arranged in chains, collapse into heads with age. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colony on OA flat, dispersed on the media, felty and pale olivaceous black at middle, membranous and white at centre and periphery, margin entire, reverse dirty white; On MEA reaching 23–25 mm diam, raised, felty, pale grey and white concentric rings, margin filiform, reverse orange with concentric rings and rust pigment; On PDA flat, dispersed on the media, felty, olivaceous black at centre with sparse whitish aerial mycelium, with dirty white edge, margin entire, reverse concolourous; On SNA reaching 18–20 mm diam, flat, felty, white, slightly olivaceous at centre, margin entire, reverse concolourous.
Description and illustration: Lechat & Fournier (2016a).
Typus: France, Martinique, Ford de France, from leaf of Cyathea sp. (Cyatheaceae), 20 Jun. 2015, C. Lechat, CBS H-24615 (holotype CLLM15060 in LIP, ex-type culture CBS 140584).
Notes: This species was originally described as Hydropisphaera znieffensis from a leaf of Cyathea sp. in Martinique based on its morphological characters along with phylogenetic analysis of its LSU sequences (Lechat & Fournier 2016a). The asexual morph of H. znieffensis resembles the characteristics of gliomastix-like species, Gliomastix fusigera (currently Monohydropisphaera fusigera), but differs in having smaller (6.3–8.3 × 1.8–3.2 μm vs 6.3–17.2 × 5–8.5 μm) and differently shaped conidia (fusiform vs obpyriform to fusiform; Lechat & Fournier 2016a). In the present study, phylogenetic analysis showed that Hydropisphaera is polyphyletic and the ex-type strain of H. znieffensis (CBS 140584) formed a distinct lineage basal to the Paragliomastix clade, distant from the genus Monohydropisphaera. As a consequence, the new combination Px. znieffensis was introduced.
Clade O17
Lasionectria (Sacc.) Cooke, Grevillea 12(no. 64): 111. 1884.
On dead woody and herbaceous substrata including basidiocarps. Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Sexual morph: Ascomata non-stromatic, superficial, orange to dark red-orange or dark brown, slightly darker in KOH but not KOH+, sub-globose to globose, collapsed slightly cupulate when dry or not, often with fasciculate and/or solitary hairs. Ascomatal wall generally more than 20 μm thick, of two regions: outer region of thick-walled, pigmented cells; inner region of elongate, thin-walled, hyaline cells. Asci clavate or cylindrical. Ascospores broadly ellipsoid, 1-septate, hyaline, smooth-walled, warty or with longitudinal striations. Asexual morph (acremonium-like): Sporulation abundant from sporodochia or conidiophores formed directly on the substratal mycelium. Conidiophores arising from submerged or superficial hyphae or from sporodochia in some spieces, (sub-)erect, or irregularly wavy, unbranched or poorly branched, hyaline, smooth-walled, with 1–2(–4) septate, occasionally with short sterile outgrowths, with cell walls usually thicker than those of vegetative hyphae. Conidiogenous cell monophialidic, terminal or lateral, cylindrical, subulate, or aculeate, hyaline, thick-, smooth-walled, with inconspicuous or conspicuous collarette and periclinal thickening at conidiogenous loci. Conidia aseptate, ellipsoid, cylindrical, drop-shaped, obovoid or obpyriform, apiculate at base, rounded at apex, hyaline, thin- or thick-, smooth- or coarse-walled, arranged in slimy heads or in chains, collapse into heads with age. Chlamydospores absent (emended from Rossman et al. 1999).
Type: Lasionectria mantuana (Sacc.) Cooke
Other accepted species with available sequences: Lasionectria antillana (Lechat & Courtec.) Schroers et al., L. atrorubra (Lechat & J. Fourn.) L.W. Hou, L. Cai & Crous, L. bisepta (W. Gams) L.W. Hou, L. Cai & Crous, L. boothii (D. Hawksw.) Lechat & J. Fourn., (Lechat & Gardiennet) L.W. Hou, L. Cai & Crous, L. castaneicola (Lechat & Gardiennet) L.W. Hou, L. Cai & Crous L. cerealis (P. Karst.) L.W. Hou, L. Cai & Crous, L. krabiense Tibpromma & K.D. Hyde, L. olida (W. Gams) L.W. Hou, L. Cai & Crous, L sylvana (Mouton) Rossman & Samuels
Notes: Lasionectria was originally introduced as Nectria subgen. Lasionectria by Saccardo (1883) for nine species of Nectria having hairs on their ascomata. This genus is based on the lectotype L. mantuana, designated by Clements & Shear (1931). Species of Lasionectria are characterised by producing yellow, pale orange, red-orange or dark brown ascomata that do not obviously change colour in 3 % KOH or lactic acid, and fusoid ascospores with striations, thus this genus was demonstrated to belong to the Bionectriaceae as defined by Rossman et al. (1999). Species of this genus are distinguished from other genera in the Bionectriaceae by the ascomatal wall composed of thick-walled cells with a small lumen, over 20 μm thick and by the abundant hairs scattered over the ascomatal surface (Rossman et al. 1999). Acremonium-like asexual morphs of Lasionectria are commonly observed in culture or occur on various woody or herbaceous substrates. Currently, there are 40 epithets listed in Index Fungorum (2020), but most of them lack molecular data. In the present study three Acremonium species, A. biseptum, A. cereale and A. olidum, are transferred to Lasionectria based on the phylogenetic analysis (Fig. 2).
Lasionectria antillana (Lechat & Courtec.) Schroers et al., PLoS ONE 12: e0180032 [24]. 2017.
Basionym: Ijuhya antillana Lechat & Courtec., Mycotaxon 113: 444. 2010.
Description and illustration: Lechat & Courtecuisse (2010).
Typus: French, West Indies, Martinique, Morne Rouge, la Propreté, on dead inflorescence of Heliconia caribaea (Heliconiaceae), 29 Aug. 2007, C. Lechat (holotype CLL7321 in LIP, ex-type culture CBS 122797).
Additional material examined: Unknown, from tropical green seaweed, unknown collection date and collector, dep. P.R. Jensen, culture CBS 114748.
Notes: This species was originally introduced as Ijuhya antillana by Lechat & Courtecuisse (2010). Recently, it was transferred to Lasionectria based on the phylogenetic analysis of the combined act, LSU and rpb1 sequences (Ashrafi et al. 2017). Our phylogenetic analysis based on ITS, LSU, rpb2 and tef-1α confirmed the result of Ashrafi et al. (2017). Ascospores of Lasionectria antillana are striated lengthwise, which are in agreement with the generic characteristics of Lasionectria.
Lasionectria atrorubra (Lechat & J. Fourn.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845831.
Basionym: Nectriella atrorubra Lechat & J. Fourn., Fungal Planet 30: [2]. 2008.
Description and illustration: Lechat & Fournier (2008).
Typus: France, Ariège, Rimont, Las Muros, 42°55’57.46” N, 1°26’36.49” O, alt. 470 m, from decorticated twig of Cornus sanguinea (Cornaceae), 25 Feb. 2005, J. Fournier (holotype JF 05026 in LIP, ex-type culture CBS 123502).
Notes: The ex-type strain of Nectriella atrorubra (CBS 123502) belongs in the genus Lasionectria (Fig. 2). Morphologically, it produces ascospores with longitudinal striations, which fit well with the generic characterisation of Lasionectria (Lechat & Fournier 2008). Nectriella atrorubra is therefore recombined as L. atrorubra.
Lasionectria bisepta (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845832. Fig. 23.
Fig. 23.

Lasionectria bisepta (culture CBS 119908). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidial heads. E. Conidiophore with conidial head. F–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Basionym: Acremonium biseptum W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 107. 1971.
Description based on culture CBS 119908: Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, up to 3 μm wide. Conidiophores solitary, (sub-)erect, straight or irregularly wavy, arising from submerged or superficial hyphae, unbranched or poorly branched, up to ca. 86 μm long, 1.6–4.5 μm wide at base, with two basal septate, hyaline, smooth-walled, rough-walled at basal part, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, 35.5–56 μm long, 2–3.5 μm wide at base, with inconspicuous collarette and periclinal thickening at conidiogenous loci. Conidia aseptate, ellipsoid to cylindrical, with rounded apices and elongated and apiculate bases, hyaline, thin-, coarse-walled, 4–7(–7.5) × 2–2.3 μm, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 55 mm diam, flat, with moderate aerial mycelium, felty or hairy, white, margin entire, reverse concolourous; On MEA reaching 40 mm diam, raised, woolly, floccose, buff, with some brown zones and buff radial lines, margin crenate, reverse rust at centre, orange at periphery; On PDA reaching 34–37 mm diam, flat, floccose and white at centre, powdery or granular and buff at periphery, margin entire, reverse buff, with some pale rust zones.
Typus: Netherlands, North Holland Province, Wieringermeer-polder, from wheat field soil, unknown collection date, isol. May 1966, coll. and isol. W. Gams, No. 371, CBS H-6638 (holotype CBS 750.69 preserved as metabolically inactive culture, ex-type culture CBS 750.69 = VKM F-2899).
Additional materials examined: Netherlands, Flevoland Province, Oostelijk Flevoland, Polder, from wheat field soil, unknown collection date, isol. May 1966, coll. and isol. W. Gams, No. 483, CBS H-8065, culture CBS 751.69; ibid. No. 484, CBS H-8066, culture CBS 752.69; North Holland Province, Wieringermeer-polder, from wheat field soil, unknown collection date, isol. May 1966, coll. and isol. W. Gams, No. 485, CBS H-8067, culture CBS 753.69. USA, Hawaii, Hue St., Kailua-Kona, from montane dry forest of Metrosideros, black stroma of pyrenomycete, 3 Nov. 2002, D.T. Wicklow, CBS H-24698, culture CBS 119908 = NRRL 40197.
Notes: According to Gams (1971), Lasionectria bisepta (basionym: Acremonium biseptum) is morphologically similar to A. olidum (currently Lasionectria olida), A. rutilum (currently Protocreopsis rutila) and A. verruculosum (currently Verruciconidia verruculosa), making it difficult to differentiate among these species. However, A. biseptum has a constant colour in several cultures and regularly has double septation in its phialides (Gams 1971). The ex-type culture clusters with A. olidum in a highly supported clade, both of which are transferred to the genus Lasionectria (Fig. 2). Based on morphological and molecular data, we propose a new combination for this name in Lasionectria.
In the present study, the culture CBS 119908 collected from the black stroma of a pyrenomycete in USA clustered slightly apart from the ex-type strain of Lasionectria bisepta (CBS 750.69) based on 28 bp changes (5 bp differences in ITS, 13 bp in rpb2 and 10 bp of tef-1α) over the four genes. Culture CBS 119908 is morphologically similar to L. bisepta, only with shorter phialides [35.5–56 μm vs 45–65(–75), Gams 1971]. Therefore, we suggest it to be strain variation within L. bisepta.
Lasionectria castaneicola (Lechat & Gardiennet) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845833. Fig. 24.
Fig. 24.

Lasionectria castaneicola (ex-type culture CBS 122792). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial chains and collapsed conidial heads. E–I. Branched conidiophores. J. Conidia. Scale bars = 10 μm.
Basionym: Hydropisphaera castaneicola Lechat & Gardiennet, Bull. Mycol. Bot. Dauphiné-Savoie 49: 5. 2009.
Description and illustration: Lechat & Gardiennet (2009).
Ascomata superficial, solitary or gathered on the substrate, globose to pyriform, with pointed papillae, orange to reddish brown, not changing colour in the KOH or in the lactic acid, smooth, collapsed cupulate when drying, measuring 230–350 μm of diameter. Ascomatal wall 50–80 μm thick, composed of two regions: external region 40–60 μm wide, composed of globose cells measuring 10–30 μm in diameter, with a wall of 1 μm of yellow thickness; internal region 10–20 μm wide, composed of elongated cells with hyaline walls. Asci cylindrical, 70–90 × 6–8 μm, with slightly flattened and thickened apex, without apical ring, containing 8 uniseriate to obliquely uniseriate ascospores. Ascospores ellipsoidal, 8–11(–12) × (4.5–)5–6 μm, hyaline to pale brownish yellow when mature, verrucous, formed by two equal cells with a guttula in each cell, not narrowed at the bulkhead. Basal hyphae few, flexuous, smooth, cloisonate, hyaline, measuring 2.5–3.5 μm in diameter (revised from Lechat & Gardiennet 2009). Asexual morph (acremonium-like): Mycelium consisting of branched, septate, hyaline, pale brown in old culture, smooth-, thin-walled hyphae, up to 3 μm wide. Sporulation abundant from sporodochia or conidiophores formed directly on the substratal mycelium, phalacrogenous or nematogenous. Conidiophores in substratal mycelium erect, straight to flexuous, unbranched or repeatedly basitonously branched, bearing 1–4 levels with 1–3 phialides per node, up to 102.5 μm long, 2–4.5(–5.2) μm wide at base, with 1–3(–4) septa at lower or upper part, hyaline, smooth-walled, occasionally coarse warty at base or lower part, with short sterile outgrowths. Phialides from solitary conidiophores mostly lateral, sub-cylindrical to subulate, hyaline, thick-, smooth-walled, (16–)19–48(–55) μm long, 2–3 μm wide at base, with inconspicuous collarette and conspicuous periclinal thickening at conidiogenous loci. Conidiophores in pale pink or orange cottony sporodochia mostly branched, hyaline, smooth-walled. Sporodochial phialides mostly lateral, aculeate or subulate, tapering on top, hyaline, thick-, smooth-walled, 21–33.5 μm long, 2–2.5 μm wide at base, with inconspicuous, cylindrical collarette and conspicuous periclinal thickening at conidiogenous loci. Conidia from solitary conidiophores and sporodochial conidiophores not significantly different, aseptate, obovoid or obpyriform, hyaline, thin-, smooth-walled, (3.4–)4–5.5(–7.5) × 2–3 μm, eguttulate, arranged in chains, later collapsed to form dry conidial heads. Crystals absent. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 55–60 cm diam, flat, membranous, with sparse aerial mycelium, pale ochreous at centre, creamy white at periphery, margin entire, reverse pale ochreous, strong geosmin odour on media; On MEA reaching 40 mm diam, flat, radially folded, rugose, felty, short hairy, brick at centre, orange in middle, and white at periphery, margin filiform, reverse rust at centre, apricot at periphery, with brown radial lines and rust coloured pigment, without any odour; On PDA reaching 57 cm diam, flat, thinly felty, rugose, pale fawn, with some pale smoke grey zones, margin crenate, reverse pale ochreous, with abundant rust pigment, without any odour; On SNA reaching 60–65 cm diam, flat, membranous sparse aerial mycelium, dusty, colourless, margin entire, reverse colourless, without any odour.
Typus: France, Fixin, Parc Noisot, 7°14’44” N, 4°57’53” E, alt. 350 m, from twig of Castanea sativa (Fagaceae), 22 Nov. 2007, A. Gardiennet, CBS H-24621 [holotype AG07NE6 in LIP, ex-type culture CBS 122792 = CLL 7144 (not CBS 122797 cited in the protologue)].
Notes: This fungus was introduced as Hydropisphaera castaneicola by Lechat & Gardiennet (2009) based on its sexual morph. However, when it was described, the ex-type strain was cited as CBS 122797, which belongs to a culture deposited in the CBS collection as the ex-type culture of Ijuhya antillana. The correct ex-type strain of H. castaneicola should be CBS 122792, recorded as “H. castaneicola” in the CBS collection.
The morphological features of the sexual morph of this species include orange to reddish brown ascomata, perithecial walls over 50 μm thick, and warty ornamentation of ascospores in agreement with the description of the genus Lasionectria (Lechat & Gardiennet 2009). Lasionectria castaneicola differs from other species in producing ellipsoid, warty ascospores, whereas other species of Lasionectria produce fusoid ascospores with striate ornamentation. According to the phylogenetic analysis in the current study, the ex-type strain of L. castaneicola (CBS 122792) clusters within the Lasionectria clade (Fig. 2). Based on the morphological and molecular evidence, this species is transferred to Lasionectria. The asexual morph of L. castaneicola is described and illustrated, thus providing a holomorphic species concept.
Lasionectria cerealis (P. Karst.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845834.
Basionym: Coniosporium cerealis P. Karst., Meddeland. Soc. Fauna Fl. Fenn. 14: 109. 1887.
Synonyms: Gliomastix guttuliformis J.C. Br. & W.B. Kendr., Trans. Brit. Mycol. Soc. 41: 499. 1958.
Gliomastix cerealis (P. Karst.) C.H. Dickinson, Mycol. Pap. 115: 19 (1968).
Acremonium cerealis (P. Karst.) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 88. 1971.
Fusarium cerealis (P. Karst.) Gruyter & J.H.M. Schneid., Jaarboek. Plantenziektenkundige Dienst, Wageningen 1989/1990, no. 168: 135. 1991. [nom. inval., Art. 41.4 (Melbourne)].
Lasionectria hilhorstii L. Lombard, Persoonia 41: 339. 2018.
Descriptions and illustrations: Gams (1971), Crous et al. (2018a).
Materials examined: Canada, culture contaminant, obtained while isolating from Hebeloma crustuliniforme tissue M 196, unknown collection date and collector, isol. 4 Sep. 1985 by A. Flis, CBS H-24622, culture CBS 461.88 = UAMH 5271. Germany, Kiel-Kitzeberg, from wheat field soil, unknown collection date and collector, isol. 1963 by W. Gams, No. C 986, culture CBS 209.70, ibid. No. C 281, CBS H-24623, culture CBS 207.70; ibid. No. C 602, CBS H-8115, culture CBS 208.70; Schleswig-Holstein, Kiel-Kitzeberg, from wheat field soil, unknown collection date and collector, isol. 1964 by W. Gams, No. C 985, culture CBS 393.66 = MUCL 9408. Norway, Troms County, Nordkapp municipality, Skarsvåg, Mefjorden, from deciduous driftwood in splash zone, 17 Aug. 2010, T. Rämä, culture TR 055cII1.1, ibid. culture TR 055cI1.1. Netherlands, from agricultural soil, unknown collection date and collector, isol. 10 Jan. 1969 by J.H. van Emden, No. 369, culture CBS 216.69; Gelderland Province, Eibergen, from soil, Mar. 2017, T. Hilhorst (holotype of Lasionectria hilhorstii CBS H-23747, ex-type culture CBS 144938 = JW85024). UK, England, Cheshire, Delamere Forest, from leaf litter of Pinus sylvestris (Pinaceae), unknown collection date and collector, isol. Dec. 1956 by W.B. Kendrick, CBS H-8114, culture CBS 206.65; Cheshire, Delamere Forest, from needle of Pinus sylvestris (Pinaceae), unknown collection date and collector, isol. Dec. 1956 by W.B. Kendrick, culture CBS 227.66 = MUCL 9407; Northumberland, Bamburgh, from sand dune soil, unknown collection date and collector, isol. Mar. 1955 by J.C. Frankland, No. D 1889 (ex-type culture of Gliomastix guttuliformis CBS 207.65 = IAM 14644 = IMI 061279 = MUCL 9443); Ireland, Co. Westmeath, Tyrellspass, from esker soil, unknown collection date and collector, isol. Feb. 1966 by E.M. Rix, No. G 23, culture CBS 215.69 = IMI 137295.
Notes: Lasionectria cerealis was originally described as the basionym Coniosporium cerealis, collected from leaves of Secalis cerealis in Finland (Karsten 1887). Later this species was transferred to the genus Gliomastix as G. cerealis (Dickinson 1968). This species is morphologically characterised by producing drop-shaped conidia, a green-black colony on malt agar and phialides occasionally with flared collarettes (Dickinson 1968). Although not all cultures used in this study match the original host and location of L. cerealis, their morphological characters agree well with the description of L. cerealis from literature (Gams 1971). Phylogenetically, all cultures formed a fully supported clade nestled in the same clade with other known Lasionectria species (Fig. 2), and the strain CBS 208.70 is considered a good representative of the species based on its morphological characteristics.
Lasionectria hilhorstii was described from soil in the Netherlands, morphologically resembling Lasionectria cerealis (Gams 1971), but differed in the production of the phialides that lack flared collarettes and basal swelling (Crous et al. 2018a). In the present study, the ex-type strain of L. hilhorstii (CBS 144938) together with CBS 393.66 clustered slightly apart from other L. cerealis cultures based on 4 bp changes over the available sequences of three genes. Therefore, we suggest that the lack of flared collarette in L. hilhorstii should be considered strain variation among cultures of L. cerealis. The presence of a collarette may not be informative at the species levels. This idea also corresponded well with the comments of Dickinson et al. (1968), stating that “The collarette may be seen only with difficulty in some collections”.
Lasionectria mantuana (Sacc.) Cooke, Grevillea 12 (no. 64): 112. 1884.
Basionym: Nectria mantuana Sacc., Michelia 1 (no. 1): 52. 1877.
Descriptions & Illustrations: Weese (1916), Rossman et al. (1999).
Typus: Italy, Mantova, Migliaretto, on decorticated poplar wood, Feb. 1873, A. Magnaguti-Rondinini (holotype PAD S00014).
Materials examined: Finland, Lempäälä, Innilä, from decorticated wood, possibly Populus sp. (Salicaceae), 27 Sep. 2003, U. Söderholm, BPI 843540, culture CBS 114291 = AR4029.
Note: This species is the type of Lasionectria.
Lasionectria olida (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845836. Fig. 25.
Fig. 25.

Lasionectria olida (ex-type culture CBS 799.69). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Slimy conidial heads. E–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Basionym:Acremonium olidum W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 108. 1971.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, up to 2.6 μm wide. Conidiophores solitary, (sub-)erect, arising from submerged or superficial hyphae, unbranched or basitonously branched, bearing 1–2 levels with 1–2 phialides per node, occasionally with short sterile outgrowths, up to ca. 92 μm long, 2–4.5 μm wide at base, with 1–3 septa at base or upper part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, 17.5–50(–63) μm long, 1.5–3 μm wide at base, with inconspicuous collarette and periclinal thickening at conidiogenous loci. Conidia aseptate, ellipsoidal with rounded ends, or with a weak apiculate base, hyaline, thin-, or thick-, smooth-walled, 3.5–5.7 × 2–2.75 μm, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 39 mm diam, flat, membranous with sparse aerial mycelium, dirty white, margin entire, reverse concolourous; On MEA reaching 27–30 mm diam, flat, spirally rugose, membranous, short hairy, orange at centre, buff to dirty white at periphery, margin entire, reverse orange; On PDA reaching 30 mm diam, flat, floccose, with greyish rose, salmon, rosy buff concentric rings, margin entire, with brown pigment, reverse with rust, apricot and saffron rings. Strong, pleasantly aromatic smell.
Typus: Poland, Augustow, from Phellinus sp. (Hymenochaetaceae), together with Ceratocystis sp. (Ceratocystidaceae) and Hypocrea pulvinata (Hypocreaceae), unknown collection date and collector, isol. Sep. 1966 by W. Gams, No. 633, CBS H-6660 (holotype CBS 799.69 preserved as metabolically inactive culture, ex-type culture CBS 799.69).
Additional material examined: Austria, Tirol, Kranebitter Klamm, from Fomitopsis pinicola (Fomitopsidaceae) on Picea abies (Pinaceae), unknown collection date and collector, isol. Dec. 1965 by W. Gams, No. 590, CBS H-8268, culture CBS 798.69.
Notes: Based on the description provided by Gams (1971), the fungus formerly known as Acremonium olidum clustered in the genus Lasionectria (BPP/MLBS = 1/100 %), supported by phylogenetic inference in this study (Fig. 2). Therefore, a new combination is provided in the genus Lasionectria.
Lasionectria sylvana (Mouton) Rossman & Samuels, Stud. Mycol. 42: 37. 1999. Fig. 26.
Fig. 26.

Lasionectria sylvana (culture CBS 566.76). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, F–J. Conidiophores. E. Conidial heads. K. Conidia. Scale bars =10 μm.
Basionym: Nectria sylvana Mouton, Bull. Soc. Roy. Bot. Belgique. 39: 49. 1900.
Synonym: Calonectria fimbriata Seaver & Waterston, Mycologia 32: 404. 1940.
Illustrations: Samuels (1976a, fig. 14; 1976b, fig. 18, both as N. sylvana); Seaver & Waterston (1940, fig. 3 lower as C. fimbriata); Rossman et al. (1999).
Description based on culture CBS 566.76: Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, up to 2.5 μm wide. Conidiophores solitary, (sub-)erect, arising from submerged or superficial hyphae, unbranched, rarely branched, occasionally with a basally sterile short outgrowth, up to ca. 55 μm long, 2.5–2.5 μm wide at base, with 1–2 septa at base, hyaline, smooth-walled, rough-walled at basal part, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or aculeate, hyaline, thick-, smooth-walled, 16.5–40.7 μm long, 1.8–3.3 μm wide at base, with inconspicuous collarette and periclinal thickening at conidiogenous loci. Conidia aseptate, cylindrical, without visible basal abscission scar, hyaline, thin-, smooth-walled, (2.5–)4–8.5 × 1.7–2.6 μm, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 36–37 mm diam, flat, membranous with sparse aerial mycelium, dusty at periphery, dirty white at centre, white at periphery, margin entire, reverse concolourous; On MEA reaching 30–35 mm diam, flat, radially folded, felty, saffron at centre, buff to creamy white at periphery, margin entire, reverse pale orange at centre, pale luteous at periphery, with buff radial lines; On PDA reaching 22–24 mm diam, flat, membranous with sparse aerial mycelium, buff, margin entire, reverse buff, with creamy white radial lines.
Typus: Belgium, near Liege, on stems of Angelica sylvestris (Apiaceae), unknown collection date and collector (holotype of N. sylvana NY01013209).
Material examined: New Zealand, Westland Province, Westland County, vic. Lake Ianthe, Lake Ianthe State Forest, SF 42, from rachis of Cyathea smithii (Cyatheaceae), unknown collection date, G.J. Samuels, G.J.S. 74-75, CBS H-15071, culture CBS 566.76.
Clade O18
Verruciconidia L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845837.
Etymology: Referring to the verrucose conidia produced by species in this genus.
Mycelium consisting of branched, septate, hyaline or pale brown, rough-, thin-walled hyphae, forming bundles or coils. Conidiophores (sub-)erect, straight or curved, arising from vegetative hyphae or from ropes formed by the mycelium, unbranched, poorly branched, septate at base or middle part, hyaline, smooth- or rough-walled, with cell walls usually thicker than those of vegetative hyphae. Conidiogenous cell monophialidic, terminal or lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, with conspicuous or inconspicuous collarette and periclinal thickening at conidiogenous loci; adelophialides occasionally present in some species, differentiating intercalary as short lateral protrusions usually from submerged hyphae. Conidia aseptate, ovoid, (broad) ellipsoid, subglobose, rounded at both ends, or with apiculate end(s), straight, hyaline, thick- and rough-walled, verrucose, or thin- and smooth-walled, eguttulate or guttulate, arranged in conidial dry heads or long chains. Chlamydospores and sexual morph not observed.
Type: Verruciconidia verruculosa (Nicot) L.W. Hou, L. Cai & Crous
Other accepted species with available sequences: Verruciconidia erythroxyli L.W. Hou, L. Cai & Crous, Ve. infuscata L.W. Hou, L. Cai & Crous, Ve. persicina (Nicot) L.W. Hou, L. Cai & Crous, Ve. quercina L.W. Hou, L. Cai & Crous, Ve. siccicapita L.W. Hou, L. Cai & Crous, Ve. unguis L.W. Hou, L. Cai & Crous
Notes: The genus Verruciconidia is proposed here for a group of cultures clustering in a fully supported clade in Bionectriaceae. Most of these cultures were previously identified as A. persicinum and A. verruculosum based on morphological characters. However, they are phylogenetically heterogeneous and distributed in different clades within the large family clade (Fig. 2). Thirteen “A. persicinum” strains, including the ex-type CBS 310.59 form a well-supported lineage, representing A. persicinum s. str. The other strains are located in other clades representing five different species (Fig. 2). Species in Verruciconidia are different from other genera in Bionectriaceae by producing warty conidia, except for Ve. erythroxyli, Ve. persicina and Ve. unguis, which produce smooth-walled conidia.
Verruciconidia erythroxyli L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845838. Fig. 27.
Fig. 27.

Verruciconidia erythroxyli (ex-type culture CBS 728.87). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial chains. E–H. Unbranched conidiophores. I, J. Branched conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the host, Erythroxylum areolatum, from which the holotype was collected.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 3 μm wide, forming bundles. Conidiophores solitary or aggregate, (sub-)erect, arising from submerged and superficial hyphae, or from ropes formed by mycelium, unbranched, poorly branched, bearing 1–3 phialides per node, up to ca. 45 μm long, 1.8–3.3 μm wide at base, with 1–3 septa, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, (15–)19.5–31.5(–37) μm long, 2–3 μm wide at base, with inconspicuous collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, broad ellipsoid, occasionally with a slightly apiculate bases and rounded apices, hyaline, thin-, smooth-walled, 3.5–4.5 × 2–3 μm, with minute guttules, arranged in dry chains, soon collapsing, forming conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 36–39 mm diam, flat, felty or dusty, white, margin fimbriate, reverse concolourous; On MEA reaching 28–30 mm diam, flat, radially folded, shortly hairy, white, margin crenate, reverse pale orange, with buff radial lines; On PDA reaching 35–38 mm diam, flat, felty, hairy at centre, white, margin filiform, reverse buff, brown at centre.
Typus: Cuba, Matanzas Province, San Miguel de los Baños, from leaf of Erythroxylum areolatum (Erythroxylaceae), unknown collection date and collector, isol. 22 Jan. 1987 by R.F. Castañeda, C87/10 (holotype CBS H-24612, ex-type culture CBS 728.87).
Additional material examined: Zaire, Bas-Zaire, Nsangi, from leaf of Urena lobata (Malvaceae), isol. 1964, isol. G.L. Hennebert, CBS H-24689, culture CBS 378.70D = MUCL 6269.
Notes: Verruciconidia erythroxyli is represented by two strains from different host plants, Erythroxylum areolatum (Erythroxylaceae) and Urena lobata (Malvaceae). This species is phylogenetically close to another new species, Ve. unguis described in this study (Fig. 2), and their morphological differences are discussed under the latter species. Although both species differ from other Verruciconidia species in their smooth-walled conidia, they are phylogenetically congeneric within Verruciconidia.
Verruciconidia infuscata L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845839. Fig. 28.
Fig. 28.

Verruciconidia infuscata (ex-type culture CBS 100888). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidia heads arising from mycelial ropes. E–H. Conidiophores. I. Brown hyphae. J, K. Conidia. Scale bars = 10 μm.
Etymology: Derived from the brown hyphae produced by this species.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, brown and thick-walled in older culture, up to 2.5 μm wide, forming bundles and coils. Conidiophores solitary or aggregate, (sub-)erect, arising from submerged and superficial hyphae, or from ropes formed by the mycelium, unbranched or rarely branched, up to ca. 64.5 μm long, 1.6–2.8 μm wide at base, with 1–2 septa in basal part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides monophialidic, mostly lateral, rarely terminal, subcylindrical or subulate, hyaline, thick-, smooth-walled, 38–50.5 μm long, 1.8–2.5 μm wide at base, with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci. Conidia aseptate, ellipsoid, subglobose, rounded at both ends, or with an inconspicuous truncate bases, hyaline, thick-, rough-walled, warty, 3–5 × 2–2.8 μm, with 1–2 large guttules, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 47–48 mm diam, flat, sparse aerial mycelium, membranous, pale olivaceous buff at centre, with dirty white margin, margin entire, pale brown pigment present in old cultures, reverse olivaceous buff, buff at centre; On MEA reaching 43 mm diam, flat, radially folded, with moderate aerial mycelium, felty, creamy white, margin lobulated, pale brown pigment present in old cultures, reverse pale orange, with buff radial lines; On PDA reaching 57–58 mm diam, flat, sparsely floccose, dirty white, few shallow striations over the colony surface, margin filiform, pale brown pigment present in old cultures, reverse creamy white; On SNA reaching 50 mm diam, flat, membranous without aerial mycelium, colourless, margin entire, reverse colourless.
Typus: Japan, Kanagawa Pref., from air, unknown collection date and collector, isol. M. Sakamoto, No. 94 (holotype CBS H-24613, ex-type culture CBS 100888).
Notes: Verruciconidia infuscata is placed on a single branch, nestling in the same clade with Ve. erythroxyli, Ve. siccicapita and Ve. unguis (Fig. 2). The species can be morphologically distinguished from Ve. erythroxyli and Ve. unguis by producing warty conidia arranged in moist slimy heads, while the last two species produce smooth-walled conidia that are arranged in chains but later collapse into conidial heads; Ve. infuscata differs from Ve. siccicapita by producing longer conidiophores (up to 64.5 μm vs up to 41 μm) and phialides (38–50.5 μm vs 25–36 μm). In addition, conidia of Ve. infuscata are arranged in moist slimy heads while Ve. siccicapita produces conidia arranged in dry conidial heads.
Verruciconidia persicina (Nicot) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845840. Fig. 29.
Fig. 29.

Verruciconidia persicina (ex-type culture CBS 310.59). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial chains. E–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Basionym: Paecilomyces persicinus Nicot, Bull. Soc. Mycol. France 74: 222. 1958.
Synonym: Acremonium persicinum (Nicot) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 75. 1971.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 3.5 μm wide, forming bundles. Sporulation abundant, phalacrogenous, nematogenous, plectonemtogenous. Conidiophores solitary or aggregate, (sub-)erect, straight to flexuous, irregularly bent at lower part, arising from submerged and superficial hyphae, or ropes formed by mycelium, unbranched or repeatedly branched, bearing 1–3 phialides per node, commonly with short sterile outgrowths, up to ca. 61(–118) μm long, 1.8–3 μm wide at base, with 1–3 septa in basal, middle and apical part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or subulate, irregularly bent, hyaline, thick-, smooth-walled, 12–36 μm long, 1.8–2.7 μm wide at base, with minute collarette and inconspicuous periclinal thickening at conidiogenous loci, polyphialides not observed. Conidia aseptate, ellipsoid, ovoid, occasionally with slightly apiculate basal end, hyaline, thin-, smooth-walled, 4.2–6.2 × 2.4–3 μm, eguttulate or with one large guttule, arranged in dry chains, soon collapsing. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 40–42 cm diam, flat, with sparse aerial mycelium, dusty, white, pale brown with age, margin entire, reverse concolourous; On MEA reaching 33–35 diam, flat, radially fold, with moderate aerial mycelium, floccose or dusty, salmon at centre, rosy buff at periphery, margin crenate, reverse saffron, with buff radial lines; On PDA reaching 40–42 cm diam, flat, with moderate aerial mycelium, felty, granular or dusty, dirty white or rosy buff, margin entire, reverse creamy white; On SNA reaching 42–45 cm diam, flat, with sparse aerial mycelium, dusty, colourless, margin entire, reverse colourless. Without odour on all media.
Description and illustration: Gams (1971).
Typus: France, Arcachon, from coastal sand under Ammophila arenaria (Poaceae), unknown collection date, J. Nicot, CBS H-6661 (ex-isotype culture of Paecilomyces persicinus CBS 310.59 = ATCC 18551 = IAM 14656 = IMI 069374 = LCP 56.1537).
Additional materials examined: Iran, Golestan Province, Gorgan, endophyte in Festuca ovina (Poaceae), unknown collection date and collector, isol G. Dehghampour, culture CBS 116385; Āz¯arbāyjān-e Sharqī Province, Tabriz, from Pleurotus ostreatus (Pleurotaceae), 2002, M.R. Asef, culture CBS 113716. Italy, from leaf of Vitis vinifera (Vitaceae), inoculated with zoospores of Plasmopara viticola (Peronosporaceae), unknown collection date, S. Burruano, Inst. Patol. Veget., Palermo, culture CBS 101712; from soil, unknown collection date and collector, isol. M.A. Pisano, No. 10C, culture CBS 169.65. Papua New Guinea, Bougainville Island, from tent canvas, unknown collection date and collector, isol. 1944, CBS H-8280, culture CBS 295.70A = QM 1b; Madang, Jais Aben, from soil along coral reef coast, Nov. 1995, A. Aptroot, isol. Nov. 1995 by A. van Iperen, No. A 152, culture CBS 218.96. USA, Hawaii, (Milepost 43) Hgw. 200 Pu’u La’au, subalpine dry forest, from a white mycelium growth of a wood decay fungus on the undersurface of a dead hardwood branch, 4 Nov. 2002, D.T. Wicklow, isol. 30 May 2002, MYC 1794, culture CBS 120889; Iowa, Steele Prairie, Cherokee County, T93N R40W S16, from soil, 1982, unknown collector, culture CBS 128826 = RMF 7540; Kansas, Konza Prairie Research Natural Area, Long Term Ecological Research site (LTER), near Manhattan, from soil, 1986, unknown collector, culture CBS 127298 = RMF 8134; Minnesota, Steele, from cyst of Heterodera glycines (nematode, Heteroderidae), unknown date, Fajun Chen, S2-81, culture CBS 102349; unknown location, from canvas legging, unknown collection date and collector, isol. 1959, CBS H-8281, culture CBS 295.70B = QM 89c. USSR, from pasture soil, unknown collection date and collector, isol. Institute of Microbiology, Moskva, CBS H-8275 & CBS H-8276, culture CBS 439.66 = VKM F-888.
Notes: Based on the multi-locus phylogenetic analyses, 13 cultures labelled as Acremonium persicinum, including its ex-isotype strain (CBS 310.59), form a fully supported clade within the clade representing the new genus Verruciconidia (Fig. 2). Acremonium persicinum is therefore transferred to Verruciconidia, and a new combination is proposed. Our morphological description of the ex-type culture slightly differs from the one in Gams (1971) by having repeatedly branched conidiophores and lacking elongated crystals. Some cultures are described as having ochreous brown to reddish brown mycelium in Gams (1971), while we only observed a white or rosy buff colour on OA and PDA media and salmon colour on MEA plates. These differences may be due to intraspecies variation or are related to culture conditions.
Verruciconidia quercina L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845841. Fig. 30.
Fig. 30.

Verruciconidia quercina (ex-type culture CBS 469.67). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial heads. F–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to Quercus, the host genus from which the ex-type culture of this fungus was isolated.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 2.7 μm wide, forming bundles. Conidiophores solitary or aggregate, (sub-)erect, straight, arising from submerged and superficial hyphae, or from ropes formed by the mycelium, unbranched or poorly branched, up to ca. 49 μm long, 1.9–3.7 μm wide at base, with 1–3-septa in basal part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, 12.8–43.5 μm long, 1.3–2.7 μm wide at base, with inconspicuous collarette and periclinal thickening at conidiogenous loci; polyphialides not observed; adelophialides present, differentiating intercalary as short lateral protrusions usually from submerged hyphae, more or less cylindrical, up to 16 μm diam. Conidia aseptate, ellipsoid, without obviously apiculate base, hyaline, thick-, rough-walled, verrucose, 2.8–5.2 × 2.5–3.5 μm, eguttulate, arranged in dry heads, conidial heads becoming pale brown in older culture. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 40–42 mm diam, flat, dusty, dirty white at centre, creamy white at periphery, margin entire, reverse saffron at centre, buff at periphery; On MEA reaching 29–30 mm diam, flat, radially folded, short hairy, white, margin filiform, reverse saffron with buff radial lines; On PDA reaching 43–45 mm diam, flat, thinly felty, dirty white, with pale greenish zones, margin entire, reverse buff.
Typus: Unknown, from leaf litter of Quercus sp. (Fagaceae), unknown collection date and collector, isol. 1965 by T. Hering, No. 642 (holotype CBS H-8278, culture CBS 469.67).
Additional materials examined: Netherlands, North Brabant Province, Haren, from agricultural soil, unknown collection date and collector, isol. G. Jager, No. M41, culture CBS 355.77; South Holland Province, Katwijk, Lysimeter 1, from sand dune soil, 95 cm depth in lysimeter at upper limit of art, Jan. 1978, W. Gams, No. 1/95 19, CBS H-1038, culture CBS 183.78.
Notes: The three cultures of Verruciconidia quercina form a distinct basal clade of Verruciconidia on the multi-locus tree (Fig. 2). Morphologically, it differs from all other species in Verruciconidia in producing short lateral adelophialides. This is the first report of acremonium-like species with verrucose conidia from Quercus spp
Verruciconidia siccicapita L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845842. Fig. 31.
Fig. 31.

Verruciconidia siccicapita (ex-type culture CBS 378.70A). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial heads. E. Conidial heads. F. Conidiophores arising from mycelial ropes. G, H. Conidiophores. I. Conidia. Scale bars = 10 μm.
Etymology: Latin, siccus = dry, caput = head, referring to the dry conidial heads produced by this fungus.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 1.7 μm wide, forming thick bundles. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregate, (sub-)erect, straight to flexuous, curved at base part, arising from submerged and superficial hyphae, or from ropes formed by the mycelium, unbranched, up to 41 μm long, 1.3–3.2 μm wide at base, with single septum at base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides most lateral, rarely terminal, cylindrical or subulate, hyaline at first, becoming pale brown with age, thick-, smooth-walled, 25–36 μm long, 1.2–2.6 μm wide at base, with flared collarette and inconspicuous periclinal thickening at conidiogenous loci. Conidia aseptate, ellipsoid or subglobose, rounded at both ends, hyaline, thick- and finely verruculose, 3.5–5 × 2.3–3 μm, guttulate, arranged in dry conidial heads, hyaline, becoming pale brown with age. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 45 cm diam, flat, with sparse aerial mycelium, dusty or membranous, dirty white, with some hairy mycelium, become fawn with age, margin entire, reverse pale fawn, later become fawn, without any odour; On MEA reaching 43 diam, flat, with moderate aerial medium, hairy, rosy buff, later become dusty or hairy and brick, margin entire, reverse pale apricot, with buff radial lines, with strong pungent odour; On PDA reaching 60 mm diam, flat, with sparse aerial mycelium, dusty, dirty white, few shallow striations over the colony surface, buff at periphery, margin fimbriate, reverse pale olivaceous buff, strong “rotten wood” odour; On SNA reaching 40–42 cm diam, flat, with sparse aerial mycelium, dusty, colourless, margin entire, reverse colourless, without any odour.
Typus: Thailand, from soil, unknown collection date and collector, isol. J. Nicot, No. BSA3 (holotype CBS H-8298, ex-type culture CBS 378.70A = LCP 2149).
Notes: Verruciconidia siccicapita is represented by a single culture isolated from soil in Thailand and differs from all other species according to the multi-locus tree (Fig. 2). It is phylogenetically different from the closely related species Ve. infuscata (98.2 % sequence similarity on ITS, 99.7 % on LSU, 91.8 % on rpb2, 98.2 % on tef-1α). Their morphological differences are discussed under Ve. infuscata.
Verruciconidia unguis L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845843. Fig. 32.
Fig. 32.

Verruciconidia unguis (ex-type culture CBS 424.93). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores and conidia chains. F–I. Conidiophores J. Conidia. Scale bars = 10 μm.
Etymology: Referring to the substrate from which the holotype culture was collected.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, up to 3.2 μm wide, forming bundles. Conidiophores (sub-)erect, arising from submerged and superficial hyphae, or from ropes of hyphae, unbranched, up to ca. 40.5 μm long, 1.5–3.5 μm wide at base, with 1–2 septa in basal part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or subulate, occasionally acuminate at top, hyaline, thick-, smooth-walled, (20.5–)22–34(–39) μm long, 2–3 μm wide at base, with inconspicuous collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, obovoid, subglobose, ellipsoid, with an inconspicuous apiculate bases, and a rounded apices, hyaline, thin-, smooth-walled, with 1–2 large guttules, 3.2–4 × 2.3–2.9 μm, arranged in dry chains and soon collapse as conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 43–45 mm diam, flat, dusty, granular, dirty white, margin fimbriate, reverse creamy white; On MEA reaching 43–45 mm diam, raised, radially folded, finely floccose, dirty white, margin crenate, reverse pale orange, with buff radial lines; On PDA reaching 45 mm diam, flat, felty or dusty, dirty white, pale fawn at centre, margin fimbriate, reverse orange at centre, buff at periphery; On SNA reaching 48 mm diam, flat, dusty, with concentric rings, dirty white, margin entire, reverse concolourous.
Typus: Netherlands, South Holland Province, Leiden, from human nail, unknown collection date, Diaconessen Hospital, No. 8192 (holotype CBS H-24611, ex-type culture CBS 424.93).
Additional material examined: Austria, Linz, from human nail, unknown collection date and collector, isol. 1968 by Kolesaric, CBS H-8301, culture CBS 378.70E.
Notes: Two cultures from nails of humans formed a fully supported and distinct clade on the tree (BPP/MLBS = 1/100; Fig. 2). Verruciconidia unguis is phylogenetically different from the closely related species Ve. erythroxyli (97 % sequence similarity on ITS, 92.8 % on rpb2, 96.7 % on tef-1α). Morphologically, Ve. unguis is similar to Ve. erythroxyli in the size and shape of phialides and conidia. However, it differs from the latter in producing unbranched conidiophores, and conidia with large guttules. Conidiophores of Ve. erythroxyli are often branched and the conidia are eguttulate.
Verruciconidia verruculosa (W. Gams & Veenb.-Rijks) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845844. Fig. 33.
Fig. 33.

Verruciconidia verruculosa (ex-type culture CBS 989.69) A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial heads. E–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Basionym: Acremonium verruculosum W. Gams & Veenb.-Rijks, Cephalosporium-artige Schimmelpilze (Stuttgart): 108. 1971.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 2 μm wide, forming mycelial ropes or coils. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores (sub-)erect, arising from vegetative hyphae or from ropes formed by the mycelium, unbranched, up to ca. 46.5 μm long, 2.5–3 μm wide at base, with single septum at base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal or lateral, cylindrical or subulate, straight or slightly curved, hyaline, thick-, smooth-walled, 25.5–43.5 μm long, 2–3 μm wide at base, with inconspicuous collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, ellipsoid, symmetrically rounded, hyaline, thick-, rough-walled, warty, 4.5–6.2 × 2.5–3.5 μm, with 1–2 large guttules, arranged in conidial dry heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: colonies on OA reaching 37–38 cm diam, flat, dusty, granular, dirty white, margin entire, with pale vinaceous pigment, reverse buff, rosy buff at periphery; On MEA reaching 30–32 diam, flat, felty and granular, radially folded, creamy white, pale vinaceous at periphery, margin crenate, reverse apricot, with buff radial lines; On PDA reaching 41–43 cm diam, flat, thinly felty, dirty white at centre, pale vinaceous at periphery, with concentric circles, margin fimbriate, reverse saffron; With vinaceous pigment on all media.
Typus: Netherlands, from agricultural soil, unknown collection date, J.W. Veenbaas-Rijks, No. 13711, CBS H-6672 (holotype CBS 989.69 preserved as metabolically inactive culture, ex-type culture CBS 989.69).
Additional materials examined: Netherlands, Flevoland Province, Oostelijk Flevoland, from agricultural soil, unknown collection date and collector, isol. 29 Sep. 1969 by J.W. Veenbaas-Rijks, CBS H-8466, culture CBS 990.69 = IPO 357; Friesland Province, Sexbierum, from clay soil under Solanum tuberosum (Solanaceae), unknown collection date and collector, isol. Feb. 1981 by G. Jager, No. 214, culture CBS 299.81B.
Notes: Verruciconidia verruculosa was originally described as Acremonium verruculosum by Gams (1971) from agricultural soil in the Netherlands. According to our phylogenetic inference, the ex-type of A. verruculosum (CBS 989.69), together with a number of cultures that were mostly labelled as A. persicinum fall in a fully supported clade representing the new genus Verruciconidia in Bionectriaceae, and are distant from Acremonium s. str. (Fig. 2). Verruciconidia verruculosa is distinct from its most closely related species V. persicina in producing vinaceous pigment on all media and in its noticeably coarse, warty chromophilic conidia in contrast to the smooth-walled conidia of Ve. persicina. Verruciconidia verruculosa is also morphologically similar to A. breve, A. pinkertoniae and A. spinosum in producing fine warty conidia (Gams 1971), but these species fall into four phylogenetically distant clades: A. breve was transferred to Parasarocladium in Sarocladiaceae (Summerbell et al. 2018); and A. pinkertoniae and A. spinosum are placed in Bulbithecium according to the phylogenetic analysis in this study (Fig. 2).
Clade O19
Lasionectriopsis Lechat & P.-A. Moreau, Ascomycete.org 11: 1. 2019.
Ascomata gregarious, non-stromatic, globose, whitish or pale yellow to pale orange becoming brownish orange, and cupulate or laterally pinched when dry, semi-immersed in a whitish subiculum, septate hyphae proliferating to cover ascomatal wall except ostiolar region. Perithecial apex with ostiolar opening, conical, slightly darker than venter, composed of subglobose to narrowly clavate cells with pale orange wall, merging with periphyses. Ascomatal wall of a single region composed of subglobose to globose or ellipsoidal cells, with pale yellow walls. Asci unitunicate, clavate, cylindrical, short-stipitate, with 8 ascospores biseriate or irregularly disposed in upper part, uniseriate in lower part, apex rounded with a ring. Paraphyses evanescent between asci, filamentous to narrowly moniliform. Ascospores narrowly ellipsoidal to fusoid, 1-septate, slightly constricted or not at septum, verruculose or finely spinulate, hyaline or pale yellow. Hyphae septate, occasionally chondroid. Conidiophores monophialidic, simple or branched, septate, flexuous, smooth, chromophilic granular substance could be present. Conidiogenous cell simple, slightly bant or wavy, septate at base, cylindrical or subulate with an unflared collarette. Conidia obovoid, ellipsoidal, subcylindrical or subglobose to globose, hyaline, smooth or finely warty, aseptate, with rounded apex, attenuated at base with a basal abscission scar, grouped at tip of phialides to form a mucous head. Chlamydospores not observed. Crystals could be present (emended from Lechat et al. 2019).
Type: Lasionectriopsis germanica Lechat et al.
Other accepted species with available sequences: Lasionectriopsis dentifera (Samuels) L.W. Hou, L. Cai & Crous, Lasionectriopsis pteridii (W. Gams & J.C. Frankland) Lechat & P.-A. Moreau
Notes: The genus Lasionectriopsis was characterised by whitish to pale orange, globose ascomata, semi-immersed in a subiculum, and verruculose ascospores (Lechat et al. 2019). Although Acremonium spinosum was classified as a member of Lasionectriopsis (Lechat et al. 2019), it is placed in Bulbithecium as Bu. spinosum based on the corrected sequences of ex-type strain CBS 136.33 in this study (Fig. 2).
Lasionectriopsis dentifera (Samuels) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845845. Fig. 34.
Fig. 34.

Lasionectriopsis dentifera (culture CBS 650.75). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial heads. E. Conidial heads. F, G. Monophialides. H, I. Branched conidiophores. J, K. Conidia. Scale bars = 10 μm.
Basionym: Nectria dentifera Samuels, New Zealand J. Bot. 14: 253. 1976.
Synonyms: Peristomialis dentifera (Samuels) Samuels, Mem. New York Bot. Gard. 48: 18. 1988.
Ijuhya dentifera (Samuels) Rossman & Samuels, Stud. Mycol. 42: 35. 1999.
Description based on culture CBS 650.75: Mycelium consisting of branched, septate, hyaline, pale brown with age, rough-, thin-walled hyphae, up to 2.8 μm wide. Conidiophores (sub-)erect, straight, arising from submerged and superficial hyphae, unbranched or basitonously branched, bearing 1–3 phialides per node, up to ca. 61 μm long, 1.5–3.5 μm wide at base, with 1–2-septa in basal and middle areas, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides monophialidic, terminal or lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, 17–48 μm long, 1.5–2.5 μm wide at base, with inconspicuous cylindrical collarette and periclinal thickening at conidiogenous loci. Conidia aseptate, globose to subglobose, or ellipsoid, without obviously apiculate scar, hyaline, thick-, rough-walled, 3–4 × 2.5–3.5 μm, arranged in dry conidial heads. Crystal present, yellow, subglobose or irregular shape. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 31–35 mm diam, flat, membranous without aerial mycelium, slightly hairy at centre, buff, margin filiform, abundant yellow crystal, reverse pale olivaceous buff; On MEA reaching 22–23 mm diam, raised, short hairy, moist, dirty white at centre and at periphery, with a buff ring in the middle, margin filiform, reverse pale orange, with buff spiral lines; On PDA reaching 20–22 mm diam, flat, creamy white and floccose at centre, membranous without aerial mycelium and buff at periphery, margin crenate, reverse saffron at centre, buff at periphery.
Typus: New Zealand, Westland, Westland County, Ianthe State Forest (S.F. 42), vic. Lake Ianthe, on bark of Dacrydium cupressinum (Podocarpaceae), 30 Apr. 1974, G.J. Samuels, G.J.S. 74-43, CBS H-7409 (holotype of Nectria dentifera PDD 32565, ex-isotype culture CBS 574.76 = ATCC 34042 = IFO 30275 = IMI 208152).
Additional material examined: Australia, Australian Capital Territory Canberra, from root of Eucalyptus sp. (Myrtaceae), unknown collection date and collector, isol. G.C. Johnson, VM11-330D, CBS H-24627, culture CBS 650.75.
Notes: Nectria dentifera was initially isolated from the bark of Dacrydium cupressinum in New Zealand and described based on both a sexual and asexual morph (Samuels 1976b). It was subsequently transferred to Peristomialis and Ijuhya based on morphological characters (Samuels 1988, Rossman et al. 1999). In the present study, the ex-type culture of N. dentifera (CBS 574.76) falls in the Lasionectriopsis clade, which is distant from Nectria and Ijuhya (Ashrafi et al. 2017). Although the ascomatal wall of N. dentifera are of two regions, differing from Lasionectriopsis in which the ascomatal wall consists of a single region, it morphologically matches the features described for Lasionectriopsis in the yellow to orange, globose ascomata, finely spinulose ascospores and (sub-)globose conidia. Therefore, a new combination is proposed here as L. dentifera. This species differs from L. germanica by producing longer conidiogenous cells [20–40(–50) μm vs 20–30 μm] and globose to broadly elliptical conidia without obviously apiculate scar, while L. germanica has ellipsoidal to subcylindrical conidia with a basal abscission scar (Samuels 1976b, Lechat et al. 2019); and it differs from L. pteridii by producing larger conidia (3–4 × 2.5–3 μm vs 2.4–2.8 × 2.2–2.4 μm) and the absence of crystals, while L. pteridii forms abundant crystals (Gams 1971, Samuels 1976b).
Phylogenetically, strain CBS 650.75 is close to the ex-type of Lasionectriopsis dentifera, with a total of 24 bp changes over the four available genes (3 bp differ in ITS, 1 bp differ in LSU, 13 bp differ in rpb2, 7 bp differ in tef-1α) (Fig. 2). Morphologically, it is similar to the protologue of L. dentifera in producing comparably shaped and sized conidia. However, CBS 650.75 slightly differs in its verruculose conidia that are arranged in dry conidial heads, while conidia in the original description of L. dentifera are flattened and held in a solitary drop of hyaline liquid (Samuels 1976b). These differences may be intraspecies variation.
Lasionectriopsis pteridii (W. Gams & J.C. Frankland) Lechat & P.-A. Moreau, Ascomycete.org 11: 1. 2019.
Basionym: Acremonium pteridii W. Gams & J.C. Frankland, Cephalosporium-artige Schimmelpilze: 81. 1971.
Description and illustration: Gams (1971).
Typus: UK, England, Cumbria, Lake District, from decaying petiole of Pteridium aquilinum (Dennstaedtiaceae), unknown collection date and collector, isol. 1964, isol. and dep. by J.C. Frankland, M4120, CBS H-24625 (holotype culture of Acremonium pteridii CBS 782.69 preserved as metabolically inactive culture, ex-type culture CBS 782.69).
Additional materials examined: Denmark, Sjaelland, from rhizosphere of Picea abies (Pinaceae), unknown collection date and collector, isol. Jun. 1969 by D.S. Malla, No. S7-32, CBS H-8347, culture CBS 738.69; Julland, soil from Fagus sp. forest, unknown collection date and collector, culture CBS 393.70. Germany, Kiel-Kitzeberg, from Trametes versicolor (Fungi, Polyporaceae), unknown collection date and collector, isol. Jul. 1965 by W. Gams, No. 527, CBS H-8346, culture CBS 737.69. Italy, Parco Nazionale del Circeo, Lago di Paola, Lazio, from soil, unknown collection date, isol. Aug. 1997, coll. and isol. O. Maggi & A. Persiani, CBS H-24626, culture CBS 102155; Torino, from ectomycorrhiza, unknown collection date and collector, isol. 1967 by A.M. Fontana, CBS H-8345, culture CBS 784.69. Netherlands, South Holland Province, Katwijk, from sand dune soil, 40 cm depth under Ammophila arenaria (Poaceae), unknown collection date and collector, isol. Feb. 1978 by W. Gams, culture CBS 182.78. Sweden, Stockholm, from skin of 78-yr-old Swedish woman, unknown collection date and collector, isol. M.-L. von Rosen, Central Microbiological Laboratory, culture CBS 579.90. UK, Cumbria, Roudsea Wood, from litter of Pteridium aquilinum (Dennstaedtiaceae), unknown collection date and collector, isol. 1965 by J.C. Frankland, No. M 3288, culture CBS 437.66; England, Cumbria, Roudsea Wood, from decaying petiole of Pteridium aquilinum (Dennstaedtiaceae), unknown collection date and collector, isol. 1964 by J.C. Frankland, No. M3288, culture CBS 783.69.
Notes: Lasionectriopsis pteridii was originally described as Acremonium pteridii from decaying petioles of Pteridium aquilinum in the UK based on its asexual morph (Gams 1971). Cultures of L. pteridii formed a moderately supported (BPP/MLBS = 1/66 %) clade containing three small clades: one clade contained six cultures isolated from diverse host plants, including the ex-type strain (CBS 782.69); one single mycophilic culture CBS 737.69 from Trametes versicolor (Polyporaceae, Basidiomycota) formed a separate lineage; and the other small clade comprised of three cultures from soil, including one isolated from ectomycorrhiza (Fig. 2).
Clade O21
Lasionectriella Lechat & J. Fourn., Ascomycete.org 8: 59. 2016.
Perithecia superficial, solitary or gregarious, non-stromatic, globose, subglobose, or tympaniform, apex conical, lacking papilla, ostiolar opening formed by narrow hyphal elements arising from the inner region of the perithecial wall, cupulate upon drying, pale yellow, dark orange to brownish orange, not changing colour in 3 % KOH or lactic acid, covered by thick-walled hairs arising from base, agglutinated and proliferating to form a fringe around upper margin of perithecia, or around the base of perithecium, hyaline to pale yellowish brown when dry, thick-walled, septate, slightly intertwined and difficult to separate. Ascomatal wall composed of two regions: outer region of globose to ellipsoid cells with hyaline to pale yellow wall; inner region of elongate, flattened, hyaline cells. Paraphyses moniliform, inserted between asci. Asci with 8 irregularly biseriate ascospores, fusoid to clavate, apex flattened, without ring. Ascospores hyaline, fusoid, rounded at ends, straight to lightly curved, 1-septate, equally 2-celled, constricted at septum, smooth, striated, or spinulose, with two yellow drops in each cell. Mycelium with hyphae branching, septate, hyaline to pale brown, smooth. Conidiophores borne on aerial hyphae, macronematous, mononematous, unbranched, 1–3-septate, flexuous, hyaline, faintly roughened or finely spinulose. Conidiogenous cells monophialidic, apex with a flared collarette. Conidia grouped at tip of phialide to form a mucous head, aseptate, allantoid, narrowly or widely ellipsoidal to subcylindrical with rounded apex, attenuated at base with an apiculate hilum, smooth, at first hyaline, becoming dark green, appearing hyaline or nearly black in mass (emended from Lechat & Fournier 2016).
Type: Lasionectriella rubioi Lechat & J. Fourn.
Other accepted species with available sequences: Lasionectriella arenuloides (Samuels) L.W. Hou, L. Cai & Crous, La. herbicola Lechat & J. Fourn., La. marigotensis (Lechat & J. Fourn.) L.W. Hou, L. Cai & Crous
Lasionectriella arenuloides (Samuels) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845846.
Basionym: Nectria arenuloides Samuels, New Zealand J. Bot. 14: 254. 1976.
Synonym: Hydropisphaera arenuloides (Samuels) Rossman & Samuels, Stud. Mycol. 42: 30. 1999.
Description and illustration: Samuels (1976b).
Typus: New Zealand, Auckland, Waitemata County, Waitakere Ranges, Huia Road, Kakamatua Stream, on rachis of Cyathea dealbata (Cyatheaceae), 5 Apr. 1974, G.J. Samuels & W.B. Kendrick, G.J.S. 74-41 (holotype PDD 32560, ex-type culture CBS 576.76 = ATCC 34041 = IMI 208150).
Notes: Nectria arenuloides was described based on its nectria-like sexual morph and acremonium-like asexual morph (Samuels 1976b). It was subsequently transferred to Hydropisphaera (Rossman et al. 1999). Based on the phylogenetic analysis in the present study, the ex-type strain of N. arenuloides (CBS 576.76) clustered apart from Hydropisphaera, but formed a lineage in Lasionectriella, sister to La. marigotensis (Fig. 2). Lasionectriella arenuloides and La. marigotensis are morphologically similar in the production of striate ascospores (Samuels 1976b, Lechat & Fournier 2013), while those of La. herbicola and La. rubioi are spinulose (Lechat & Fournier 2016b).
Lasionectriella marigotensis (Lechat & J. Fourn.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845847.
Basionym: Lasionectria marigotensis Lechat & J. Fourn., Mycotaxon 121: 276. 2013 (2012).
Description and illustration: Lechat & Fournier (2013).
Typus: France, French West Indies, Guadeloupe, Vieux Habitants, Marigot, l’Anse à la barque, on decaying leaf of Cocos nucifera (Arecaceae), 3 Aug. 2011, C. Lechat (holotype CLLGUAD11002 in LIP, ex-type culture CBS 131606).
Clade O22
Ramosiphorum L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845848.
Etymology: Referring to the production of multiply branched conidiophores.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, occasionally forming bundles. Conidiophores arising from submerged and superficial hyphae, (sub-)erect or curved, repeatedly verticillately or basitonously branched, bearing 1–3(–5) levels with 1–3 phialides per node, aggregated as sporodochial-like or not, or unbranched, occasionally proliferating sympodially, hyaline, smooth-walled, septate and occasionally coarse at lower part, with cell walls usually thicker than those of vegetative hyphae. Conidiogenous cell monophialidic, terminal or lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci. Conidia aseptate, subglobose, broadly ellipsoid or ellipsoid, obovoid, occasionally with a slightly apiculate base, hyaline, thin-, smooth-walled, or thick- and rough-walled, arranged in moist slimy heads. Chlamydospores and sexual morph not observed.
Type: Ramosiphorum polyporicola L.W. Hou, L. Cai & Crous
Other accepted species with available sequences: R. echinoporiae L.W. Hou, L. Cai & Crous, R. thailandicum L.W. Hou, L. Cai & Crous
Notes: The genus Ramosiphorum is proposed for a group of strains clustering in a fully supported clade (Fig. 2). Although all these cultures were labelled as “Nectriopsis oropensoides”, morphological characters reveal they are different species. Conidiophores of N. oropensoides are described as monophialidic and unbranched or once branched (Samuels et al. 1988), while all strains examined in our study produced repeatedly branched conidiophores, even aggregated as sporodochia. Therefore, all cultures are reidentified and three new species are introduced.
Ramosiphorum echinoporiae L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845849. Fig. 35.
Fig. 35.

Ramosiphorum echinoporiae (ex-type culture CBS 115288). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the host genus from which this fungus was isolated, Echinoporia hydnophora.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 2.5 μm wide. Conidiophores (sub-)erect, straight to flexuous or curved, arising from submerged and superficial hyphae, repeatedly verticillately or basitonously branched, bearing 1–3(–5) whorls of 1–3 phialides, aggregated as sporodochium-like structures, or unbranched, up to ca. 105.5 μm long, 1.5–3.2 μm wide at base, with 1–3 septa in lower part, hyaline, smooth-walled, occasionally coarse in lower part, with cell walls usually thicker than those of vegetative hyphae. Phialides monophialidic, lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, 11.5–41.5 μm long, 1.4–2.8 μm wide at base, with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci. Conidia aseptate, subglobose to ellipsoid, straight, hyaline, thin-, smooth-walled, 1.8–3.6 × 1.8–3.3 μm, arranged in moist slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 45–50 mm diam, flat, with sparse aerial mycelium, thinly felty, granular, white, margin crenate, reverse concolourous; On MEA reaching 24–27 mm diam, flat, radially folded, rosy buff, margin crenate, reverse pale orange, with buff radial lines; On PDA reaching 25–35 mm diam, flat, felty, creamy white, with olivaceous buff concentric rings, margin crenate, reverse buff to pale saffron; On SNA reaching 38–40 mm diam, flat, dusty, dirty white, margin filiform, reverse white.
Typus: China, Taiwan, Pingtung, from basidiocarp of Echinoporia hydnophora (Schizoporaceae), 21 Apr. 2002, isol. 24 Apr. 2002, coll. and isol. R. Kirschner, RoKi 1176 (holotype CBS H-24599, ex-type CBS 115288).
Notes: Ramosiphorum echinoporiae is phylogenetically closely related but clearly distinct from R. thailandicum based on the multi-locus phylogenetic analysis (Fig. 2). Morphologically, R. echinoporiae differs from R. thailandicum by producing relatively erect conidiophores while those of R. thailandicum are irregularly curved with a coarse base at the lower part. In addition, conidiophores of R. echinoporiae are repeatedly verticillately or basitonously branched and mostly have three phialides per node, while those of R. thailandicum are less frequently branched with mostly two phialides per node. Ramosiphorum echinoporiae could also be differentiated from R. thailandicum by lacking crystals, while R. thailandicum produces abundant, globose or irregular shaped, brown crystals.
Ramosiphorum polyporicola L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845850. Fig. 36.
Fig. 36.

Ramosiphorum polyporicola (ex-type culture CBS 123779). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial heads. E–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the host from which this fungus was isolated, polypore.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 2.8 μm wide, occasionally forming bundles. Conidiophores (sub-)erect, straight or curved, arising from submerged and superficial hyphae, repeatedly verticillately or basitonously branched, bearing 1–3(–5) whorls of 1–3 phialides per node, or unbranched, occasionally proliferating sympodially, with short sterile outgrowths, up to ca. 70.5 μm long, 1.8–3.5 μm wide at base, with 1–2 septa in basal or upper part, hyaline, smooth-walled, occasionally coarse in lower part, with cell walls usually thicker than those of vegetative hyphae. Phialides monophialidic, terminal or lateral, subulate, rarely cylindrical, hyaline, thick-, smooth-walled, 9.5–34 μm long, 1.3–2.3 μm wide at base with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, subglobose, broadly ellipsoid, or obovoid, occasionally with a slightly apiculate base and obtuse apices, hyaline, thin-, smooth-walled, 2–3.5 × 1.8–2.5 μm, arranged in moist slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 27–28 mm diam, flat, thinly felty, dirty white, pale olivaceous buff pigment, margin lobate, reverse pale olivaceous buff; On MEA reaching 23–24 mm diam, flat, deeply radially folded, felty, rosy buff, margin fimbriate, reverse saffron at centre, straw at periphery, with buff radial lines; On PDA reaching 20–25 mm diam, flat, thinly felty, dirty white, with pale olivaceous rings at periphery, with buff edge, margin crenate, reverse pale ochreous at centre, buff at periphery.
Typus: USA, Illinois, from polypore, unknown collection date, G.J. Samuels, G.J.S. 94-55 (holotype BPI 1107175, ex-type culture CBS 123779).
Additional materials examined: Japan, University of Tsukuba, Sugadaira Montane Research Center, Daimyojin waterfall, alt. 1 300 m, from dead decorticated wood on ground, 11 Aug. 1997, H.-J. Schroers, W. Gams, T. Gräfenhan & M. Klamer, isol. Sep. 1997 by H.-J. Schroers, No. H.J.S. 217, CBS H-24722, culture CBS 100282. Venezuela, from bark, unknown collection date, K.P. Dumont, isol. G.J. Samuels, CBS H-24721, culture CBS 109.87 = C.T.R. 72-209 = VE 6209.
Notes: The strains CBS 109.87 and CBS 100282 were stored as “Nectriopsis oropensoides” in CBS culture collection. According to our phylogenetic inference based on the ITS, LSU, tef-1α and rpb2 four loci, both strains clustered with CBS 123779 from polypore in USA (Fig. 2). These strains differ from N. oropensoides by producing repeatedly verticillately branched conidiophores, while those of N. oropensoides are mostly unbranched and monophialidic or once branched (Samuels 1988); thus, CBS 123779 is considered to represent a different species. Strains of Ramosiphorum polyporicola form a fully supported clade (BPP/MLBS = 1/100 %), phylogenetically different from other Ramosiphorum species. Morphologically, R. polyporicola resembles R. echinoporiae and R. thailandicum in producing repeatedly branched conidiophores. However, it can be distinguished from the latter in lacking sporodochia-like structure; R. polyporicola also could be differentiated from R. thailandicum by lacking of crystal, while R. thailandicum produces abundant, globose or irregular shaped, brown crystal.
Ramosiphorum thailandicum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845851. Fig. 37.
Fig. 37.

Ramosiphorum thailandicum (ex-type culture CBS 101914). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidial heads. E. Conidiophores and conidial heads. F–H. Branched conidiophores. I, J. Unbranched conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Named after the location where the fungus was collected, Thailand.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 3 μm wide. Sporulation abundant, phalacrogenous, nematogenous, rarely plectonematogenous. Conidiophores (sub-)erect, straight or irregularly curved and flexuous, arising from submerged and superficial hyphae, mostly repeatedly verticillately, basitonously branched, bearing up to 5 whorls of 1–2(–3) phialides, aggregated as sporodochia-like structures, rarely unbranched, up to ca. 114 μm long, 1.5–3.1 μm wide at base, with 1–3 septa in basal, middle or upper part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides monophialidic, lateral or terminal, subulate, hyaline, thick-, smooth-walled, 9–35 μm long, 1–2.3 μm wide at base, with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, subglobose to ellipsoid, hyaline, thick-, rough-walled, 2.5–4 × 1.8–2.3 μm, arranged in moist slimy heads, becoming salmon with age. Crystals abundant, globose or irregular shaped, brown. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: colonies on OA reaching 35–36 mm diam, flat, with moderate aerial mycelium, floccose or felty, with mycelial ropes and granule, white, margin crenate, reverse pale olivaceous buff, with strong geosmin odour; On MEA reaching 28–29 mm diam, flat, radially folded, with moderate aerial mycelium, felty, white, margin filiform, reverse pale orange, with buff radial lines; On PDA reaching 39–40 mm diam, flat, with moderate aerial mycelium, felty or floccose, with mycelial ropes and granule, with concentric rings, dirty white, creamy white and pale fawn at centre, margin filiform, reverse olivaceous buff; On SNA reaching 25–26 mm diam, flat, with sparse aerial mycelium, dusty, with mycelial ropes and granule, white, margin entire, reverse concolourous. Without odour on all media.
Typus: Thailand, from bark of dead tree, unknown collection date, G.J. Samuels, G.J.S. 97-71 (holotype CBS H-24598, isotype BPI 745614, ex-type culture CBS 101914).
Note: See notes under Ramosiphorum echinoporiae and R. polyporicola.
Clade O23
Protocreopsis Yoshim. Doi, Kew Bull. 31: 551. 1977.
Ascomata superficial on substrata, densely gregarious, less commonly solitary, surrounded by white to tan hyphae arising from ascomatal wall with few to many free ends visible, thus appearing hypocrea-like, often extensive; ascomata hyaline to orange, KOH-; cells at ascomatal surface completely obscured by investing hyphae; ascomatal wall more than 20 μm thick, comprising a single region of small, brick-like cells. Asci clavate to fusoid, apex simple or with an obscure ring; ascospores bi- to pluriseriate. Ascospores ellipsoid to fusoid, 1-septate, hyaline, typically striate, also smooth, punctate-striate, or tuberculate. Asexual morph where known, acremonium-like. On dead monocotyledonous substrata (Doi 1977, Rossman et al. 1999, Lechat et al. 2016b).
Type: Protocreopsis fusigera (Berk. & Broome) Yoshim. Doi
Other accepted species with available sequences: Pt. caricicola Lechat & J. Fourn., Pt. euphorbiae Crous, Pt. finnmarkica L.W. Hou, L. Cai, Rämä & Crous, Pt. freycinetiae (Samuels) Samuels & Rossman, Pt. pertusa (Pat.) Samuels & Rossman, Pt. phormiicola (Samuels) Samuels & Rossman, Pt. rutila (W. Gams) L.W. Hou, L. Cai & Crous
Notes: The genus Protocreopsis was introduced by Doi (1977) for a group of nectria-like species that have ascomata completely enclosed in long, white to tan or green, flexuous hyphal stroma, ascomatal walls that are more than 20 μm thick, typically striate ascospores and acremonium-like asexual morphs (Rossman et al. 1999, Lechat et al. 2016b). Although molecular sequences of the type Pt. fusigera are not available in GenBank, phylogenetical analysis of four Protocreopsis species including Pt. caricicola, Pt. freycinetiae, Pt. pertusa and Pt. phormiicola demonstrate that this genus belongs to Bionectriaceae.
Protocreopsis caricicola Lechat & J. Fourn., Ascomycete.org 8: 30. 2016.
Description and illustration: Lechat et al. (2016b).
Typus: Germany, Westmecklenburg, 5 km NE of Rhena, meadow pond 1 km E-SE of Strohkirchen, from last year’s leaf residues of Carex acutiformis (Cyperaceae), 25 May 2015, T. Richter, isol. 26 May 2016 by C. Lechat (holotype CLL 15081 in LIP, ex-type culture CBS 140572).
Protocreopsis finnmarkica L.W. Hou, L. Cai, Rämä & Crous, sp. nov. MycoBank MB 845852.
Etymology: Name reflects the most north-eastern county of Norway, Finnmark, where the species was collected.
Culture sterile. Protocreopsis finnmarkica differs from its closest phylogenetic neighbour Pt. euphorbiae (culture: CPC 38896) by unique fixed alleles in three loci based on alignments of the concatenated three loci deposited in Figshare (doi: 10.6084/m9.figshare.22258765): ITS position shares similarity: 97(C), 152(C), 180(C), 183(G), 443(T), 444(G), 513(T), 528(T), 529(C), 535(gap), 540(C), 560(C), 618(A), 621(T); LSU position: 1 459 (C); tef-1α position: 2 376(G), 2 403(C), 2 415(A), 2 439(T), 2 504(C), 2 508 (C), 2 546 (G), 2 575 (C), 2 576 (T), 2 578 (T), 2 590 (C), 2 656 (T), 2 665 (C), 2 674 (T), 2 681 (C), 2 684 (T).
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 35 mm diam, flat, hairy at centre, felty at periphery, white, margin entire, reverse creamy white. On MEA reaching 23 mm diam, raised, felty, white, margin entire, reverse orange. On PDA reaching 27 mm diam., raised, hairy and buff at centre, flat, felty and white at periphery, margin entire, reverse pale luteous at centre, buff at periphery. On SNA reaching 28 mm diam, flat, aerial mycelium sparse, white, margin fimbriate, reverse concolourous.
Typus: Norway, Troms og Finnmark county, Kvænangen municipality, Alteidet, from old dock made of Pinus sylvestris (Pinaceae) in the sea, 9 Aug. 2010, T. Rämä 041cS1.2 (holotype CBS H-24724, ex-type culture CBS 147428 = CPC 40316 = TR041cS1.2).
Additional materials examined: Norway, Troms County, Kvænangen municipality, Alteidet, from old dock of P. sylvestris in the sea, 9 Aug. 2010, T. Rämä, culture CBS 147427 = CPC 40315 = TR041bE1.1; ibid., culture CBS 147429 = CPC 40318 = TR042bE1.2; ibid., culture TR042cE1.2, ibid., culture TR041aN1.1.
Notes: This fungus was isolated from wood as explained in Rämä et al. (2014). The strains from an old dock of Pinus sylvestris in the Norwegian Sea were examined in this study. They cluster on a separate branch close to Pt. euphorbiae, from which they are phylogenetically distinct, which supports this species as unique (Fig. 2). However, all strains remained sterile in all culture media tested in this study. Considering that it is phylogenetically distinct, a new species, Pt. finnmarkica, is introduced here and the molecular differences based on the sequence data are provided.
Protocreopsis freycinetiae (Samuels) Samuels & Rossman, Stud. Mycol. 42: 65. 1999.
Basionym: Nectria freycinetiae Samuels, New Zealand J. Bot. 14: 243. 1976.
Description and illustration: Samuels (1976b).
Typus: New Zealand, Auckland, Thames County, Coromandel Forest Park, Kauaeranga Valley, vic. Thames, from leaf of Freycinetia banksia (Pandanaceae), 17 Aug. 1974, J.M. Dingley et al., isol. G.J. Samuels, CBS H-718 (holotype of Nectria freycinetiae PDD 32577, ex-isotype culture CBS 573.76 = ATCC 34044 = IMI 208153).
Note: Protocreopsis freycinetiae clustered within the Protocreopsis clade but with low support values.
Protocreopsis pertusa (Pat.) Samuels & Rossman, Stud. Mycol. 42: 66. 1999.
Basionym: Nectria pertusa Pat., Bull. Soc. Mycol. France. 11: 227. 1895.
Synonym: Cucurbitaria pertusa (Pat.) Kuntze, Revis. Gen. Pl. (Leipzig) 3: 461. 1898.
Description and illustration: Rossman et al. (1999).
Material examined: New Zealand, Auckland, Waitakere Ranges, West Coast Road, Marguerite Track, from rachis of Cyathea medullaris (Cyatheaceae), 21 Aug. 1974, J.M. Dingley & Haydon, isol. G.J. Samuels, PDD 32584, culture CBS 568.76.
Protocreopsis phormiicola (Samuels) Samuels & Rossman, Stud. Mycol. 42: 67. 1999.
Basionym: Nectria phormiicola Samuels, New Zealand J. Bot. 14: 244. 1976.
Description and illustration: Rossman et al. (1999).
Typus: New Zealand, Auckland, Waitemata County, Waitakere Ranges, vic. Piha, Marowhara Loop Track, from dead leaf of Phormium tenax (Asphodelaceae), 17 Dec. 1974, J.M. Dingley et al., G.J.S. 74-133 (holotype of Nectria phormiicola PDD 32684, ex-type culture CBS 567.76 = ATCC 34049 = IFO 30281 = IMI 208158).
Protocreopsis rutila (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845853. Fig. 38.
Fig. 38.

Protocreopsis rutila (strain CBS 394.70). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidial heads on OA media. E. Conidiophores and conidial heads. F–H. Monophialides. I, J. Degraded conidiophores. K. Conidia. Scale bars = 10 μm.
Basionym: Acremonium rutilum W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 105. 1971.
Description based on culture CBS 394.70: Mycelium consisting of branched, septate, hyaline, pale brown in old cultures, smooth-, thin-walled hyphae, up to 2 μm wide, often formed mycelial coils. Sporulation abundant, phalacrogenous, nematogenous, rarely plectonematogenous. Conidiophores (sub-)erect, straight to flexuous, arising from submerged and superficial hyphae, unbranched or poorly branched, up to ca. 68 μm long, 1.3–3.8 μm wide at base, with short sterile outgrowths, with 1–2(–3)-septa at basal or upper part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae; degenerated to very short conidiophores with age. Phialides monophialidic, lateral, subulate, always narrowed at base, hyaline, thin-or thick-, smooth-walled, 10.5–60.5 μm long, 1.5–3 μm wide at base, with inconspicuous collarette and conspicuous periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, long cylindrical, with an inconspicuous truncate hilum at basal end, hyaline, thin-, smooth-walled, with two large inconspicuous guttules, 3.5–8.7 × 2.2–3 μm, arranged in moist slimy heads, mostly confluent, salmon. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: colonies on OA reaching 30–32 mm diam, flat, thinly felty, white at centre and periphery, pale salmon in middle, margin entire, reverse buff; On MEA reaching 25–27 mm diam, flat, radially folded, felty, dirty white, undulant margin, reverse ochreous, with radially buff lines; On PDA reaching 27–30 mm diam, flat, thinly felty, sienna at centre, peach at middle, salmon at periphery, margin entire, with yellowish pigment, reverse orange, with buff edge; On SNA reaching 32–33 mm diam, flat, dusty, white, margin entire, reverse concolourous.
Description and illustration: Gams (1971).
Typus: Germany, Kiel-Kitzeberg, from moist wall in greenhouse, unknown collection date and collector, isol. 1966 by W. Gams, No. 599, CBS H-24701 (holotype CBS 396.66 preserved as metabolically inactive culture, ex-type culture CBS 396.66 = IAM 14661).
Additional materials examined: Belgium, Haasrode, Meerdael Bos, from soil, unknown collection date and collector, isol. Nov. 1963 by G.L. Hennebert, CBS H-8300, culture CBS 378.70C = MUCL 6094. Denmark, Zealand, Graese, from agricultural soil, coll. 17 Nov. 2004, isol. 22 Dec. 2004, coll. and isol. S. Ronhede, Gr 161, culture CBS 118526. Germany, Kiel-Kitzeberg, from moist wall in greenhouse, unknown collection date and collector, isol. W. Gams, No. 602, culture CBS 229.70; Kiel-Kitzeberg, from scab of Malus domestica (Rosaceae), unknown collection date and collector, isol. W. Gams, No. 564, culture CBS 226.70; near Braunschweig, from agricultural löss soil, unknown collection date and collector, isol. H. Nirenberg, No. 208, culture CBS 263.89; from wheat field soil, unknown date and collector, culture CBS 395.66. Netherlands, Utrecht Province, Baarn, from humus rich soil, unknown collection date and collector, isol. Apr. 1964 by G.L. Hennebert, CBS H-8368, culture CBS 394.70 = MUCL 6903.
Notes: According to the phylogenetic analysis (Fig. 2), the ex-type strain of Acremonium rutilum (CBS 396.66) is placed in Protocreopsis, and it is therefore recombined as Pt. rutila. Eight cultures representing Pt. rutila form two subclades: subclade I contains two strains from the moist wall in the greenhouse in Germany, including the ex-type CBS 396.66; subclade II comprises six strains mainly from soil of various European countries. Morphologically, the size of the structures observed in CBS 394.70 (subclade II) differs slightly from those described in the protologue of Pt. rutila (Gams 1971): phialides and conidia are larger than those of Pt. rutila [phialides 10.5–60.5 μm × 1.5–3 μm vs (15–)20–40(–55) × 2.0–2.5(–3.0); conidia: 3.5–8.7 × 2.2–3 μm vs 3.0–6.0 × 2.0–3.3 μm]. However, we consider that these morphological differences may reflect intraspecific variability and are insufficient to allow confident splitting of this group into more than one species.
Clade O24
Nectriopsis Maire, Ann. Mycol. 9: 323. 1911. (nom. cons.).
Synonym: Dasyphthora Clem., Gen. Fung. (Minneapolis): 45. 1909.
Perithecia superficial or immersed in substratum, generally not conspicuously stromatic, generally less than 200 μm diam., nearly white to pale yellow or orange, rarely violet or purple, KOH-. Ascomatal wall less than 20 μm thick, usually comprising of a single region of small thin-walled, non-descript cells; wall cells at the surface forming textura epidermoidea. Asexual morph where known, acremonium-like, gliocladium-like, or verticillium-like. On free-living fungi, lichens, and myxomycetes, less frequently on herbaceous substrata (Samuels 1988, Rossman et al. 1999).
Type: Nectriopsis violacea (J.C. Schmidt ex Fr.) Maire
Other accepted species with available sequences: Nectriopsis candicans (Plowr.) Maire, N. ellipsoidea L.W. Hou, L. Cai & Crous, N. rexiana (Sacc.) Rossman, L. Lombard & Crous, N. lindauiana (Bubák) Zare & W. Gams, N. fuliginicola Zare & W. Gams, N. microspora (Jaap) L.W. Hou, L. Cai & Crous, N. sporangiicola (Samuels) Samuels
Notes: Nectriopsis was originally established for four hypocrealean fungi with ascomata in a byssoid stroma, which were considered intermediate between Nectria and Hypomyces (Maire 1911; Rossman et al. 1999). Samuels (1988) described and illustrated 43 species of this genus that were recognised three intergrading subgroups. This genus can be easily distinguished from Nectria s. str. in its thin perithecial wall with textura epidermoidea at the surface, combined with yellow pigmentation, small perithecial and a fungicolous habit (Samuels 1988). The monotypic genus Peloronectriella was introduced for a fungus (Pe. sasae) on bamboo having elongate, tuberculate stroma with nectria-like ascomata, and 1-septate ascospores (Doi 1968). Rossman et al. (1999) regarded it as synonym of Nectriopsis based on morphological characters. However, the ex-type strain of the type Pe. sasae (CBS 333.69) clustered with Cosmospora in Nectriaceae, which are distant from Nectriopsis s. str. (Supplementary Fig. S1). Therefore, it would seem that it is not a synonym of Nectriopsis.
Nectriopsis candicans (Plowr.) Maire, Ann. Mycol. 9: 324. 1911. Fig. 39.
Fig. 39.

Nectriopsis candicans (ex-epitype culture CBS 701.79). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Ascomata on OA. E, F. Ascomata. G, H. Asci. I. Ascospores. Scale bars: E, F = 20 μm; G–I = 10 μm.
Basionym: Hypomyces candicans Plowr., Grevillea 11: 50. 1882.
Synonyms: Nectria candicans (Plowr.) Samuels, Mycologia 65: 412. 1973.
Hypolyssus candicans (Plowr.) Kuntze, Revis. Gen. Pl. 3: 488. 1898.
Description based on ex-epitype culture CBS 701.79: Perithecia superficial, solitary, gregarious or scattered, globose, sub-globose or broadly pyriform, pale yellow, becoming orange, dull, smooth, uniloculate, covered with abundant white, cylindrical, septate, unbranched hyphae, woolly except the ostiolum, 121–197 × 104–165 μm. Ostiole single, central, papillate, formed of thin-walled, septate hyphae; hyphae arising from upper half of perithecial wall, branching, forming a network around papillae. Perithecial wall 8.5–16 μm wide, composed of several layers of thick-walled, hyaline to pale brown, cylindrical cells, forming textura angularis. Paraphyses not observed. Asci 8-spored, unitunicate, cylindrical, apex simple, 37–55 × 3–4.5 μm. Ascospores uniseriate, with overlapping ends, oblong, cylindrical, rounded at both ends, 1-septate, not constricted at septa, hyaline, smooth-walled, eguttulate, without mucilaginous sheath, 4.5–6.8 × 2–2.7 μm. Asexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 42–47 mm diam, flat, felty, granular, dirty white, margin dendritic, reverse pale olivaceous buff; On MEA reaching 38–39 mm diam, flat, radially fold at centre, felty, dirty white, pale olivaceous buff at centre, with concentric rings, margin dendritic, reverse pale ochreous, with dirty white radial lines; On PDA reaching 49 mm diam, flat, thinly felty, granulose, dirty white, creamy white at periphery, margin dendritic, reverse pale olivaceous buff; On SNA reaching 18 mm diam, flat, sparse aerial mycelium, felty, white, margin dendritic, reverse concolourous.
Other illustrations: Maire (1911), Samuels et al. (1973).
Typus: UK, Great Britain, England, Bathford Down, on Mycetozoa, Aug. 1880, Leziate (K, lectotype in 1973).
Additional materials examined: Germany, former West-Germany, from Fuligo septica (Protozoa), unknown collection date and collector, isol. W. Gams, culture CBS 440.65; Holzdorf, from Fuligo septica (Protozoa), unknown collection date and collector, isol. 15 Sep. 1961 by G. Arnold (MW m 43), culture CBS 424.64. Netherlands, Gelderland Province, Doorwerth, from Leocarpus fragilis (Protozoa) on Prunus laurocerasus (Rosaceae), 7 Aug. 1972, N.E. Nannenga-Bremekamp, culture CBS 627.72. ; Flevoland Province, Noordoostpolder, Kuinderbos, L perceel KB 72, from Fuligo sp. (Protozoa), Sep. 1979, W. Gams, CBS H-15093, culture CBS 701.79.
Notes: According to the original literature, Hypomyces candicans was initially isolated from a kind of Mycetozoa in the UK described as having globose, gregarious perithecia, 200 × 150 μm, cylindrical asci 50–60 × 3–5 μm, ovoid to oblong, uniseptate ascospores measuring 8 × 3 μm, blunt at both ends, and surrounded by white, floccose mycelium (Plowright 1882). Later, it was transferred to Nectriopsis as N. candicans (Maire 1911). No holotype specimen was cited in the original description. Samuels et al. (1973) designated the collection from Bathford Downs in England as lectotype, because it agreed in all respects with the original description of H. candicans. In the present study, four strains received as N. candicans and isolated from Mycetozoa Fuligo spp. and Leocarpus fragilis in Europe were examined. One of the strains, CBS 701.79, morphologically matches the original description by producing comparable sized and shaped ascomata, asci and ascospores. Therefore, CBS 701.79 is considered a representative culture of H. candicans, and typification of this species awaits further collections.
Nectriopsis ellipsoidea L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845854. Fig. 40.
Fig. 40.

Nectriopsis ellipsoidea (ex-type culture CBS 358.78). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores and conidial heads. F. Percurrently proliferated conidiophores. G. Unbranched conidiophore. H–K. Branched conidiophores. L. Conidia. Scale bars = 10 μm.
Etymology: Referring to the ellipsoid conidia produced by this fungus.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 2.3 μm wide. Sporulation abundant, phalacrogenous or nematogenous. Conidiophores (sub-)erect, arising from submerged and superficial hyphae, orthogonally, unbranched or basitonously branched, bearing 1–3 levels with 1–3 phialides per node, commonly proliferating sympodially, up to ca. 254 μm long, 1.6–3.4 μm wide at base, 1–2-septate, hyaline, smooth-walled, coarse in lower part. Phialides mono- or polyphialidic, lateral or terminal, orthotropic, cylindrical or subulate, hyaline, thick-, smooth-walled, (9.0–)15.2–51.8 μm long, 1–2.1 μm wide at base, with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci; polyphialides with up to three conidiogenous loci commonly present; with percurrent or subterminal proliferations. Conidia aseptate, ellipsoid, with obviously apiculate bases and rounded apices, hyaline, thin-, smooth- or rough-walled, 4.3–7 × 2.5–3.5 μm, guttulate, arranged in moist slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 85 mm diam, flat, felty, slightly floccose in some sections, dirty white, margin filiform, reverse colourless; On MEA reaching 85 mm diam, flat, woolly at centre, felty at periphery, mycelial ropes abundant, creamy white, aerial mycelium arranged radially, margin filiform, reverse luteous; On PDA reaching 85 mm diam, flat, aerial mycelium arranged radially, floccose, mycelial ropes abundant, dirty white, margin filiform, reverse buff; On SNA reaching 52 mm diam, flat, membranous without aerial mycelium, white, margin fimbriate, reverse concolourous. With strong geosmin odour on PDA and MEA media, without odour at OA and SNA media.
Typus: USA, Louisiana, along Chappepeela Creek, Tangipahoa Parish, from Polyporus pargamenus (Polyporaceae) on log, unknown collection date, isol. 5 Jun. 1976, coll. and isol. C.T. Rogerson (holotype CBS H-24628, ex-type culture CBS 358.78 = C.T.R. 76-39).
Notes: Nectriopsis ellipsoidea was associated with Polyporus pargamenus on a log of an unknown tree. It is phylogenetically different from the closely related species N. rexiana. Morphologically, it differs from N. rexiana in producing longer and ellipsoid conidia (4.3–7 × 2.5–3.5 μm), while conidia of the latter are shorter and ovoid (5 × 3 μm) (Saccardo 1882).
Nectriopsis fuliginicola Zare & W. Gams, Mycol. Progr. 15: 1027. 2016.
Description and illustration: Zare & Gams (2016).
Typus: Russia, St. Petersburg region, Puschkin, from Fuligo septica (Protozoa), unknown collection date and collector, isol. 11 Oct. 1969 by G. Arnold, No. 11 (holotype CBS H-22407, ex-type culture CBS 400.82).
Nectriopsis lindauiana (Bubák) Zare & W. Gams, Mycol. Progr. 15: 1023. 2016.
Basionym: Verticillium lindauianum Bubák, Ann. Mycol. 12: 210. 1914.
Description and illustration: Zare & Gams (2016).
Typus: Germany, Kr. Plön, Schüttbrehm, from Fuligo septica (Protozoa), 30 Aug. 1965, W. Gams (neotype CBS H-22408, ex-neotype culture MUCL 7926 = CBS 897.70).
Nectriopsis microspora (Jaap) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845855.
Basionym:Verticillium microsporum Jaap, Verh. Bot. Ver. Prov. Brandenb. 58: 38. 1916.
Synonyms: Sesquicillium microsporum (Jaap) Veenb.-Rijks & W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 226. 1971.
Tolypocladium microsporum (Jaap) Bissett, Canad. J. Bot. 61: 1318. 1983.
Gliocladium microsporum (Jaap) Arx, Mycotaxon 25: 157. 1986. (nom. illegit.)
Sesquicillium parvulum Veenb.-Rijks, Acta Bot. Neerl. 19(3): 323 (1970)
Description and illustration: Gams (1971).
Material examined: Canada, Ontario, Petawawa, from forest soil under Populus tremuloides (Salicaceae), Oct. 1968, G.C. Bhatt, No. PET 160, CBS H-18229, culture CBS 355.70 = DAOM 175216. Netherlands, Flevoland Province, Oostelijk Flevoland, from wheat field soil, 12 Mar. 1969, J.W. Veenbaas-Rijks, No. 223 (holotype of Sesquicillium parvulum CBS H-7751, isotypes CBS H-1022, CBS H-7753, CBS H-7754 & CBS H-7755, ex-type culture CBS 933.69 = ATCC 18932 = DAOM 175219 = IMI 152204 = IPO 738).
Notes: According to Gams (1971), the type material of Verticillium capitatum Ehrenb. in B no longer shows the original fungus. The substrate was indicated as “small dead insects and larvae, and then transferred to rotten wood”, which is why identity with the present fungus is unlikely. In the present study, the representative strain and type strain of its synonym (Sesquicillium microsporum) clustered within the genus Nectriopsis.
Nectriopsis rexiana (Sacc.) Rossman et al., Stud. Mycol. 80: 243 (2015)
Basionym: Verticillium nanum subsp. rexianum Sacc., Michelia 2: 577. 1882.
Synonyms: Verticillium rexianum (Sacc.) Sacc., Syll. Fung. 4: 153. 1886.
Hypomyces exiguus Pat., Bull. Soc. Mycol. France. 18: 180. 1902.
Nectriopsis exigua (Pat.) W. Gams, Netherlands J. Pl. Pathol. 88: 73. 1982.
Verticillium niveostratosum Lindau, Verh. Bot. Vereins Prov. Brandenburg. 45: 158. 1904 (1905).
Nectria myxomyceticola Samuels, Mycologia 65: 409. 1973.
Description: Saccardo (1882).
Materials examined: Germany, Kr. Rendsburg, Enkendorfer Gehölz, from Arcyria sp. (Protozoa), unknown collection date, W. Gams, No. 1090, culture CBS 305.70C. UK, England, Derbyshire, Haddon Hall, from Physarum nutans (Protozoa), unknown collection date, W. Gams, No. 1075, culture CBS 305.70A.
Nectriopsis sporangiicola (Samuels) Samuels, Mem. New York Bot. Gard. 48: 49. 1988.
Basionym: Nectria sporangiicola Samuels, Mycologia 65: 416. 1973.
Descriptions: Samuels (1973, 1988).
Typus: USA, New Jersey, Cape May County, 5 km SE of Woodbine, from Physarum polycephalum (Protozoa), 14 Sep. 1967, C.T. Rogerson (isotypes CBS H-7415, CBS H-7416 & CBS H-7417, ex-isotype culture CBS 166.74 = ATCC 26542 = C.T.R. 67-136).
Nectriopsis violacea (J.C. Schmidt ex Fr.) Maire, Ann. Mycol. 9: 323. 1911. Fig. 41.
Fig. 41.

Nectriopsis violacea (ex-epitype culture CBS 914.70). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Ascomata on OA. E. Ascoma. F. Ostiole formed of thick-walled hyphae. G–I. Asci. J–L. Conidiophores. M. Conidia. Scale bars: E = 50 μm; F–G = 20 μm; H–M = 10 μm.
Basionym: Sphaeria violacea J.C. Schmidt ex Fr., Syst. Mycol. (Lundae) 2: 441. 1823.
Synonyms: Nectria violacea (J.C. Schmidt ex Fr.) Fr., Summa Veg. Scand., Sectio Post. (Stockholm) 1: 388. 1849.
Hypomyces violaceus (J.C. Schmidt ex Fr.) Tul. & C. Tul, Ann. Sci. Nat., Bot., sér. 4 13: 14. 1860.
Peckiella violacea (J.C. Schmidt ex Fr.) Sacc., Syll. Fung. (Abellini) 9: 945. 1891.
Hypolyssus violaceus (J.C. Schmidt ex Fr.) Kuntze, Revis. Gen. Pl. (Leipzig) 3: 488. 1898.
Byssonectria violacea (J.C. Schmidt ex Fr.) Seaver, Mycologia 2: 65. 1910.
Hyphonectria violacea (J.C. Schmidt ex Fr.) Petch, J. Bot. 74: 220. 1937 (1936).
Description based on ex-epitype culture CBS 914.70: Sexual morph: perithecia immersed in mycelium, becoming collabent when dry, solitary, gregarious or scattered, globose, sub-globose or broadly pyriform, violet, vinaceous, uniloculate, covered with abundant cylindrical, septate, hyaline hyphae, 148.5–304 × 126.5–238.5 μm. Ostiole single, central, papillate, formed of thick-walled, septate, unbranched hyphae; hyphae extending outwardly as hairs, 25–55 μm long, 3–4 μm diam at rounded tips, forming a fringe around papillae. Perithecial wall 14–19 μm wide, composed of several layers of thick-walled, cylindrical, hyaline to pale brown cells forming textura angularis. Paraphyses not observed. Asci unitunicate, 8-spored, cylindrical, apex simple, 46–70.5 × 3–4.5 μm. Ascospores obliquely uniseriate with overlapping ends, oblong, cylindrical, rounded at both ends, 1-septate, not constricted, hyaline, spinulose, without mucilaginous sheath, 4.7–8 × 2–2.7 μm. Asexual morph: sporulation abundant, phalacrogenous or nematogenous. Conidiophores (sub-)erect, arising from submerged and superficial hyphae, unbranched or branched, bearing 1–3 levels with 1–3 phialides per node, up to ca. 355 μm long, with 1–2 septa in basal and upper part, hyaline, smooth-walled. Phialides monophialidic, lateral or terminal, cylindrical or subulate, hyaline, thin-, smooth-walled, 18–55 μm long, 1.3–2.3 μm wide at base, with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci. Conidia variable in size, aseptate, ellipsoid, cylindrical or oblong, rounded at both ends, or with a truncate basal end, hyaline, thin-, smooth-walled, 2.8–9.3 × 1.9–2.8 μm, guttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 70–72 mm diam, flat, felty, with some floccose zones, dirty white, margin entire, reverse buff; On MEA reaching 60–62 mm diam, flat, radially fold at centre, floccose and white at centre, felty and pale luteous at periphery, margin entire, reverse orange at centre, pale luteous at periphery, with white radial lines; On PDA reaching 65 mm diam, flat, felty, slightly floccose at centre, white, dirty white and filiform margin, reverse buff; On SNA reaching 33 mm diam, flat, sparse aerial mycelium, membranous, colourless, margin entire, reverse colourless.
Typus: Germany, Sachsen, Bernstadt, Fuligo septica (Protozoa), 1817, G. Kunze (holotype UPS: BOT: F-004721 as Sphaeria violacea); Eifel, Gerolstein, Gerolsteiner Wald, from Fuligo septica (Protozoa), 15 Sep. 1970, W. Gams (epitype designated here, CBS H-15101, MBT10009440, ex-epitype culture CBS 914.70).
Additional material examined: Germany, Eifel, Pelmer Wald bei Gerolstein, from Fuligo septica (Protozoa), 18 Sep. 1970, W. Gams, CBS H-15102, CBS H-15104 & CBS H-15105, culture CBS 849.70.
Notes: Several nectria-like species had been reported to develop on sporangia, commonly of Fuligo and Stemonitis, including N. candicans, N. hirsuta, N. myxomyceticola, N. sporangiicola and N. violacea (Samuels et al. 1973). Nectria violacea, N. candicans and N. myxomyceticola are closely related, having cylindrical or oblong ascospores which are less than 10 μm long, cylindrical asci averaging less than 60 μm long, and thin perithecial walls with modified hyphae or hairs. Nectriopsis violacea (basionym: Sphaeria violacea) was originally reported on Fuligo septica in Germany (Fries 1823), and is characterised by having violet to purple perithecia. In the present study, two cultures isolated from Fuligo septica in Germany were examined. Culture CBS 914.70, which is from the same host and country and agreed well in morphology with the protologue (Fig. 41), is herewith designated as ex-epitype culture, and the specimen CBS H-15101 as epitype of Sphaeria violacea. This species formed a fully supported clade in Bionectriaceae (BPP/MLBS = 1/100 %; Fig. 2).
Clade O27
Mycocitrus Möller, Bot. Mitt. Trop. 9: 297. 1901.
Stroma well-developed, buff to rufous, clasping and surrounding the substratum. Ascomata immersed, with apices barely visible, densely gregarious, forming a single layer. Asci cylindrical, ascal apex simple. Ascospores ellipsoid, 1-septate, hyaline, spinulose. Asexual morph acremonium-like. On living stems of bamboo (Rossman et al. 1999).
Type: Mycocitrus aurantium Möller
Other accepted species with available sequences: Mycocitrus odorus L.W. Hou, L. Cai & Crous, M. phyllostachydis (Syd. & P. Syd.) Yoshim, M. zonatus (Sawada) L.W. Hou, L. Cai & Crous
Notes: Mycocitrus was originally described based on the type M. aurantium from culms of living bamboo (Guadua) and on Microstachys sp. (Euphorbiaceae) in the city of Blumenau, Santa Catarina, in southern Brazil (Möller 1901). This genus is characterised by its large fleshy orange stromata that clasp and surround bamboo stems, and perithecial ascomata partially to fully immersed in the upper region of the stromata. Mycocitrus was originally placed in “Hypocreaceen, Didymosporae” (Möller 1901), or in Hypocreaceae (Doi 1967). Rossman et al. (1999) included Mycocitrus in the newly introduced family Bionectriaceae based on morphology. Recently, two bamboo-inhabiting Mycocitrus aurantium strains (BAFC 3843 and BAFC 51693) found in South America were discovered to form an independent lineage within Hypocreales based on ITS phylogenetic tree and limited taxa. This lineage was distinct from Bionectriaceae, Nectriaceae, Cordycipitaceae, Clavicipitaceae and Hypocreaceae, and suggesting Mycocitrus represents an additional lineage within Hypocreales (Leite et al. 2018). However, only ITS sequences were available for these strains, and may affect the support values of clades and result in unreliable phylogenies, we therefore excluded these strains from the dataset for the phylogenetic analysis of Bionectriaceae (Fig. 2). A dataset that included more strains was used to construct a more complete phylogeny, showing that the two strains of M. aurantium clustered in a well-supported lineage together with strain CBS 330.69 that representing Mycocitrus phyllostachydis. This cluster lies within the Bionectriaceae (Supplementary Fig. S2), a placement which conforms to the proposition of Rossman et al. (1999).
Mycocitrus odorus L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845856. Fig. 42.
Fig. 42.

Mycocitrus odora (ex-type culture CBS 100104). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores and conidial heads. F–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Etymology: Referring to the prominent odour this fungus produces in culture.
Mycelium consisting of branched, septate, hyaline, rough-, thin-walled hyphae, up to 2.5 μm wide. Sporulation abundant, phalacrogenous or nematogenous. Conidiophores (sub-)erect, straight or curved, arising from submerged and superficial hyphae, or radiating out from the coils formed by mycelium, unbranched or basitonously, verticillately branched, bearing 1–4 levels with 2–3 phialides per node, up to ca. 133 μm long, 2–3.8 μm wide at base, with 1–2(–3) septa at basal, middle or upper part, coarse at lower part, hyaline, smooth-walled. Phialides monophialidic, lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, 14.5–60 μm long, 1.5–2.8 μm wide at base, with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, oblong, symmetrically rounded, hyaline, thin-, smooth-walled, 3.5–7.5 × 2–4.5 μm, with 2–4 minute guttules, arranged in slimy heads, mostly confluent. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 78–80 mm diam, flat, moderate aerial mycelium, thinly felty, abundant mycelial granula, dirty white, margin entire, reverse dirty white, strong fresh-leaf odour (aromatic hydrocarbon); On MEA reaching 78–80 mm diam, raised, abundant aerial mycelium, floccose, granular, abundant mycelial ropes, white at centre and periphery, with a buff ring, margin entire, reverse luteous; strong fresh-leaved odour (aromatic hydrocarbon); On PDA reaching 80 mm diam, flat, moderate aerial mycelium, floccose at centre, felty at periphery, dirty white, margin filiform, reverse concolourous, strong geosmin odour; On SNA reaching 80 mm diam, flat, with sparse aerial mycelium, membranous, white, margin filiform, reverse white (no obvious odour).
Typus: Netherlands, North Holland Province, Amsterdam, Slotervaart Hospital, on onychomycosis (human), unknown collection date, W.C. van Dijk & W. Pauw (holotype CBS H-24690, ex-type culture CBS 100104).
Additional material examined: Netherlands, human skin, unknown collection date and collector, culture CBS 120610. Sweden, Stockholm, from human skin, unknown collection date and collector, No. BF 220/74c, culture CBS 232.75B.
Notes: Three cultures from human skin or nails formed a fully supported clade sister to Mycocitrus zonatus, representing M. odorus. These cultures differ from M. zonatus in producing aseptate, relatively wide conidia (3.5–7.5 × 2–4.5 μm), while those of M. zonatus are occasionally 1-septate, narrower [(3.6–)5.0–6.7(–8.1) × (1.6–)2.0–2.7 μm]. In addition, M. odorus appears to be associated with human infection, though no case has been clinically ascertained, while cultures and specimens of M. zonatus are from diverse plant hosts (Gams 1971). As many fungi frequently isolated as clinical contaminants from skin and nail lesions are common in household dust and garden soil fungi, this ecological possibility should be considered. Many non-dermatophytic fungi may cause or contribute to nail infections, but the ability to infect unwounded skin is extremely rare.
Mycocitrus phyllostachydis (Syd. & P. Syd.) Yoshim. Doi, Bull. Natl. Sci. Mus., Tokyo 10: 31. 1967.
Basionym: Ustilaginoidea phyllostachydis Syd. & P. Syd., Mém. Herb. Boissier 4: 5. 1900.
Synonyms: Hypocreopsis phyllostachydis (Syd. & P. Syd.) I. Miyake & Hara, Bot. Mag., Tokyo 24: 333. 1910.
Shiraiella phyllostachydis (Syd. & P. Syd.) Hara, Bot. Mag., Tokyo 28: 402. 1914.
Illustration: Doi (1967).
Material examined: Japan, from Phyllostachys sp. (Poaceae), unknown collection date, W. Gams, CBS H-14839, culture CBS 330.69 = IFO 8912.
Notes: Gams (1971) examined the strain of Mycocitrus phyllostachydis (CBS 330.69), which was isolated from the same host (Phyllostachys sp.) in Japan. Based on the phylogenetic analyses, CBS 330.69 clustered within Bionectriaceae and it is closely related to the four strains that were recorded as “Acremonium zonatum” and were currently described as M. odorus and M. zonatus (Fig. 2).
Mycocitrus zonatus (Sawada) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845857.
Basionym: Cephalosporium zonatum Sawada, Rep. Dept. Agric. Gov. Res. Inst. Formosa. 19: 603. 1919.
Synonym: Acremonium zonatum (Sawada) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 119. 1971.
Description and illustration: Gams (1971).
Typus: China, Taiwan, from leaves of Morus acidosa (Moraceae), 30 Oct. 1908 K. Sawada, (holotype IMI 93328).
Material examined: Zaire, Bas-Zaire, Nsangi, from leaf and pericarp of Citrus reticulata (Rutaceae), unknown collection date and collector, isol. May 1968 by G.L. Hennebert, CBS H-8481, culture CBS 400.70 = IAM 14665 = MUCL 11517.
Notes: Three living cultures were examined and identified as Acremonium zonatum (basionym: Cephalosporium zonatum) by Gams (1971). Later, one of the three cultures (CBS 565.67) illustrated by Gams (1971) under A. zonatum was confirmed by sequencing as Sarocladium strictum (Summerbell et al. 2011). Another culture, CBS 145.62, was stated to be possibly isolated from the natural substrate that was mixed up with the real A. zonatum by accident and had an LSU sequence identical to that of S. kiliense (Summerbell et al. 2011). The third culture CBS 400.70 was isolated from a leaf and pericarp of Citrus reticulata in Zaire. It morphologically resembles the holotype specimen of Cephalosporium zonatum, but does not match the original collection information. Therefore, CBS 400.70 is considered a representative culture of A. zonatum, and typification of this species awaits further collections. Based on the multi-locus phylogenetic analysis, CBS 400.70 clustered within the genus Mycocitrus and was therefore recombined as M. zonatus.
Clade O28
Emericellopsis J.F.H. Beyma, Antonie van Leeuwenhoek 6: 264. 1940.
Synonyms: Stilbella Lindau, in Engler & Prantl, Nat. Pflanzenfam., Teil. I (Leipzig) 1: 489. 1900.
Peyronellula Malan, Mycopathol. Mycol. Appl. 6: 173. 1952.
Saturnomyces Cain, Canad. J. Bot. 34: 136. 1956.
Mycelium consists septate, hyaline, often fasciculate, smooth-walled vegetative hyphae, swelling at maturity, becoming barrel-shaped in some species. Ascomata cleistothecial, spherical, submerged or immersed in agar, transparent, hyaline and membranaceous, but appearing brown or brownish black when the ascospores mature, clad with a wall consisting of several layers of tightly intertwined hyphae, glabrous, non-ostiolate, or opening at maturity with particular pore. Cleistothecial wall pseudoparenchymatous, composed of thin, hyaline, flattened cells. Asci saccate, spherical, subspherical, hyaline, indistinct and evanescent, containing 8-spores, with a thin, evanescent wall, unitunicate, disposed and scattered irregularly in the ascocarp. Ascospores ellipsoidal to ovoid, hyaline, turning slightly brown, pale greenish brown, or dark olivaceous black in colour, with uneven surfaces, with large droplets of oil, gelatinous layers collapsing to form 2–6 longitudinal, subhyaline wings or flanges at maturity, each of which emanates a hyaline, star-shaped or triangular, patterned hem, in older spores they disintegrate and become very irregular in shape. These seams are visible in front view of the spores as whip-shaped spurs; ascospores black in mass (Van Beyma 1940, Rossman et al. 1999). Conidiation abundant, phalacrogenous, nematogenous, plectonematogenous, synnematougenous. Synnemata scattered or gregarious, cylindrical-capitate, unbranched, smooth or hirsute, white, with cylindrical stipe and globose head, walls smooth to slightly roughened, marginal hyphae coiled, smooth-walled. Conidiophores erect, solitary or aggregated, erect, straight or curved, directly arising from aerial or substratal mycelium, sometimes radiating out from sterile coils or ropes formed by the mycelium, or from synnemata, unbranched and orthotropic, or branched, septate in basal, middle or upper part, hyaline, with a chromophilic base in some species, smooth-walled, rough- and thick-walled in some species, with cell walls usually thicker than those of vegetative hyphae. Conidiogenous cells mono- or polyphialidic, terminal, lateral, acicular, subulate, (sub-)cylindrical, ampulliform, awl-shaped, hyaline, thin- or thick- and smooth-walled, commonly with conspicuous or inconspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides and adelophialides present in some species. Conidia aseptate, globose, cylindrical, allantoid, oblong-elliptical, ellipsoid, ovoid, obovoid, clavate, pyriform, with rounded or truncate ends, hyaline, thin- or thick-, smooth-walled, guttulate or eguttulate, with minute brown guttules in some species, arranged in slimy heads or in chains which may collapse into heads. Conidia and hyphae vacuolate and containing a few orange-tinged granules. Chlamydospores present in some species, terminal or intercalary, mostly single or in chains, rapidly collapsing pieces of hyphae, sub-globose, (broadly-) ellipsoid, ovoid, limoniform, with truncate base at one end or at both ends, hyaline, rough-, thick-walled, covered with chromophilic warts.
Type: Emericellopsis terricola J.F.H. Beyma
Other accepted species with available sequences: E. alkalina Bilanenko, E. brunneiguttula L.W. Hou, L. Cai & Crous, E. donezkii Beliakova., E. exuviara (Sigler et al.) L.W. Hou, L. Cai & Crous, E. fimetaria (Pers.) L.W. Hou, L. Cai & Crous, E. fuci (Summerb. et al.) L.W. Hou, L. Cai & Crous, E. glabra (J.F.H. Beyma) Backus & Orpurt, E. humicola (Cain) Cain ex Grosklags & Swift, E. maritima Beliakova, E. microspora Backus & Orpurt, E. minima Stolk, E. mirabilis (Malan) Stolk, E. moniliformis (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, E. pallida Beliakova, E. pusilla P.N. Mathur, Sukapure & Thirum., E. robusta Emden & W. Gams, E. salmosynnemata Grosklags & Swift, E. salmonea (W. Gams & Lodha) L.W. Hou, L. Cai & Crous, E. stolkiae D.E. Davidson & M. Chr., E. tubakii (Gams) L.W. Hou, L. Cai & Crous
Notes: The genus Emericellopsis was established to accommodate the soilborne species E. terricola (Van Beyma 1940). Emericellopsis is characterised by the production of globose cleistothecia with globose or subglobose asci that fill the cavity, always 8-spored, and ellipsoid, ovate and brown ascospores, initially with a wide hyaline sheath, which collapses at maturity to leave several longitudinal wings (Van Beyma 1940, Gams et al. 1971). Species of Emericellopsis commonly have conspicuous acremonium-like conidial morphs, but rarely have stilbella-like synnemata (Gams et al. 1971). This study revisits the taxonomy of the five “Acremonium” names that cluster within the Emericellopsis clade based on both phylogenetic analysis and morphological characters (Fig. 2). The genus Stilbella was originally introduced by Engler & Prantl in 1900. It is well known among field biologists and amateurs by its special habitat and the morphological characters (producing stilbella-like synnemata). Although the generic name Stilbella is list as conserved name, it only against Botryonipha (Rossman et al. 2013). In the present study, the genus Stilbella (Engler & Prantl 1900, published in 1900) is synonymous with another conserved genus Emericellopsis (Van Beyma 1940, published in 1940) based on the lectotype of Stilbella, S. erythrocephalum, now regarded as S. fimetaria. In the present study, the ex-neotype culture of S. erythrocephalum (CBS 558.84) falls within the Emericellopsis clade (Fig. 2). This S. erythrocephalum culture is closely related to the ex-type culture of E. synnematicola (CBS 176.60), which was found to be connected to S. emericellopsis by single ascospore and single conidium isolations.
Emericellopsis alkalina Bilanenko, IMA Fungus 4: 222. 2013.
Description and illustration: Grum-Grzhimaylo et al. (2013).
Typus: Russia, Altai, Kulunda steppe, from soda soil (total salts 73 g/kg, pH 10.1) on the edge of the basin of Tanatar Lake, Aug. 2002, D. Sorokin, E101 (holotype CBS H-21412, ex-type culture CBS 127350 = VKM F-4108).
Additional material examined: Russia, Eastern Siberia, Baikal Lake region, unknown substrates, unknown collection date and collector, culture CBS 120049.
Emericellopsis brunneiguttula L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845858. Fig. 43.
Fig. 43.

Emericellopsis brunneiguttula (ex-type culture CBS 111360). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial heads. E–H. Conidiophores. I, J. Chlamydospores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the brown guttules in conidia produced by this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.8–3.5 μm wide. Sporulation moderate, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight, arising directly from aerial or substratal mycelium, or from mycelial ropes, usually reduced to single phialides, unbranched, poorly branched, up to 119 μm long, 1.5–4.5 μm wide at base, with 1–2 septa, hyaline, rough- and thick-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate or cylindrical, hyaline, thick-, rough-walled, (21.7–)34.5–70(–89.5) μm long, 1.5–3 μm wide at base, commonly with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, diverse shape and size, clavate, (broadly-) ellipsoid, cylindrical, both ends rounded, hyaline, thin-, smooth-walled, with several minute brown guttules, 4–14(–15.5) × 1.7–4.5 μm, arranged in slimy heads. Chlamydospores terminal or intercalary, mostly single or in chains, sub-globose, (broadly-) ellipsoid, ovoid, limoniform, with apiculate or truncate base at one end or at both ends, hyaline, rough-, thick-walled, with several brown guttules, 4.7–10(–12.5) × 3.6–7.3 μm. Sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 37–40 mm diam, flat, with moderate aerial mycelium, with floccose, alternated buff or rosy buff concentric rings, margin filiform, reverse rosy buff; On MEA reaching 20 mm diam, raised, radially folded, with moderate aerial mycelium, felty, hairy at periphery, peach at centre, rosy buff at periphery, margin lobate, reverse pale orange, with buff radial lines; On PDA reaching 27 mm diam, flat, aerial mycelium abundant, floccose, creamy white, buff at periphery, margin entire, reverse pale orange at centre, buff at periphery; On SNA reaching 30–33 mm diam, flat, sparse aerial mycelium, dusty, dirty white or colourless, margin entire, reverse concolourous.
Typus: Germany, Helgoland, from Fucus serratus (Algae), unknown collection date, A. Zuccaro (holotype CBS H-24594, ex-type culture CBS 111360).
Notes: Emericellopsis brunneiguttula associated with an alga (Fucus serratus) in Germany is described here as a new species. This fungus is phylogenetically different from the related species E. glabra and E. terricola (Fig. 2). Morphologically, E. brunneiguttula can be distinguished from E. glabra and E. terricola and other known species of Emericellopsis by its larger conidia, [4–14(–15.5) × 1.7–4.5 μm in E. brunneiguttula, 6–8 × 3.3–4 μm (most 5.3–6.7 × 3–4 μm) in E. glabra and E. terricola; van Beyma 1940], and by its longer conidiophores (up to 119 μm in E. brunneiguttula, 30–40 × 2–3 μm in E. glabra and E. terricola; van Beyma 1940). In addition, E. brunneiguttula differs from E. glabra and E. terricola in producing thick-walled chlamydospores with brown guttules (van Beyma 1940).
Emericellopsis exuviara (Sigler et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845859.
Basionym: Acremonium exuviarum Sigler et al., Stud. Mycol. 50: 411. 2004.
Description and illustration: Sigler et al. (2004).
Typus: USA, California, San Francisco, from shed dorsal skin of Corucia zebrata (Scincidae), unknown collection date, J. Paré, UW014A-B (holotype UAMH 9995, ex-type culture CBS 113360).
Emericellopsis fimetaria (Pers.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845860. Fig. 44.
Fig. 44.

Emericellopsis fimetaria (culture CBS 382.56). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Cleistothecium. E. Asci. F. Ascospores. G, I. Synnemata. H. Synnemata and cleistothecia. J–M. Conidiophores. N. Conidia. Scale bars: D, I = 50 μm; E–F, J–N = 10 μm.
Basionym: Leotia fimetaria Pers., Observ. Mycol. (Lipsiae) 2: 21. 1800 (1799).
Synonyms: Helotium fimetarium (Pers.) Pers., Syn. Meth. Fung. (Göttingen) 2: 678. 1801.
Stilbum fimetarium (Pers.) Pers., Mycol. Eur. (Erlanga) 1: 352. 1822.
Peziza fimetaria (Pers.) Fr., Syst. Mycol. (Lundae) 2(1): 157. 1822.
Sarea fimetaria (Pers.) Schwein., Trans. Am. Phil. Soc., New Series 4: 178. 1832 (1834).
Botryonipha fimetaria (Pers.) Kuntze, Revis. Gen. Pl. (Leipzig) 2: 845. 1891.
Calycina fimetaria (Pers.) Kuntze, Revis. Gen. Pl. (Leipzig) 3: 448. 1898.
Stilbella fimetaria (Pers.) Lindau, Verh. Bot. Ver. Prov. Brandenb. 47: 75. 1905.
Dendrostilbella fimetaria (Pers.) Höhn., Öst. bot. Z. 66: 110. 1916.
Clavaria mucerdae Schumach., Enum. Pl. (Kjbenhavn) 2: 405. 1803.
Mitrula mucerdae (Schumach.) Fr., Syst. Mycol. (Lundae) 1: 492. 1821.
Stilbum mucerdae (Schumach.) Hornem., Fl. Danic. 11: tab. 1852. 1825.
Hydrophora mucerdae (Schumach.) Fr., Syst. Mycol. (Lundae) 3: 315. 1832.
Stilbum erythrocephalum Ditmar, Deutschl. Fl., 3 Abt. (Pilze Deutschl.) 1: 91. 1816.
Botryonipha erythrocephala (Ditmar) Kuntze, Revis. Gen. Pl. (Leipzig) 2: 845. 1891.
Stilbella erythrocephala (Ditmar) Lindau, Nat. Pflanzenfam., Teil. I (Leipzig) 1: 489. 1900.
Stilbum leiopus Ehrenb. [as ‘lejopus’], Sylv. Mycol. Berol. (Berlin): 24. 1818.
Botryonipha leiopus (Ehrenb.) Kuntze, Revis. Gen. Pl. (Leipzig) 2: 845. 1891.
Stilbum coprophilum P. Karst., Enum. Fungorum et Myxomycetum in Lappio orientali: 219. 1866.
Stilbum leiopus var. majus Thüm. [as ‘lejopus’], Fungi Austr. Exsicc. 11–13: no. 1184. 1874.
Tubercularia lichenicola Sacc., Michelia 2(8): 561. 1882.
Knyaria lichenicola (Ces.) Kuntze, Revis. Gen. Pl. (Leipzig) 2: 856. 1891.
Stilbum caninum Cooke & Massee, Grevillea 20(no. 94): 36. 1891.
Stilbella dielsiana Reichert, Botanische Jahrbücher für Systematik Pflanzengeschichte und Pflanzengeographie 56: 725. 1921.
Emericellopsis synnematicola P.N. Mathur & Thirum., Mycologia 52: 695. 1961 (1960) [nom. inval., Art. 40.1 (Shenzhen)].
Cephalosporium incoloratum Sukapure & Thirum., Sydowia 19: 171. 1966 (1965).
Acremonium incoloratum (Sukapure & Thirum.) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 50. 1971.
Description based on culture CBS 382.62. Sexual morph: Cleistothecia submerged or immersed in agar, globose, hyaline at beginning, became black as ascospores mature, smooth, without papilla and ostiole, 120–340 × 110–310 μm. Cleistothecial wall 12.5–21 μm thick, cells hyaline. Paraphyses not observed. Asci 8-spored, scattered, (sub-)globose, 10–13(–15) × 9.5–13.5 μm, with a thin, hyaline wall. Ascospores aseptate, ellipsoid with three flanges, olive-brown to black, 5.5–8.5 × 3.7–5.5 μm. Asexual morph: Synnemata scattered or gregarious, cylindrical-capitate, unbranched, smooth or hirsute, white, 330–730 μm tall, 54–102 μm wide. Hyphae of stipe 1.8–2.5 μm wide, walls smooth to slightly roughened. Marginal hyphae coiled, smooth-walled. Conidiophores solitary or aggregated, erect, straight or curved, directly arising from aerial or substratal mycelium, or from synnemata, unbranched or branched, bearing 1–2 levels with 1–3 phialides per node, up to 82 μm long, 1.2–2.6 μm wide at base, 2–3(–4)-septate in basal, middle or upper part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate or subcylindrical, hyaline, thick-, smooth-walled, 11–35 μm long, 1.2–2.2 μm wide at base, commonly with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, ellipsoid or ovoid, rounded at both ends, hyaline, thin-, smooth-walled, eguttulate, 3.3–4.8 × 2.3–2.9 μm, arranged in slimy heads.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 40 mm diam, flat, floccose and rosy buff at centre, felty and creamy white at periphery, margin entire, reverse rosy buff; On MEA reaching 45–48 mm diam, flat, felty, radially folded, colour changed from centre with pale grey, straw, rosy buff, creamy white at periphery, margin entire, reverse pale orange, with dirty white radial lines; On PDA reaching 25–27 mm diam, flat, thinly felty, pale olivaceous buff, margin dendritic, reverse pale olivaceous buff; On SNA reaching 31–33 mm diam, flat, membranous without aerial mycelium, white, margin dendritic, reverse concolourous.
Other illustration: Mathur & Thirumalachar (1960).
Additional materials examined: Belgium, from soil, unknown collection date, J. Remacle, CBS H-12405, CBS 382.62 = DSM 3158 = VKM F-1483. India, Maharashtra, Poona, Hingne, from soil, unknown collection date, M.J. Thirumalachar, isol. 25 Jan. 1959 by R.S. Sukapure, CBS H-6644 (ex-isotype culture of Cephalosporium incoloratum CBS 146.62 = ATCC 14613 = IMI 091577 = MUCL 9722); Rajasthan, Mount Abu, from soil, unknown collection date, P.N. Mathur & M.J. Thirumalachar, isol. M.J. Thirumalachar, CBS H-12406 & CBS H-7797 (deposited as type culture of Emericellopsis synnematicola nom. inval. CBS 176.60 = ATCC 16540 = HACC 189 = IMI 081600 = MUCL 11495). Netherlands, Utrecht Province, Baarn, Groeneveld, from dung of rabbit, 9 Sep. 1984, K.A. Seifert, K.A.S. 372, CBS H-3610, culture CBS 628.85; Utrecht Province, Baarn, Groeneveld, from dung of rabbit, 23 Sep. 1967, W. Gams, No. 1216 & 1217, CBS H-18593 & CBS H-18594, culture CBS 511.67; Utrecht Province, Baarn, Groeneveld, from dung of rabbit, 9 Sep. 1984, K.A. Seifert, K.A.S. 294 (neotype of Stilbella erythrocephala CBS H-3597, ex-neotype culture CBS 558.84). Papua New Guinea, Goroka, from dung, unknown collection date and collector, isol. 20 Jan. 1970 by T. Matsushima (ex-type culture of Stilbum coprophilum CBS 117.84 = MFC 2872). Unknown, unknown substrates, unknown collection date and collector, CBS 115625. USA, Pennsylvania, Chester County, from dung, Nov. 1975, C.T. Rogerson, culture CBS 621.85 = C.T.R. 75-210.
Notes: This species was originally introduced as Leotia fimetaria (Persoon 1800). The taxonomic history of this species is complex and has been addressed by multiple authors (Persoon 1801, Fries 1822, Berkeley & Broome 1850, Kuntze 1891, Lindau 1905, Höhnel 1916). Seifert (1985) examined and designated a neotype for S. erythrocephala, a synonym of E. fimetaria, which was selected as lectotype of Stilbella by Clements & Shear (1931). Although type materials of S. fimetaria were not located and could not be examined in this study, phylogenetic analysis based on the ex-type cultures of its synonyms S. erythrocephala (CBS 558.84) and Stilbum coprophilum (CBS 117.84) places this species in a highly supported clade within the Emericellopsis clade (Fig. 2), and is therefore recombined as E. fimetaria.
Most recorded Emericellopsis species have only the cephalosporium (currently acremonium) conidial type. Emericellopsis synnematicola was the first species described that is characterised by producing the stilbaceous type of conidiophores in its asexual morph, a feature that is peculiar to Emericellopsis (Mathur & Thirumalachar 1960). However, according to the Shenzhen code, E. synnematicola is invalid because the type material was not been indicated in the protologue, which only left an “ex-type culture” deposited in CBS culture collection (Mathur & Thirumalachar 1960). In the present study, the ex-type culture of Cephalosporium incoloratum (CBS 146.62), which was isolated from the same country and same substrates as E. synnematicola, proved to be genetically identical to “ex-type culture” of E. synnematicola (CBS 176.60). Both types were placed in a highly supported clade together with cultures of E. fimetaria. Morphologically, E. fimetaria and E. synnematicola are exactly the same. The size of synnemata tends to vary among specimens from a variety of dung, but can be influenced by external factors. Additionally, E. fimetaria was observed only to produce an acremonium-like asexual morph in vitro or, in some retrogressed cultures that had lost the capacity to produce synnemata, then having asexual morphological characters matching those of C. incoloratum (Sukapure & Thirumalachar 1965). Therefore, we concluded that these types are conspecific, with E. fimetaria having priority.
The culture CBS 382.62 was originally recorded as E. salmosynnemata, but is phylogenetically distinct from the ex-type culture of E. salmosynnemata but clustered together with Emericellopsis fimetaria (Fig. 2). This culture is morphologically comparable to E. salmosynnemata and differs from the latter by the production of larger ascospores (5.5–8.5 × 3.7–5.5 μm vs 5–5.5 × 3–3.5 μm) and conidia (3.3–4.8 × 2.3–2.9 μm vs 3–4 × 1.5–2 μm).
Emericellopsis fuci (Summerb. et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845861.
Basionym: Acremonium fuci Summerb. et al., Stud. Mycol. 50: 288. 2004.
Description and illustration: Zuccaro et al. (2004).
Typus: Germany, Helgoland, from thalli of Fucus serratus (Fucaceae, brown algae), unknown collection date and collector, isol. A. Zuccaro, T.U.B. 264 (holotype CBS H-7997, ex-type culture CBS 112868).
Additional materials examined: Canada, British Columbia, from Fucus distichus (Fucaceae), 8 Aug. 1980, R.C. Summerbell, culture CBS 550.86; British Columbia, Vancouver, Littoral, from decaying thalli of Fucus distichus, 1 Jan. 1987, R.C. Summerbell, culture CBS 113887 = UAMH 6506, ibid. culture CBS 113888 = UAMH 6507; New Brunswick, Charlotte County, St. Andrews, Bar Rd., from composting seaweed, mostly Ascophyllum nodosum (Fucaceae, brown algae), unknown collection date, D. Malloch & Scott, isol. 2 Sep. 1991 by D. Malloch, No. OA6, culture CBS 485.92. Germany, Baltic Sea, Travemunde, from Ceramium sp. (Ceramiaceae, algae), unknown collection date and collector, isol. May 2004 by S. Draeger, culture CBS 116467 = TUB 406. Norway, Berlevåg municipality, Tanafjorden, Store Molvik, from driftwood (Pinus sp.) in tidal zone, 6 Sep. 2010, T. Rämä, culture TR070cS1.1; Båtsfjord municipality, Ytre Syltevika, from driftwood in splash zone, 8 Sep. 2010, T. Rämä, culture TR080bE3.1; Båtsfjord municipality, Hamningberg, Skjåvika, from coniferous driftwood in splash zone, 9 Sep. 2010, T. Rämä, culture TR085aW1.2; Kvænangen municipality, Alteidet, from old dock of Picea sp. (Pinaceae) in the sea, 9 Aug. 2010, T. Rämä, culture TR043cU1.1; Nordkapp municipality, Skarsvåg, Mefjorden, from driftwood (Pinus sylvestris) in upper tidal zone, 18 Aug. 2010, T. Rämä, culture TR061aN1.1, ibid. cultures TR061aN1.2, TR061aS1.1, TR061aS1.2, TR061cN1.1; Troms County, Nordkapp municipality, Skarsvåg, Tufjorden, from driftwood (Pinus sp.) in upper tidal zone, 18 Aug. 2010, T. Rämä, culture TR058aW2.1; ibid. cultures TR058bD1.1, TR058cD1.2, TR058cW1.2; Vadsø municipality, Varangerfjorden, Ekkerøy, from construction wood in tidal zone, 10 Sep. 2010, T. Rämä, culture TR090aU1.2; ibid cultures TR090bU1.1, TR090cD1.2. USA, California, Monterey County, South of Carmel Highlands, from composting seaweed (mostly Macrocystis pyrifera, Laminariaceae, brown algae), unknown collection date, isol. 3 Jan. 1992, coll. and isol. D. Malloch, No. OA1, culture CBS 484.92; Maine, Sagadahoc County, Bailey Island, from wrack of eelgrass (probably Zostera marina, Zosteraceae, seagrasses), 10 Aug. 2005, unknown collector, culture CBS 120611; Maine, Kittery Point, York County, from Ascophyllum nodosum (Fucaceae, brown algae), 10 Aug. 2005, D. Malloch, ACR 8-1, culture CBS 120530; Massachusetts, Paine’s Creek Beach, from wrack of Fucus vesiculosus, 9 Aug. 2005, D. Malloch, ACR 3-7, culture CBS 120529.
Notes: Emericellopsis fuci is characterised by its relatively large, ovate conidia with a truncate basal hilum and a slightly flattened apex (Zuccaro et al. 2004). Zuccaro et al. (2004) stated that the basionym, A. fuci, was the second Acremonium species with distinctively large conidia to have been described from the marine environment. Phylogenetically, the algal-associated A. fuci clustered within Emericellopsis, representing a different ecological niche for this genus (Zuccaro et al. 2004). In this study, 11 strains from diverse algae (including the type of A. fuci) and 16 strains from the coast of Norway formed a fully supported clade in the “marine clade” of Emericellopsis. Therefore, a new combination is introduced, E. fuci (Fig. 2).
Emericellopsis glabra (J.F.H. Beyma) Backus & Orpurt, Mycologia 53: 75. 1962 (1961).
Basionym: Emericellopsis terricola var. glabra J.F.H. Beyma, Antonie van Leeuwenhoek 6: 266. 1940 (1939–1940).
Description and illustration: van Beyma (1940).
Typus: Netherlands, Utrecht Province, Baarn, from soil, 1937, F.H. van Beyma, isol. 1937 by N. Schierbeek, CBS H-12388 (holotype culture CBS 119.40 preserved as metabolically inactive culture, ex-type culture CBS 119.40 = IAM 14674 = IAM 14675 = JCM 10470 = MUCL 11486).
Emericellopsis microspora Backus & Orpurt, Mycologia 53: 67. 1962 (1961).
Description and illustration: Backus & Orpurt (1961).
Typus: USA, Wisconsin, from wet prairie soil, summer 1953, unknown collector, isol. P.A. Orpurt, CBS H-12400 (holotype WSF 47 in WIS, ex-isotype culture CBS 380.62 = ATCC 14645 = IFO 9241 = IMI 092625 = MUCL 11494 = VKM F-1301 = WSF 47).
Emericellopsis minima Stolk, Trans. Brit. Mycol. Soc. 38: 419. 1955.
Description and illustration: Stolk (1955).
Typus: Mozambique, Inhaca, from mangrove soil, unknown collection date, isol. Jul. 1953, coll. and isol. H.J. Swart, CBS H-7056 (holotype K(M) 36100, ex-type culture CBS 190.55 = JCM 10472 = VKM F-1484).
Emericellopsis mirabilis (Malan) Stolk, Trans. Brit. Mycol. Soc. 38: 421. 1955.
Basionym: Peyronellula mirabilis Malan, Mycopathol. Mycol. Appl. 6: 173. 1952.
Typus: Italy, from damp soil, dead protonema of moss, isol. 1952 by C.E. Malan, No. 1 (ex-syntype of Peyronellula mirabilis CBS 177.53 = JCM 10471 = MUCL 11482); unknown substrate and collection date, isol. C.E. Malan (ex-syntype culture of Peyronellula mirabilis CBS 176.53).
Emericellopsis moniliformis (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 846143.
Basionym: Acremonium moniliforme A. Giraldo et al., Mycol. Progr. 16: 357. 2017.
Description and illustration: Giraldo et al. (2017).
Typus: Spain, Aragón, Huesca Province, Ordesa y Monte Perdido National Park, from forest soil, 15 Mar. 2011, A. Giraldo, M. Hernández, & J. Capilla, isol. A. Giraldo (holotype CBS H-22022, dried culture on OA, ex-type culture CBS 139051 = FMR 11785).
Additional materials examined: Chile, from volcanic ash soil, unknown collection date and collector, isol. J. Grinbergs, No. 520/73, culture CBS 864.73. Netherlands, Overijssel Province, Kloosterhaar, from human nail, unknown collection date and collector, isol. Streeklab. voor de Volksgezondheid, Groningen, No 1984, culture CBS 463.92.
Emericellopsis pallida Beliakova, Mikol. Fitopatol. 8: 386. 1974.
Description and illustration: Beljakova (1974).
Typus: Ukraine, Crimea, Black Sea, from water, unknown collection date and collector, isol. L.A. Belyakova (holotype CBS 490.71 preserved as metabolically inactive culture, ex-type culture CBS 490.71 = VKM F-925).
Additional material examined: Canada, Manitoba, Delta, University Field Station, from organic soil under Phragmites communis (Poaceae), unknown collection date, J. Reid, CBS H-12403, culture CBS 624.73 = UM 194.
Emericellopsis salmonea (W. Gams & Lodha) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845862.
Basionym: Acremonium salmoneum W. Gams & Lodha, Trans. Brit. Mycol. Soc. 64: 399. 1975.
Description and illustration: Gams (1975).
Typus: India, Rajasthan, Jaipur, from dung of deer, unknown collection date, B.C. Lodha, CBS H-6667 (holotype CBS 721.71 preserved as metabolically inactive culture, isotype IMI 185379 = ATCC 32185, ex-type culture CBS 721.71).
Emericellopsis salmosynnemata Grosklags & Swift, Mycologia 49: 305. 1957.
Description and illustration: Grosklags & Swift (1957).
Typus: USA, Michigan, laboratory contaminant, unknown collection date and collector, isol. 1953 by J.M. Roberts, (holotype MDH 3590A, ex-type culture VKM F-1260 = CBS 182.56 = ATCC 11661 = DAOM 64321 = IFO 9239 = IMI 058330 = NRRL 2271).
Emericellopsis stolkiae D.E. Davidson & M. Chr., Trans. Brit. Mycol. Soc. 57: 385. 1971.
Description and illustration: Davidson & Christensen (1971).
Typus: USA, South East Wyoming, from mud in saline lake, unknown collection date, M. Christensen, CBS H-7059 (holotype IMI 155476, ex-holotype culture CBS 159.71 = ATCC 22761 = IFO 9604 = RMF 3002).
Additional material examined: USA, from soil in shore of permanent alkali lake, unknown collection date and collector, culture CBS 139531.
Emericellopsis terricola J.F.H. Beyma, Antonie van Leeuwenhoek 6: 265. 1940 (1939).
Description and illustration: Beyma (1940).
Typus: Netherlands, Utrecht Province, Baarn, from soil, unknown collection date, J.F.H. Beyma isol. 1937 by H. Schierbeek, CBS H-12407 (holotype CBS 120.40 preserved as metabolically inactive culture, ex-type culture CBS 120.40 = JCM 10474).
Emericellopsis tubakii (Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845863.
Basionym: Acremonium tubakii W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 55. 1971.
Synonyms: Cephalosporium polyaleurum Tubaki, Mycologia 65: 939. 1973.
Acremonium polyaleurum (Tubaki) E.B.G. Jones et al., Fungal Diversity 35: 147. 2010.
Description and illustration: Gams (1971).
Typus: Japan, coast near Wakayama City, from marine sediment, collection date unknown, isol. 1967, coll. and isol. K. Tubaki, No. VII-4, CBS H-8458 (holotype of Acremonium tubakii CBS 790.69 preserved as metabolically inactive culture, ex-type culture CBS 790.69 = IAM 14664 = IFO 874).
Additional materials examined: India, Andhra Pradesh, Hyderabad, from Oryza sativa (Poaceae), rhizosphere, unknown collection date, B.K. Vaidehi, CBS H-8462, culture CBS 791.69 = IMI 131943. Japan, coast of Wakayama City, from seashore, unknown collection date and collector, isol. 1967 by K. Tubaki, No. IV-8, CBS H-8464 & CBS H-8465, culture CBS 824.69 = IFO 788; Wakayama Bay, from coastal mud, unknown collection date, K. Tubaki, MM-199 (holotype of Cephalosporium polyaleurum IFO-H11637, ex-type culture CBS 555.74 = IFO 9394). Netherlands, Flevoland Province, Zuidelijk Flevoland, from soil, unknown collection date and collector, isol. Sep. 1970 by G.M. Tichelaar, No. 295, culture CBS 822.70.
Notes: Emericellopsis tubakii was originally reported as Acremonium tubakii from marine sediment in Japan, near Wakayama City, which is characterised by its cylindrical conidia (3.5–5.7 × 1.5–2.3 μm), arranged in heads, and sub-globose to ovoid chlamydospores covered with chromophilic warts and arranged in chains (Gams 1971). Two years later Cephalosporium polyaleurum was described from the same kind of substrates and country as E. tubakii during a study of Emericellopsis species from the marine environment (Tubaki 1973). Morphologically, C. polyaleurum is similar with E. tubakii in its shape and size of conidia (3–6 × 1.5–2 μm) and chlamydospores (Tubaki 1973). In our molecular analyses, the ex-type of C. polyaleurum (CBS 555.74) and A. tubakii (CBS 790.69) are phylogenetically identical, grouping in a fully supported lineage in Emericellopsis (Fig. 2). Therefore, they are conspecific with E. tubakii having priority, and a new combination was introduced.
Clade O29
Stanjemonium W. Gams et al., Canad. J. Bot. 76: 1579. 1999 (1998).
Colonies slow growing, densely woolly to felty. Sporulation abundant. Conidiophores on single or slightly fasciculate aerial hyphae. Conidiogenous cells flask-shaped or cylindrical, scattered along subtending cells, either singly or in pseudowhorls (i.e., whorls not concentrated near a septum), each producing a single conidium, or producing multiple conidia arranged in slimy heads; cells collapsing soon after maturation of conidia, with short low projections on subtending hyphae. Conidia with rotational symmetry, ellipsoid to cylindrical, with apiculate base, hyaline to distinctly pigmented. Chlamydospores and sexual morph absent. (emended from Gams 1998).
Type: Stanjemonium grisellum W. Gams et al.
Other accepted species with available sequences: Stanjemonium dichromosporum (Gams & Sivasith.) L.W. Hou, L. Cai & Crous, S. fuscescens W. Gams, Schroers & Abdullah, S. ochroroseum W. Gams et al.
Notes: Stanjemonium is an asexual morph genus, and not known to be associated with any sexual morph. This genus is quite different from Acremonium and is known to have a close relationship with Emericellopsis (Fig. 2), as shown by Gonçalves et al. (2020). It was characterised by producing slow-growing colonies and pigmented conidia from flask-shaped conidiogenous cells which collapse soon after conidial maturation, leaving low projections on the subtending hyphae (Gams et al. 1998). In addition, each conidiogenous cell produces a single conidium (Gams et al. 1998). Based on the published molecular data and phylogenetic analysis, Gams et al. (1998) demonstrated the affinity between Stanjemonium and Emericellopsis. Our phylogenetic analysis results agree well with the previous study showing that Stanjemonium formed a fully supported clade in Bionectriaceae, showing a close relationship with Emericellopsis (Fig. 2).
Stanjemonium dichromosporum (Gams & Sivasith.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845864.
Basionym: Acremonium dichromosporum W. Gams & Sivasith., Trans. Brit. Mycol. Soc. 64: 397. 1975.
Synonym: Gliomastix dichromospora (W. Gams & Sivasith.) Subram., Kavaka 5: 98. 1978 (1977).
Description and illustration: Gams (1975).
Typus: Australia, Western Australia, Nedlands, from rhizosphere of Triticum aestivum (Poaceae), unknown collection date and collector, dep. K. Sivasithamparam, CBS H-6604 (holotype of Acremonium dichromosporum CBS 638.73 preserved as metabolically inactive culture, isotype IMI 185377, ex-type culture CBS 638.73 = ATCC 32181 = IMI 185377).
Notes: This species was originally reported as Acremonium dichromosporum in section Gliomastix (Gams 1975). The multi-locus phylogenetic analysis placed the ex-type strain (CBS 638.73) in the genus Stanjemonium (Fig. 2). Morphologically, S. dichromosporum differs from other known Stanjemonium species in its cylindrical conidiogenous cells and abundant conidia arranged in orange, slimy heads (Gams 1975), while the others have flask-shaped conidiogenous cells giving rise to only a single conidium (Gams et al. 1998). The uniconidial blastic cells or aphanophialides proved to coexist with typical phialides even within individual species (Zare & Gams 2001), and thus appear to be less important at the generic level. The described morphology of A. dichromosporum is consistent with the generic description of Stanjemonium in its unbranched, densely arranged phialides, and pigmented conidia (Gams 1975).
Stanjemonium fuscescens W. Gams, Schroers & Abdullah, Canad. J. Bot. 76: 1580. 1999 (1998).
Description and illustration: Gams et al. (1998).
Typus: Iraq, from desert soil, unknown collection date and collector, isol. S. Abdullah on actidione medium, dep. J. Guarro (holotype of Stanjemonium fuscescens CBS H-6076, isotypes CBS H-5711 & CBS H-8778, ex-type culture CBS 264.96 = NRRL 26546).
Stanjemonium grisellum W. Gams et al., Canad. J. Bot. 76: 1580. 1999 (1998).
Description and illustration: Gams et al. (1998).
Typus: USA, Wyoming, ca. 11 km west of Rock Springs, Sweetwater County, alt. 1 921 m, soil from native Artemisia tridentata (Asteraceae) grassland, Sep. 1978, M. Christensen, RMF 05 (holotype CBS H-6077, isotype CBS H-8777, ex-type culture CBS 655.79 = NRRL 26538).
Stanjemonium ochroroseum W. Gams et al., Canad. J. Bot. 76: 1580. 1999 (1998).
Description and illustration: Gams et al. (1998).
Typus: USA, Wyoming, approximately 11 km west of Rock Springs, Sweetwater County, alt. 1 921 m, soil from native Artemisia tridentata (Asteraceae) grassland, Sep. 1978, M. Christensen, RMF G27 (holotype CBS 656.79 preserved as metabolically inactive culture, ex-type culture CBS 656.79 = NRRL 26539).
Clade O30
Proliferophialis L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845865.
Etymology: Referring to the proliferation of conidiophores and phialides of the type.
Mycelium consisting of branched, septate, hyaline, smooth- or rough-, thin-walled hyphae. Conidiophores solitary or aggregated, erect, usually reduced to single phialides, arising from aerial, substratal mycelium, or from mycelial ropes, unbranched, basitonously branched, repeatedly proliferating sympodially or percurrently, straight or curved, hyaline, smooth- or rough-walled, with cell walls usually thicker than those of vegetative hyphae. Conidiogenous cells enteroblastic, mono- and polyphialidic, terminal or lateral, subulate, hyaline, thick-, smooth-walled, commonly with apical percurrent proliferation, and with inconspicuous periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, ovoid, obpyriform, or fusoid, with hilar spot at both apices and bases, hyaline, thick-, smooth-walled, arranged in long chains, often irregularly collapsing into conidial heads. Chlamydospores and sexual morph not observed.
Type: Proliferophialis apiculata L.W. Hou, L. Cai & Crous
Notes: The monotypic genus Proliferophialis is proposed here to accommodate four cultures clustering in a fully supported lineage in Bionectriaceae (Fig. 2). Three cultures were formerly identified as Acremonium egyptiacum and one was received as A. potronii. However, they are phylogenetically distant from the ex-type of A. egyptiacum and A. potronii, and form an independent clade that differs from all other known genera in Bionectriaceae (Fig. 2). Morphologically, Proliferophialis is characterised by producing abundant phialides with repeatedly apical percurrent proliferation.
Proliferophialis apiculata L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845866. Fig. 45.
Fig. 45.

Proliferophialis apiculata (ex-type culture CBS 303.64). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial chains. E–I. Branched or unbranched conidiophores and phialides with repeatedly apical percurrent proliferation (arrows). J. Conidia. Scale bars = 10 μm.
Etymology:Referring to its terminal conidia, which are apiculate at both ends.
Mycelium consisting of branched, septate, hyaline, smooth- or rough-, thin-walled hyphae, 1–2.5 μm wide. Conidiophores solitary or aggregated, erect, straight or irregularly curved, arising directly from aerial or substratal mycelium, or from ropes formed by mycelium, usually reduced to single phialides, unbranched, or basitonously branched, repeatedly proliferating sympodially or percurrently, up to 162 μm long, 1.8–3.2 μm wide at base, hyaline, smooth- or rough-walled 1–2-septate, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate, hyaline, thick-, smooth-walled, 17–94 μm long, 1–2 μm wide at base, commonly with apical percurrent proliferation, and with minute collarette and inconspicuous periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, ovoid, obpyriform or fusoid, with apiculate thicken hilar spot at both ends, hyaline, thick-, smooth-walled, 3.2–6.6(–7.3) ×1.9–2.7 μm, arranged in long chains, often irregularly collapsing in conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 26–27 mm diam, flat, moderate aerial mycelium, thinly felty, dusty, with mycelium ropes at centre, white, margin entire, reverse creamy white; On MEA reaching 25–26 mm diam, raised, radially folded, moderate aerial mycelium, felty, white, margin entire, reverse ochreous, with buff radial lines; On PDA reaching 30 mm diam, flat, felty, white, margin entire, reverse olivaceous at centre, rosy buff at periphery; On SNA reaching 22–23 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse concolourous.
Typus: France, Rennes, from Triticum aestivum (Poaceae), stored grain, unknown collection date and collector, isol. Sep. 1962 by J. Pelhâte, No. X3 (holotype CBS H-8155, ex-holotype culture CBS 303.64).
Additional materials examined: France, near Rennes, from Triticum aestivum (Poaceae), stored grain, unknown collection date and collector, isol. Sep. 1962 by J. Pelhâte, No. X4, CBS H-8157, culture CBS 365.64. USA, Wisconsin, from Acer saccharum (Sapindaceae), leaf litter, unknown collection date and collector, isol. G.A. Kuter, No. 1175, culture CBS 397.78 = IAM 14646; Wyoming, appr. 11 km west of Rock Springs, soil from native Artemisia tridentata (Asteraceae) grassland, unknown collection date and collector, isol. Jun. 1978 by M. Christensen, No. S 104, culture CBS 542.79.
Notes: The four cultures of Proliferophialis apiculata form a distinct clade basal to Emericellopsis and Stanjemonium (Fig. 2). Morphologically, Pro. apiculata is characterised by the production of abundant phialides with repeatedly apical percurrent proliferation.
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hits using the ITS sequence of Pro. apiculata are sequences of Stanjemonium ochroroseum [culture CBS 125923; GenBank MH864099.1, identity = 469/516 (91 %), no gaps] (except culture CBS 127849, which was probably misidentified as “A. egyptiacum”, as the ITS sequences only shares 88.5 % similarity to the ex-type strain of A. egyptiacum CBS 114785). The closest hits using the LSU sequence is a culture of Acremonium brachypenium [culture CBS 866.73; GenBank MH872547.1, identities = 759/780 (97 %), 5 gaps (0 %)]. The closest hits using the rpb2 sequence is sequence of Fusarium solani [culture CBS 102429; GenBank KM232376.1, identity = 305/403 (75.68 %), 20 gaps (4 %)]. The closest hits using the tef sequence is sequence of Amphichorda guana [culture LC5815; GenBank KX855211.1, identities = 745/808 (92 %), no gaps].
Clade O31
Acremonium Link, Mag. Gesell. Naturf. Freunde, Berlin 3(1–2): 15. 1809.
Synonyms: Monoconidia Roze, Bull. Soc. Mycol. France 13: 83. 1897.
Mastigocladium Matr., C. R. Hebd. Séanc. Acad. Sci., Paris 152: 325. 1911.
Pseudofusidium Deighton, Mycol. Pap. 118: 26. 1969.
Mycelium consisting of branched, septate, hyaline, smooth-, thin- or thick-walled hyphae, occasionally chondroid. Sporulation abundant, phalacrogenous, nematogenous, synnematogenous, plectonematogenous. Conidiophores solitary, erect, straight or irregularly curved, lateral or terminal, arising directly from aerial and substratal mycelium, or from ropes formed by mycelium, or radiating out from coils formed by mycelium, unbranched or repeatedly basitonously, verticillately or asymmetrically branched, bearing 1–10 levels with 1–4 phialides per node, proliferating sympodially percurrently in some species, 1–3(–10)-septate at apex, middle and base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Conidiogenous cells mono- or polyphialidic, lateral or terminal, awl-shaped, subulate, (sub-)cylindrical or acicular, hyaline, thin- or thick-, smooth-walled, with distinct or inconspicuous collarette and periclinal thickening at conidiogenous loci, with percurrent or subterminal proliferations in some species; chromophilic on the basal septum in some species; polyphialides with two conidiogenous loci occasionally present in some species. Conidia aseptate, fusiform, short fusoid, cylindrical, bacilliform, ovoid, obovoid, tear-shaped, pyriform, ellipsoidal, (sub-)globose, occasionally shape and size vary between those borne internally and peripherally of conidial heads in some species, with or without hilum, truncate, apiculate or rounded at apices or bases, or both ends, thin- or thick-, smooth-walled, hyaline or slightly pigmented, eguttulate or guttulate, arranged in slimy heads or in long dry chains, or arranged in long chains, soon collapsing into slimy heads. Crystals present in some species, elongated. Chlamydospores present in some species, intercalary or terminal, mostly in single chains, subglobose, hyaline, becoming pale brown with age, smooth-, thick-walled. Sexual morph not observed.
Type: Acremonium alternatum Link
Other accepted species with available sequences: Acremonium aerium L.W. Hou, L. Cai & Crous, A. acutatum W. Gams, A. brachypenium W. Gams, A. brunneisporum L.W. Hou, L. Cai & Crous, A. charticola (Lindau) W. Gams, A. chlamydosporium L.W. Hou, L. Cai & Crous, A. egyptiacum (J.F.H. Beyma) W. Gams, A. ellipsoideum L.W. Hou, Rämä, L. Cai & Crous, A. longiphialidicum L.W. Hou, L. Cai & Crous, A. gamsianum L.W. Hou, L. Cai & Crous, A. multiramosum L.W. Hou, Rämä, L. Cai & Crous, A. mycoparasiticum L.W. Hou, L. Cai & Crous, A. psychrophilum C. Möller & W. Gams, A. purpurascens (Sukapure & Thirum.) L.W. Hou, L. Cai & Crous, A. sordidulum W. Gams & D. Hawksw., A. subulatum L.W. Hou, L. Cai & Crous, A. stroudii K. Fletcher, F.C. Küpper & P. van West, A. synnematoferum L.W. Hou, Rämä, L. Cai & Crous
Acremonium acutatum W. Gams, Trans. Brit. Mycol. Soc. 64: 394. 1975.
Synonym: Cephalosporium acremonium var. cereum Sukapure & Thirum., Sydowia 19: 173. 1966 (1965).
Description and illustration: Gams (1975).
Typus: India, Varanasi, mycoparasite on Cercospora atromarginalis (Mycosphaerellaceae) on Solanum nigrum, Jun. 1971, M.S. Pavgi, strain 3, dep. W. Gams, CBS H-6634 (holotype of Acremonium acutatum CBS 682.71 preserved as metabolically inactive culture, isotype IMI 185374 = ATCC 32209, ex-type culture CBS 682.71).
Additional materials examined: India, Koyna valley, from soil, 21 May 1958, unknown collector, isol. 22 Jan. 1960 by R.S. Sukapure & M.J. Thirumalachar, dep. M.J. Thirumalachar, CBS H-24580 (holotype of Cephalosporium acremonium var. cereum HACC 117, ex-type culture CBS 140.62 = ATCC 14610 = IMI 091574 = MUCL 9730). Netherlands, South Holland Province, Rotterdam, from skin scraping of a patient with clinical eczema, unknown collection date, A. Notowicz, CBS H-8093, culture CBS 829.73.
Notes: Cephalosporium acremonium var. cereum differs from C. acremonium in topography, texture of the colonies, and measurement of conidia and conidiophores (Sukapure & Thirumalachar 1965). Sequences of the ex-type of C. acremonium var. cereum (CBS 140.62) are identical to the ex-type of Acremonium acutatum (CBS 682.71) and form a fully supported lineage within the Acremonium s. str. clade (Fig. 2). However, their morphology differs in the shape and size of conidia: Acremonium acutatum produces fusiform conidia with symmetrically elongated and truncated ends, 4–6(–8) × 1.5–2 (–2.5) μm; C. acremonium var. cereum has oblong conidia, 2.7–3.7 × 1.2–1.5 μm (Sukapure & Thirumalachar 1965, Gams 1975). In addition, hyaline chlamydospores were observed in A. acutatum, while absent in C. acremonium var. cereum. These might result from the different media used for morphological observation.
Acremonium aerium L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845867. Fig. 46.
Fig. 46.

Acremonium aerium (ex-type culture CBS 189.70). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–F. Conidiophores. G. Conidiophores radiating out from coils formed by the mycelia. H. Conidia. Scale bars = 10 μm.
Etymology: Referring to the air sample, from which the type strain was collected.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.1–2.2 μm wide. Conidiophores solitary, erect, straight, arising directly from aerial and substratal mycelium, rarely radiating out from coils formed by mycelium, usually reduced to single phialides, unbranched, straight, up to 38.5 μm long, 1.3–2 μm wide at base, hyaline, smooth-walled, often with single septum, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate, short, hyaline, thick-, smooth-walled, (7.3–)14.7–35.5 μm long, 1.3–1.8 μm wide at base, with inconspicuous periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, thin-, smooth-walled, hyaline, shape and size vary between those borne internally and peripherally of conidial heads: internal conidia (sub-)globose or ellipsoid, 3.5–5.6 × 2–3 μm; peripheral conidia oblong or cylindrical, 3–4.5 × 1.5–2.3 μm. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 21 mm diam, flat, membranous with sparse aerial mycelium, white, undulate margin, reverse buff; On MEA reaching 18–19 mm diam, flat, radially folded, rugose, felty, rosy buff, margin crenate, reverse saffron, with creamy white radial lines; On PDA reaching 30 mm diam, flat, radially folded, membranous, dirty white, margin entire, reverse creamy white, with buff radial lines.
Typus: Morocco, Rabat, from air, unknown collection date and collector, isol. M. Chabert, No. 43-G, dep. J. Nicot (holotype CBS H-8331, ex-type culture CBS 189.70 = LCP 2130).
Additional material examined: Netherlands, North Holland Province, Hilversum, from human toenail, unknown collection date and collector, isol. 1969 by J. Hoogendoorn, CBS H-8333, culture CBS 379.70C.
Notes: Acremonium aerium is fully supported in the phylogenetic analysis (BPP/MLBS 1/100 %) and closely related with A. purpurascens (Fig. 2, BPP/MLBS 1/100 %). It differs from A. purpurascens in producing straight conidiophores, while the later occasionally producing curved conidiophores. Acremonium aerium and A. purpurascens are similar by producing variably shaped and sized conidia that differ within the same conidial head, however, the internal conidia of the conidial heads of A. aerium are longer than that of A. purpurascens (3.5–5.6 μm vs 2.7–3.6 μm), while conidia at the periphery of the heads of A. aerium are smaller than that of A. purpurascens [3–4.5 μm vs (3.2–)3.8–8.3 μm].
Acremonium alternatum Link, Mag. Gesell. Naturf. Freunde, Berlin 3(1–2): 15. 1809. Fig. 47.
Fig. 47.

Acremonium alternatum (ex-type culture CBS 407.66). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Descriptions: Link (1809), Gams (1971).
Typus: Austria, Innsbruck, Stangensteig, from Hypoxylon deustum (Hypoxylaceae), Dec. 1965, isol. 22 Dec. 1965, coll. and isol. W. Gams, No. C 584 (epitype CBS H-20525, ex-epitype culture CBS 407.66; Summerbell et al. (2011)).
Notes: Gams (1968) designated Acremonium alternatum as the lectotype of Acremonium and chose four strains as representative cultures of this species. Summerbell et al. (2011) designated CBS 407.66 with a dried culture as the ex-epitype for A. alternatum. Our present study confirms the results of Summerbell et al. (2011) that the culture CBS 407.66 groups with A. charticola, A. sclerotigenum and A. sordidulum, based on which the genus Acremonium was restricted in Bionectriaceae (Fig. 2).
Acremonium brunneisporum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845868. Fig. 48.
Fig. 48.

Acremonium brunneisporum (ex-type culture CBS 413.76). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial heads and chains. F, G. Unbranched conidiophores. H. Conidiophore with apical percurrent proliferation. I. Conidia. Scale bars = 10 μm.
Etymology: Referring to the brown conidia produced by this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–2 μm wide. Conidiophores solitary, erect, straight or curved, arising directly from aerial and substratal mycelium, unbranched, poorly branched at lower part, with up two septa at base, some with apical percurrent proliferation, slightly hackly, 27.5–61.5 μm long, 1.1–2.5 μm wide at base, hyaline, smooth-walled. Phialides mostly lateral, subcylindrical or acicular, hyaline, thick-, smooth-walled, 23–40 μm long, 1.5–2 μm wide at base, with inconspicuous cylindrical collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, fusoid, with slightly thicken and truncate hilar spot at both apices and bases, thick-, smooth-walled, hyaline at first, becoming pale brown with age, 4–5.5 × 1.3–2 μm, eguttulate, arranged in long chains, soon collapsing into slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 25–26 mm diam, flat, felty, greenish olivaceous at centre, white at periphery, margin entire, reverse creamy white; On MEA reaching 24–25 mm diam, flat, radially folded, felty or hairy, with ropes of mycelial strands, dirty white, margin entire, reverse pale ochreous, with buff radial lines; On PDA reaching 33–35 mm diam, flat, felty, dusty at periphery, dark brick at centre, rosy buff at periphery, margin entire, reverse dark olivaceous at centre, pale umber to buff at periphery; On SNA reaching 27–28 mm diam, flat, felty, white, margin entire, reverse concolourous.
Typus: India, Varanasi, on Colletotrichum dematium (Glomerellaceae) on pod of Albizzia lebbek (Leguminosae), unknown collector and collection date, dep. U.P. Singh (holotype CBS H-24591, ex-type culture CBS 413.76).
Additional material examined: Russia, Moscow, from plaster, 13 Nov. 2013, V. Ponizovskaya, N. Rebrikova, CBS 142823.
Notes: In our phylogenetical analysis, Acremonium brunneisporum grouped with A. gamsianum and A. stroudi. Morphologically, A. brunneisporum differs by producing fusoid, pale brown conidia arranged in long dry chains, while A. gamsianum and A. stroudi produce globose to oblong or cylindrical, hyaline conidia arranged in slimy heads (Fletcher et al. 2017). In addition, A. brunneisporum can be distinguished from the last two species by its conidia having distinct hilar spots at both ends.
Acremonium charticola (Lindau) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 46. 1971. Fig. 49.
Fig. 49.

Acremonium charticola (culture CBS 117.25). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidial heads. E–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Basionym: Cephalosporium charticola Lindau, Rabenh. Krypt.-Fl., Edn 2 (Leipzig) 1.8: 107. 1904 (1907).
Synonym: Cephalosporium malorum Kidd & Beaumont, Trans. Brit. Mycol. Soc. 10: 110. 1924.
Description: Kidd & Beaumont (1924) (based on CBS 117.25); Gams (1971).
Materials examined: Germany, Kiel-Kitzeberg, from cellar wall, unknown collection date and collector, isol. Feb. 1966 by W. Gams, No. 610, culture CBS 402.66. UK, England, from rotten apple (Malus sp.), unknown collection date and collector, isol. M.N. Kidd, CBS H-24584 (ex-type culture of Cephalosporium malorum CBS 117.25 = MUCL 9710). Unknown, unknown host, collection date and collector, isol. J. Lacey, (171)1403, CBS H-24585, culture CBS 547.86.
Notes: Acremonium charticola was originally described as Cephalosporium charticola from damp wallpaper by Lindau (1904). Gams (1971) examined the type specimen from the S herbarium, as well as several cultures from diverse substrates, and concluded that this species is generally distributed on mouldy wallpaper and damp cellar walls. Acremonium charticola is characterised by the multiply branched conidiophores, relatively short conidia and crystal formation (Gams 1971), which could be differentiated from its closely related species A. sordidulum and A. subulatum on the phylogenetic tree (Fig. 2). Acremonium sordidulum and A. subulatum produces unbranched or poorly branched conidiophores and lack of crystals (Gams 1975). Cephalosporium malorum together with C. ballagii were synonymised under A. charticola based on morphological characters (Gams 1971). Our multi-locus phylogenetic analysis agrees with the results of Gams (1971), showing that the ex-type culture of C. malorum is genetically identical to cultures of A. charticola (Fig. 2). However, phylogenetic analysis locates the ex-type culture of C. ballagii in Niessliaceae (Fig. 1), distant from Acremonium s. str., as demonstrated previously by Summerbell et al. (2011). Therefore, C. ballagii is resurrected as a separate species treated under Niessliaceae in future studies.
Acremonium chlamydosporium L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845869. Fig. 50.
Fig. 50.

Acremonium chlamydosporium (ex-type culture CBS 414.76). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads. E. Conidial heads. F–I. Conidiophores. J, K. Chlamydospores. L. Conidia. Scale bars = 10 μm.
Etymology: Referring to the chlamydospores produced by this species.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, occasionally chondroid, 1.8–2.5 μm wide. Conidiophores solitary, erect, straight, lateral, arising directly from aerial and substratal mycelium, unbranched or basitonously branched, bearing 1–3 levels with 1–2(–3) phialides per node, 10–107.5 μm long, 1.4–2.2 μm wide at base, 1–4-septate in lower and upper part, hyaline, smooth-walled. Phialides lateral or terminal, aciculate, cylindrical, hyaline, thick-, smooth-walled, 10.8–69(–84.5) μm long, 1.2–2.2 μm wide at base, with inconspicuous cylindrical collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, cylindrical with inconspicuous truncate bases and obtuse apices, thin-, smooth-walled, hyaline, 3.3–5.8 × 1.5–2.4 μm, eguttulate, arranged in slimy heads. Chlamydospores intercalary or terminal, mostly in single chains, subglobose, hyaline, becoming pale brown with age, smooth-, thick-walled, 2.9–6 × 3–4.7 μm. Sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 20–21 mm diam, flat, dusty, white, margin entire, reverse dirty white; On MEA reaching 20–22 mm diam, radially folded, flat, membranous, rosy buff, margin entire, reverse saffron, with buff radial lines; On PDA reaching 26–27 mm diam, flat, radially folded at centre, thinly felty, creamy white, margin fimbriate, reverse creamy white, with buff radial lines; On SNA reaching 18–20 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse colourless.
Typus: India, Uttar Pradesh, Varanasi, on Colletotrichum dematium (Glomerellaceae) on pods of Albizia lebbek (Fabaceae), unknown collection date and collector, dep. U.P. Singh (holotype CBS H-24592, ex-type culture CBS 414.76).
Notes: Both Acremonium chlamydosporium and its closely related species A. acutatum are facultative or obligate mycoparasitic fungi. Acremonium chlamydosporium is morphologically similar to A. acutatum in the shape of conidiophores and the size of conidia, but it could be distinguished by its multi-septate conidiophores (up to 4-septate vs 1-septate), longer phialides [10.8–69(–84.5) μm vs 20–40 μm], different shape of conidia (cylindrical with truncate bases and obtuse apices vs fusiform with more or less symmetrically elongated and minutely truncated ends) (Gams 1975).
Acremonium egyptiacum (J.F.H. Beyma) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 64. 1971. Fig. 51.
Fig. 51.

Acremonium egyptiacum (ex-type culture CBS 114785). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Basionym: Oospora egyptiaca J.F.H. Beyma, Centbl. Bakt. ParasitKde, Abt. II 89: 243. 1933.
Synonyms: Cephalosporium sclerotigenum Moreau & R. Moreau ex Valenta, Acta Acad. Sci. Nat. Morav.-Siles. 20: 4. 1948.
Acremonium sclerotigenum (Moreau & R. Moreau ex Valenta) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 45. 1971.
Description and illustration: Gams (1971).
Typus: Egypt, Cairo, from soil, unknown collection date and collector, isol. Sabet, G5, CBS H-24583, ex-type culture of Oospora egyptiaca CBS 114785 = IFO 4607 = NBRC 4607.
Materials examined: Canada, Ontario, Toronto, from soft contact lens from inflamed human eye, unknown collection date and collector, isol. 1989 by R.C. Summerbell, culture CBS 109041. France, Dunkerque, fishmeal, unknown collection date and collector, isol. Aug. 1964 by G.L. Hennebert, culture CBS 740.69 = MUCL 6966; Nancy, from human toenail, unknown collection date and collector, isol. E. Kiffer, culture CBS 270.86; Pointe de Chemoulin, from dune sand, under Ammophila and Convolvulus sp. (Convolvulaceae), unknown collection date and collector, dep. F. Moreau, CBS H-24762 (ex-type culture of Cephalosporium sclerotigenum CBS 124.42 = ATCC 22616 = IAM 14662); Torbali, Izmir, from root of Lycopersicon esculentum (Solanaceae), unknown collection date and collector, isol. I. Karaca, No. 106B, CBS H-8158, culture CBS 734.69. Germany, former DDR, unknown substrate, collection date and collector, isol. G. Sörgel, No. 4081, CBS H-8377, culture CBS 286.70B; former West-Germany, Hamburg, from human toenail, unknown collection date and collector, isol. H. Listemann, culture CBS 391.89; Kiel-Kitzeberg, from mouldy cellar wall, unknown collection date and collector, isol. 4 Nov. 1965 by W. Gams, No. 568, CBS H-24581, culture CBS 403.66. Greece, Thessaloniki, from human peritoneal fluid, Dec. 2002, E. Bibashi, culture CBS 112783. Iran, Sarab, from leaf of Hordeum vulgare (Poaceae), unknown collection date, B. Askari, culture CBS 114321; unknown substrate, collection date and collector, dep. R. Zare, Department of Botany, Plant Pests & Diseases Research Institute (Tehran, Iran), culture CBS 113719. Netherlands, North Holland Province, Amsterdam, from bark of apple and pear trees (Rosaceae), unknown collection date and collector, isol. J. Scheepens, culture CBS 500.82. Syria, from grape vines, unknown collection date and collector, dep. K.A. Halim, No. Syrie 1, culture CBS 121405. USA, unknown substrate, collection date and collector, isol. Aug. 1960 by M.A. Pisano, No. A-29c, CBS H-24582, culture CBS 159.61. UK, Bristol, from human toenail, isol. 9 Oct. 1972 by M.P. English, CBS H-8389, culture CBS 629.73 = IMI 171213.
Notes: Acremonium sclerotigenum and A. egyptiacum were frequently reported to be associated with food contamination and human disease. Acremonium sclerotigenum was synonymised to A. egyptiacum by Summerbell et al. (2018) based on the examination of 72 isolates on morphological characters and phylogenetic analysis of ITS and ACT sequences. According to our phylogenetic analysis based on ITS, LSU, rpb2 and tef 1-α four genes, three well-supported subclades were recognised in A. egyptiacum, corresponding to the three ITS barcode groups delimited by Summerbell et al. (2018). The ex-type culture of A. sclerotigenum (basionym: Cephalosporium sclerotigenum) and A. egyptiacum (basionym: Oospora egyptiaca) clustered in the first clade, consistent with the ITS barcode group 1 illustrated by Summerbell et al. (2018), which contains strains commonly from sand, mouldy cellar wall, plant sources (Hordeum vulgare, tomato root, grapevine, bark of apple and pear trees), food-related sources (fishmeal), rarely from human (toe nail of man). The remaining isolates of A. egyptiacum were recognised as comprising two small clades, consistent with ITS barcode group 2 and 3 in Summerbell et al. (2018). Strains in these groups were commonly reported as clinical agents associated with disseminated infection in immunocompromised patients and with onychomycosis cases. Also, a few isolates were from soil, plants or marine substrates.
Acremonium ellipsoideum L.W. Hou, Rämä, L. Cai & Crous, sp. nov. MycoBank MB 845870. Fig. 52.
Fig. 52.

Acremonium ellipsoideum (ex-type culture CBS 147433). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–F. Conidiophores and conidial heads. G, H. Unbranched conidiophores. I, J. Conidiophores with mono- and polyphialides, and proliferating phialides. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the ellipsoid conidia produced by this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–1.8 μm wide. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregate, erect, straight or bent, arising directly from aerial and substratal mycelium, or from ropes formed by the mycelium, often reduced to single phialides, unbranched, poorly branched, occasionally proliferating sympodially, 11.5–29 μm long, 1.3–2.1 μm wide at base, 1–2-septate at base, hyaline, smooth-walled. Phialides mostly lateral, subulate, hyaline, straight or irregularly curved, thick-, smooth-walled, 10–27 μm long, 1.2–2 μm wide at base, with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci, percurrent sympodialy proliferating phialides can be present; polyphialides with up to two conidiogenous loci commonly present. Conidia aseptate, subglobose to broadly ellipsoid, or cylindrical, occasionally constricted at middle, both ends rounded, thin-, smooth-walled, hyaline, 2.5–4.1 × 1.8–2.4 μm, eguttulate, arranged in slimy conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics: Colonies on OA reaching 15–17 mm diam, flat, with moderate aerial mycelium, felty, white, mycelial ropes present, margin entire, reverse creamy white; On MEA reaching 15 mm diam, raised, radially folded, with dense aerial mycelium, felty, buff, margin crenate, reverse luteous, with buff radial lines; On PDA reaching 16–17 mm diam after 14 d at 25 °C, raised, with moderate aerial mycelium, floccose, buff at centre, creamy white at periphery, margin crenate, reverse buff; On SNA reaching 18–20 mm diam, flat, with sparse aerial mycelium, dusty, dirty white, margin crenate, reverse concolourous. Without odour on all media.
Typus: Norway, Troms County, Kvænangen municipality, Alteidet, from old dock of Pinus sylvestris (Pinaceae) in the sea, 9 Aug. 2010, T. Rämä (holotype CBS H-24725, ex-type culture CBS 147433 = CPC 40317 = TR042aS1.1).
Additional materials examined: Norway, Troms County, Kvænangen municipality, Alteidet, from old dock of Pinus sylvestris in the sea, 9 Aug. 2010, T. Rämä, culture CBS 147434 = CPC 40329 = TR042aE1.1; Troms County, Sørøya, Hasvik municipality, Sørsandfjorden, from coniferous driftwood in tidal zone, 14 Aug. 2010, T. Rämä, culture CBS 147435 = CPC 40320 = TR049aU1.2.
Notes: The fungus was isolated from wood in the sea as explained in Rämä et al. (2014). In the phylogenetic tree (Fig. 2), the ex-type strain of Acremonium ellipsoideum as well as CBS 147434 and CBS 147435, which all have been isolated from coniferous wood in marine environment, formed a fully supported, independent branch. This fungus is clearly separated from the other three species found in the seacoast in Norway, A. multiramosum, A. synnematoferum and Protocreopsis finnmarkica (Fig. 2). Morphologically, A. ellipsoideum differs from its closest species, A. alternatum and A. psychrophilum, in the production of shorter phialides [10–27 μm long in A. ellipsoideum vs 16–30(–38) μm long in A. alternatum and 20–70 μm long in A. psychrophilum] and shorter conidia (2.5–4.1 × 1.8–2.4 μm, subglobose to broadly ellipsoid in A. ellipsoideum vs 4.3–5 × 1.9–2.1 μm, elongated obovate, rounded at the apex, clearly apiculate at the base in A. alternatum; 5–13.5 × 2–2.5 μm, cylindrical or ellipsoidal in A. psychrophilum; Gams 1971).
Acremonium gamsianum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845873. Fig. 53.
Fig. 53.

Acremonium gamsianum (A–I from ex-type culture CBS 881.73, J–L from culture CBS 145769). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Hyphal rope with conidiophores. E–G. Conidiophores. H. Moniliform mycelium. I, L. Conidia. J, K. Conidiophores radiating out from coils formed by the mycelia. Scale bars = 10 μm.
Etymology: Named in honour of Prof. K. Walter Gams, who was an avid collector of microfungi, and collected this species in India.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 0.8–2.4 μm wide, abundant moniliform, thick-walled mycelium present. Sporulation moderate, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary, (sub-)erect, straight or curved, arising directly from aerial and substratal mycelium, or from ropes formed by mycelium, or radiating out from sterile coils formed by mycelium in some strains, unbranched, poorly branched, 20.5–56 μm long, 1–1.7 μm wide at base, 1–2-septate in basal or apical parts, hyaline, smooth-walled. Phialides mostly lateral, subcylindrical or acicular, hyaline, thick-, smooth-walled, (8.5–)20–43.5 μm long, 1–1.6 μm wide at base, up to 55 μm × 1.9 μm in some strains, with inconspicuous cylindrical collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, thin-, smooth-walled, hyaline, short cylindrical, ellipsoidal, with obtuse apices and apiculate bases, 2.6–6.8 × 1.5–2 μm, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 30–31 mm diam, flat, slightly rugose at centre, membranous without aerial mycelium, dusty at periphery, white, margin entire, reverse concolourous; On MEA reaching 26–30 mm diam, flat, hairy, with radially ropes formed of mycelial strands, white, with irregular-shaped crystals, margin entire, reverse saffron; On PDA reaching 40 mm diam, flat, felty, hairy at centre, rugose, dirty white, margin filiform, reverse dirty white; On SNA reaching 32 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse concolourous. Without odour on all media.
Typus: India, Bangalore, Hortus Lal Bagh, from decaying leaf of Dracaena sp. (Asparagaceae) besides Zygosporium oscheoides (Zygosporiaceae), Jan. 1973, W. Gams (holotype CBS H-24590, ex-type culture CBS 881.73).
Additional material examined: Malta, Gozo, from leaf litter of Citrus sinensis (Rutaceae), 10 Jul. 2016, V. Guarnaccia, CBS H-24324, culture CBS 145769 = CPC 31177.
Notes: Acremonium gamsianum is represented in the phylogenetic tree by two cultures from India and Malta, and grouped in a distinct clade close to A. stroudii (Fig. 2). It differs morphologically from A. stroudii by having moniliform mycelium while no such structures are formed by A. stroudii. In addition, A. gamsianum produces cylindrical, ellipsoidal, relatively large conidia (2.6–6.8 × 1.5–2 μm) while conidia of A. stroudii are globose to oblong [1.2–2.0(–3) μm diam] (Fletcher et al. 2017). Although culture CBS 145769 has 32 bp (ITS 5 bp, rpb2 20 bp, tef-1α 7 bp) differences with the ex-type strain CBS 881.73, the morphological characters of both strains show no significant differences, thus the cultures are treated as conspecific.
Acremonium longiphialidicum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845871. Fig. 54.
Fig. 54.

Acremonium longiphialidicum (ex-type culture CBS 451.70). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores and conidial heads. F–H. Conidiophores. I. Conidia. Scale bars = 10 μm.
Etymology: Relating to the long phialides produced by this species.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–1.8 μm wide. Sporulation phalacrogenous, nematogenous. Conidiophores solitary, erect, straight, lateral, arising directly from aerial and substratal mycelium, unbranched or branched, commonly proliferating sympodially, bearing 1–2(–5) levels with 1–2 phialides per node, up to 290(–340) μm long, 1.4–2.8 μm wide at base, 1–3(–5) septate in basal and middle part, occasionally up to 10-septate, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, cylindrical or acicular, hyaline, thick-, smooth-walled, 51–123(–138.5) μm long, 1.3–2 μm wide at base, with conspicuous flared collarette and periclinal thickening at conidiogenous loci, occasionally with percurrent or subterminal proliferations; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, variable in shape and size, ovoid, ellipsoidal, oblong or cylindrical, thin-, smooth-walled, hyaline, variable in size, 4–10(–13) × 1.8–2.9 μm, eguttulate or two medium guttules, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics: after 14 d at 25 °C: Colonies on OA reaching 26–28 mm diam, flat, sparse aerial mycelium, dusty, white, margin entire, reverse creamy white; On MEA reaching 22–24 mm diam, flat, radially folded, with moderate aerial mycelium, hairy at centre, felty at periphery, creamy white at centre, white at periphery, margin crenate, reverse saffron, with buff radial lines at periphery; on PDA reaching 30 mm diam, flat, with abundant aerial mycelium, hairy and white at centre, felty and vinaceous buff at periphery, margin entire, reverse buff, with creamy white radial lines; On SNA reaching 27–28 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse concolourous.
Typus: UK, England, London, Hampton Court Park, from Daldinia concentrica (Hypoxylaceae), unknown collection date and collector, isol. 1967 by W. Gams, No. 1278 (holotype CBS H-24654, isotype CBS H-8124, ex-type culture CBS 451.70).
Notes: Culture CBS 451.70 was previously identified as Acremonium charticola based on morphological characters (Gams 1971). According to the multi-locus phylogenetic analysis, this culture forms an independent branch in the genus Acremonium, but was clearly separate from other species and distant from the ex-type of A. charticola (Fig. 2). Morphologically, CBS 451.70 could be clearly distinguished from other known Acremonium species by producing relatively long, multi-septate conidiophores [up to 290(–340) μm], and variably sized conidia.
Acremonium multiramosum L.W. Hou, Rämä, L. Cai & Crous, sp. nov. MycoBank MB 845876. Fig. 55.
Fig. 55.

Acremonium multiramosum (ex-type culture CBS 147436). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores and conidial heads. F–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the multiply branched conidiophores produced by this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.8–3.6 μm wide. Sporulation abundant, phalacrogenous, nematogenous. Conidiophores solitary, erect, straight or irregularly curved, lateral or terminal, arising directly from aerial and substratal mycelium, alternately arranged, repeatedly basitonously, verticillately or asymmetrically branched, bearing 1–10 levels with 1–4 phialides per node, up to 505 μm long, 2–2.5 μm wide at base, with (2–)3–5(–8) septa at middle and base, hyaline, smooth-walled. Phialides mostly lateral, subcylindrical or acicular, hyaline, thick-, smooth-walled, (12.3–)22.5–63.5 μm long, 1.5–2.8 μm wide at base, with inconspicuous collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, cylindrical, bacilliform, symmetrically rounded, or with a truncate and elongated hilum at basal end, thin-, smooth-walled, hyaline, 3.3–7.7(–9.2) × 1.3–2.5 μm, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 13–15 mm diam, flat, with sparse aerial mycelium, thinly felty, buff at centre, white at periphery, margin entire, reverse creamy white; On MEA reaching 15 mm diam, raised, radially and deeply folded, rugose, with densely aerial mycelium, felty, white, margin crenate, reverse apricot, with saffron edges, with creamy white radial lines; On PDA reaching 15 mm diam, raised at centre, flat at periphery, radially folded, thinly felty, rosy buff, margin crenate, reverse buff, with creamy white radial lines; On SNA reaching 10 mm diam, flat, with sparse aerial mycelium, dusty, creamy white, margin crenate, reverse concolourous. Without odour on all media.
Typus: Norway, Troms County, Sørøya, Hasvik municipality, Norsandfjorden, from wood (Betula sp., Betulaceae) in tidal zone, 15 Aug. 2010, T. Rämä (holotype CBS H-24726, ex-type culture CBS 147436 = CPC 40321 = TR052cE1.1).
Notes: This fungus was isolated from intertidal wood as explained in Rämä et al. (2014). Phylogenetically, Acremonium multiramosum is represented by a single strain forming a basal branch and is distant from other species in Acremonium (Fig. 2). The morphology of this species is unique: it can be distinguished by its repeatedly branched conidiophores up to 505 μm long and by its long conidia [3.3–7.7(–9.2) μm]. Although it is positioned on a very long basal branch and possibly represents a different genus, we decided to retain it in the genus Acremonium until more information and cultures become available.
Acremonium mycoparasiticum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845877. Fig. 56.
Fig. 56.

Acremonium mycoparasiticum (ex-type culture CBS 188.80). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Mycelial ropes with conidiophores and conidial heads. E–H. Conidiophores. I. Conidia. Scale bars = 10 μm.
Etymology: Referring to the mycoparasitic habit of this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–1.6 μm wide. Conidiophores solitary, erect, straight or curved, lateral or terminal, arising directly from aerial and substratal mycelium, or from the ropes formed by the mycelium, unbranched, poorly basitonously branched, 22.5–42 μm long, 1.4–2.3 μm wide at base, 1-septate at base, hyaline, smooth-walled. Phialides mostly lateral, rarely terminal, subcylindrical or acicular, hyaline, thin-, smooth-walled, 28.5–40.5 μm long, 1.4–2 μm wide at base, with conspicuous cylindrical collarette and inconspicuous periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, thin-, smooth-walled, hyaline, tear-shaped or obovoid, with obtuse apices and elongated, apiculate bases, 2.7–5.3 × 1.6–2.3 μm, eguttulate, arranged in dry conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 16–17 mm diam, flat, with sparse aerial mycelium, dusty, white, margin entire, reverse creamy white; On MEA reaching 17–19 mm diam, raised, radially and deeply folded, with moderate aerial mycelium, felty, creamy white, margin crenate, reverse pale luteous, with buff radial lines; On PDA reaching 20–21 mm diam, flat, radially folded, with moderate aerial mycelium, felty to granulose, creamy white, margin entire, reverse buff, with creamy white radial lines. Without odour on all media.
Typus: Colombia, Cundinamarca, bosque sobre Cogua, alt. ca. 3 000 m, from ascomycete on decaying leaf, unknown collection date, isol. 6 Dec. 1979, coll. and isol. W. Gams (holotype CBS H-24588, ex-type culture CBS 188.80).
Additional material examined: Netherlands, Utrecht Province, Baarn, Cantonspark, from Phytoptus pyri (=Eriophyes pyri, plant mite) on Pyrus communis (Rosaceae), unknown collection date and collector, isol. H.A. van der Aa, CBS H-24589, culture CBS 684.71.
Notes: Based on the phylogenetic analyses, Acremonium mycoparasiticum is represented by two strains from fungus and mites, respectively, forming a distinct clade related to A. alternatum, A. ellipsoideum and A. psychrophilum (Fig. 2). Morphologically, A. mycoparasiticum can be differentiated from all these species in its tear-shaped or obovoid conidia with one end slightly elongated and apiculate (2.7–5.3 × 1.6–2.3 μm), arranged in dry conidial heads. The conidia of A. alternatum are elongated obovate, rounded at the apex, clearly apiculate at the base (4.3–5 × 1.9–2.1 μm) and arranged in chains (Gams 1971), those of A. ellipsoideum are subglobose to broadly ellipsoid or cylindrical (2.5–4.1 × 1.8–2.4 μm), and the conidia of A. psychrophilum cylindrical to ellipsoid (5–13.5 × 2–2.5 μm; Möller & Gams 1993).
Acremonium psychrophilum C. Möller & W. Gams, Mycotaxon 48: 445. 1993.
Description and illustration: Möller & Gams (1993).
Material examined: Antarctica, King George Island, slope near Argentinian station Jubany (current name Carlini Base), from Turgidosculum complicatum (lichen, Verrucariaceae), Dec. 1991, C. Möller, No. 6/22, CBS H-5321, culture CBS 139.93.
Notes: Acremonium psychrophilum isolated from the lichen Turgidosculum complicatum in Antarctica differs from other known lichen-inhabiting Acremonium species (including A. antarcticum, A. lichenicola, A. rhabdosporum, A. spegazzinii) based on morphological characteristics (Möller & Gams 1993). Acremonium psychrophilum was originally classified in Acremonium section Gliomastix because of the production of chondroid hyphae and thick-walled phialides (Möller & Gams 1993). Although the ex-type culture, CBS 732.92, is unavailable to us, the strain CBS 139.93 was examined, which had the same host, location and was identified by the original authors as this species (Möller & Gams 1993). It was recognised as a possible ex-type culture of A. psychrophilum based on the information recorded in the CBS culture collection. Phylogenetically, the strain CBS 139.93 clusters within the genus Acremonium, and is closely related to the type, A. alternatum (Fig. 2).
Acremonium purpurascens (Sukapure & Thirum.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845878. Fig. 57.
Fig. 57.

Acremonium purpurascens (ex-type culture CBS 149.62). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–H. Conidiophores. I. Conidia. Scale bars = 10 μm.
Basionym: Cephalosporium purpurascens Sukapure & Thirum., Bull. Torrey Bot. Club. 93: 310. 1966.
Illustration: Sukapure & Thirumalachar (1966).
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–1.6(–2.5) μm wide. Conidiophores solitary or aggregate, erect, straight or waved, arising directly from aerial and substratal mycelium, usually reduced to single phialides, unbranched, poorly branched, up to 40 μm long, 1.3–2.1 μm wide at base, hyaline, smooth-walled, 1-septate at base, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate, hyaline, thin-, smooth-walled, 12–31 μm long, 1.2–2 μm wide at base, with inconspicuous periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, thin-, smooth-walled, eguttulate, hyaline, arranged in slimy heads, shape and size vary between those borne internally and peripherally of conidial heads: internal conidia (sub-)globose or ellipsoid, 2.7–3.6 × 2.5–3.2 μm; peripheral conidia are cylindrical, (3.2–)3.8–8.3 × 1.7–2.4 μm; conidial heads confluent in old cultures. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 22 mm diam, flat, dusty or membranous with sparse aerial mycelium, white, pale salmon at centre, margin entire, reverse concolourous; On MEA reaching 27–29 mm diam, flat, radially and deeply folded, hairy and buff at centre, felty and rosy buff at periphery, margin slightly crenate, reverse pale ochreous, with buff radial lines at periphery; On PDA reaching 35–36 mm diam, flat, radially folded at centre, dusty, rosy buff at centre, dirty white towards periphery, margin fimbriate, reverse creamy white.
Typus: India, Koyna Valley, Chiplun, from soil, unknown collection date and collector, isol. 1 Aug. 1957 by R.S. Sukapure & M.J. Thirumalachar, dep. W. Gams, CBS H-8273 (holotype HACC 104, ex-type culture CBS 149.62 = ATCC 14611 = IMI 091575 = MUCL 9709 = HACC 104).
Additional material examined: Unknown, from human skin, unknown collection date and collector, isol. 1966 by Vink, No. 354, CBS H-8327, culture CBS 780.69.
Notes: Sukapure & Thirumalachar (1966) described Cephalosporium purpurascens from soil in India. This species was once synonymised with Acremonium persicinum (Gams 1971). We re-examined the ex-type culture of C. purpurascens (CBS 149.62) and concluded that the size of the structures observed in this study differs slightly from those described in the protologue (Sukapure & Thirumalachar 1966). The conidiophores are slightly larger than those recorded in the protologue (up to 40 μm vs 15–25 μm). In addition, the shape and size of conidia differ among conidia held internally in the conidial heads and those held on the periphery. The conidia found internally are (sub-)globose, consistent with the original description; the external conidia are cylindrical, a finding not described in the protologue. According to the multi-locus phylogenetic analysis, the ex-type culture of C. purpurascens clustered within Acremonium s. str. (Fig. 2), distant from A. persicinum (currently Verruciconidia persicina). Thus, C. purpurascens is transferred to Acremonium as A. purpurascens. It differs from the closest species A. brachypenius in its conidia with variable shape and size internal and peripheral of the conidial heads. Morphologically differences of A. purpurascens and A. aerium were discussed under A. aerium.
Acremonium sordidulum W. Gams & D. Hawksw., Trans. Brit. Mycol. Soc. 64: 392. 1975.
Description and illustration: Gams (1975).
Typus: India, Tamil Nadu, from old stem of Euphorbia tirukalli, Jan. 1973, W. Gams (holotype CBS H-6669, ex-type living culture CBS 385.73 = ATCC 32186 = IMI 185373).
Additional material examined: Brazil, Fortaleza, from Ravenelia sp. (Raveneliaceae) on Stryphnodendron coriaceum (Fabaceae), 1999, F. Freire, culture CBS 102413.
Note: Acremonium sordidulum has a strong resemblance to A. egyptiacum, but differs in the production of shorter phialides and grey pigmentation (Gams 1975).
Acremonium stroudii K. Fletcher, F.C. Küpper & P. van West, sp. nov. MycoBank MB 848120.
Etymology: Named after the collector (Stedson Stroud) of the initial field material.
Description and illustration: Fletcher et al. (2017).
Typus: UK, Ascension Island, Whale Point, from environmental sample taken from a seawater blow hole, 31 Aug. 2012, S. Stroud & J. Sim (holotype CBS 138820 preserved as metabolically inactive culture, ex-type culture CBS 138820).
Notes: Acremonium stroudii was invalidly described (Fletcher et al. 2017), because the citation of the identifier issued for the name was not included, and the type of the name was not indicated in the protologue (International Code of Nomenclature for algae, fungi, and plants ShenZhen, Art. F.5.1. and Art. 40.1). The taxon is thus validated here.
Acremonium subulatum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845879. Fig. 58.
Fig. 58.

Acremonium subulatum (ex-type culture CBS 588.73A). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads and conidial chains. E–H. Conidiophores. I. Conidia. Scale bars = 10 μm.
Etymology: Referring to the subulate shape of its phialides.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–1.5 μm wide, mycelial ropes present. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary, erect, straight or curved, arising directly from aerial and substratal mycelium, or from ropes formed by mycelium, usually reduced to single phialides, unbranched or poorly basitonously branched, up to 37.5 μm long, 1.2–1.8 μm wide at base, hyaline, smooth-walled, 1-septate at base, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate, hyaline, thick-, smooth-walled, 13.7–35.5 μm long, 1.3–1.9 μm wide at base, with inconspicuous periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, thin-, smooth- or rough-walled, short fusoid, with thick and truncate hila at apices and bases, or with rounded apices and apiculate bases, hyaline, 2.8–5.3 × 1.5–2.3 μm, arranged in long chains, irregularly collapsing into heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 23–25 mm diam, flat, dusty, salmon at centre, white at periphery, margin entire, reverse buff; On MEA reaching 15–18 mm diam, flat, radially folded, rugose, felty, salmon, creamy white at periphery, margin crenate, reverse pale ochreous, with buff radial lines; On PDA reaching 23–24 mm diam, flat, radially folded and hairy at centre, membranous at periphery, white, margin entire, reverse buff; On SNA reaching 20–22 mm, flat, dusty, white, margin entire, reverse white.
Typus: Sri Lanka, Anuradhapura, from fruit of Annona reticulata (Annonaceae), unknown collection date, isol. Jan. 1973, coll. and isol. W. Gams (holotype CBS H-24587, ex-type culture CBS 588.73A).
Additional material examined: South Africa, Western Cape Province, Paarl, Zandrift, from Vitis vinifera (Vitaceae), unknown collection date, isol. L. Mostert, LM 89, CBS H-24586, culture CBS 115996 = CPC 5434.
Notes: Strain CBS 588.73A was initially received as ‘A. sordidulum’ based on morphological characters (Gams 1975). However, it formed an independent branch together with strain CBS 115996. This branch was found to be distinct from that bearing the ex-type culture of A. sordidulum (Fig. 2). Morphologically, A. subulatum differs from the closely related A. sordidulum by its colony that is salmon on OA and MEA, and whitish at PDA after two weeks. The colony of A. sordidulum is white to pale greenish grey. Although culture CBS 115996 has 33 bp (ITS 4 bp, LSU 3 bp, rpb2 10 bp, tef-1α 16 bp) differences with CBS 588.73A, the morphological characters of the cultures show no significant differences and the two are thus treated as conspecific.
Acremonium synnematoferum L.W. Hou, Rämä, L. Cai & Crous, sp. nov. MycoBank MB 845880. Fig. 59.
Fig. 59.

Acremonium synnematoferum (ex-type culture CBS 147431). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Mycelial ropes and conidiophores. F–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the conidiophores arranged in synnemata.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.1–2 μm wide, abundant mycelial ropes formed. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous, synnematogenous. Conidiophores solitary or aggregate, (sub-)erect, straight or slightly bended, lateral or terminal, arising directly from aerial and substratal mycelium, or from ropes formed by the mycelium, unbranched, poorly branched, bearing up to two phialides per node, forming synnemata-like ropes, 27–68.6(–99) μm long, 1.1–2.5 μm wide at base, 1–4-septate at base, hyaline, smooth-walled. Phialides mostly lateral, acicular, hyaline, thick-, smooth-walled, 20–51 μm long, 1.1–2 μm wide at base, with conspicuous cylindrical collarette and periclinal thickening at conidiogenous loci; polyphialides not observed. Conidia aseptate, oblong, apices rounded, with a slightly truncate hilum at bases, thin-, smooth-walled, hyaline, 2.8–5.8 × 1.6–2.3 μm, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 15 mm diam, flat, with moderate aerial mycelium, felty, short hairy, white, creamy white or salmon at centre, margin entire, reverse creamy white; On MEA reaching 14–16 mm diam, raised, radially folded, with moderate aerial mycelium, felty, rosy buff, margin crenate, reverse pale apricot at centre, saffron at periphery, with buff radial lines; On PDA reaching 17–19 mm diam, flat, with moderate aerial mycelium, felty, buff, margin entire, reverse buff; On SNA reaching 15–17 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse concolourous. Without odour on all media.
Typus: Norway, Troms County, Vadsø municipality, Varangerfjorden, Ekkerøy, from construction wood in tidal zone, 10 Sep. 2010, T. Rämä (holotype CBS H-24727, CBS 147431 = CPC 40324 = TR090cU1.1).
Additional materials examined: Norway, Troms County, Berlevåg municipality, Tanafjorden, Store Molvik, from driftwood (Pinus sp., Pinaceae) in tidal zone, 6 Sep. 2010, T. Rämä, culture TR070aS1.2; Troms County, Berlevåg municipality, Sandfjorden, from driftwood (Larix sp., Pinaceae) in tidal zone, 7 Sep. 2010, T. Rämä, culture CBS 147430 = CPC 40330 = TR076aD1.1; Troms County, Båtsfjord municipality, Ytre Syltevika, from driftwood (Pices sp.) in splash zone, 8 Sep. 2010, T. Rämä, culture CPC 40326 = TR079cD1.1; Troms County, Berlevåg municipality, Sandfjorden, from driftwood (Larix sp., Pinaceae) in tidal zone, 7 Sep. 2010, T. Rämä, culture CBS 147432 = CPC 40325 = TR076cU1.1.
Notes: Acremonium synnematoferum is represented by five cultures from intertidal wood in Norway that were isolated as explained in Rämä et al. (2014). According to the phylogenetic inference, they fall into a separate clade and differ from other known species of Acremonium and from the other two novel species described from this location and habitat. Morphologically, sporulation of A. synnematoferum is abundant and synnematogenous, forming synnemata-like ropes, which is rare in Acremonium.
Clade O32
Waltergamsia L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845881.
Etymology: Named after Dr Walter Gams, in acknowledgement for the tremendous contribution that he made to the taxonomy of acremonium-like fungi.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, chondroid hyphae present. Conidiophores solitary or aggregated, erect or flexuous, arising directly from submerged or superficial hyphae, sometimes radiating out from sterile coils or ropes formed by mycelium, unbranched or poorly branched, septate, hyaline, smooth-walled. Phialides lateral or terminal, subulate, acicular, ampulliform, or sub-cylindrical, occasionally widened and flat at lower part, hyaline, thin- or thick-, smooth-walled, with inconspicuous or conspicuous periclinal thickening at conidiogenous loci, percurrently proliferating phialides can be present; polyphialides present in some species. Conidia aseptate, pyriform, obpyriform, clavate, lanceolate, (narrowly) ovoid, obovoid, fusoid, ellipsoid, cylindrical, or sub-globose, hyaline or subhyaline, weakly pigmented, thin- or thick-, smooth-walled, with apiculate or obtuse end(s), guttulate or eguttulate, arranged in slimy heads or dry chains, or arranged in chains at first, later collapsing soon as conidial heads. Chlamydospores and sexual morph unknown.
Type: Waltergamsia fusidioides (Nicot) L.W. Hou, L. Cai & Crous
Other accepted species with available sequences: Waltergamsia alkalina L.W. Hou, L. Cai & Crous, W. catenata L.W. Hou, L. Cai & Crous, W. citrina (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, W. dimorphospora (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, W. epimycota (Samuels) L.W. Hou, L. Cai & Crous, W. hennebertii (W. Gams) L.W. Hou, L. Cai & Crous, W. moroccensis L.W. Hou, L. Cai & Crous, W. obpyriformis L.W. Hou, L. Cai & Crous, W. parva (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, W. pilosa (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, W. zeylanica (Petch) L.W. Hou, L. Cai & Crous
Notes: Waltergamsia is proposed to accommodate eight Acremonium s. lat. species that form a fully supported clade. Morphologically, most species in this genus produce conidia arranged in slimy heads or in chains at first but soon collapsing to form conidial heads. However, W. pilosa and W. zeylanica were only recorded to have conidia arranged in chains.
Waltergamsia alkalina L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845882. Fig. 60.
Fig. 60.

Waltergamsia alkalina (ex-type culture CBS 741.94). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores and conidial heads. E, F. Conidiophores radiating from coils formed by mycelium. G–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the substrate from which the holotype culture was collected.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.7–2.4 μm wide. Conidiophores solitary or aggregated, (sub-)erect, straight or flexuous, arising directly from submerged or superficial hyphae, sometimes radiating from sterile coils formed by mycelium, usually reduced to single phialides, unbranched, poorly branched, bearing up to two phialides per node, 21.5–39.5 long, 1.5–2.5 μm wide at base, 1–2-septate, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal, lateral, subulate, hyaline, thin-, smooth-walled, 13–32.8 μm long, 1.3–2.5 μm wide at base, with inconspicuous periclinal thickening and cylindrical collarette at conidiogenous loci, occasionally with a percurrent proliferation; polyphialides not observed. Conidia aseptate, cylindrical, symmetrically rounded, hyaline, thin-, smooth-walled, 3–5.4 × 1.2–1.8 μm, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 30 mm diam, flat, felty to dusty, white, margin entire, reverse creamy white; On MEA reaching 28 mm diam, flat, felty, white, margin entire, reverse umber at centre, pale apricot at periphery; On PDA reaching 25 mm diam, flat, dusty, white at centre, hazel at periphery, margin entire, reverse greenish glaucous at centre, olivaceous buff at periphery.
Typus: Indonesia, from alkaline soil, unknown collection date and collector, isol. K. Nagai, Drug Serendipity Research laboratories, Yamanouche Pharmaceutical Co., Tokyo, Japan, No. 577 (holotype CBS H-24603, ex-type culture CBS 741.94).
Notes: Waltergamsia alkalina is represented by an isolate from alkaline soil collected in Indonesia. According to phylogenetic inference from the ITS, LSU, rpb2, and tef-1α loci (Fig. 2), W. alkaline occupies a single long branch that is clearly different from other species in Waltergamsia. Morphologically, W. alkalina differs from its closely related species, W. citrina, by producing cylindrical conidia with both ends rounded, arranged in slimy heads, while conidia of W. citrina are obovoid or ellipsoid, arranged in long chains (Giraldo et al. 2014).
Waltergamsia catenata L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 848119. Fig. 61.
Fig. 61.

Waltergamsia catenata (ex-type culture CBS 102462). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Monophialidic conidiophores. F. Branched conidiophore. G. Proliferating conidiophore. H. Conidia. Scale bars = 10 μm.
Etymology: Referring to the conidial chains produced by this species.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–1.9 μm wide. Conidiophores solitary, (sub-)erect, straight, arising directly from submerged or superficial hyphae, usually reduced to single phialides, unbranched, poorly branched, bearing up to two phialides per node, up to 51 μm long, 1.6–2.8 μm wide at base, hyaline, smooth-walled, 1–2-septate at base, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal, lateral, subulate or cylindrical, hyaline, thin-, smooth-walled, 21–39 μm long, 1.5–2.5 μm wide at base, with inconspicuous periclinal thickening and conspicuous cylindrical collarette at conidiogenous loci, occasionally with a percurrent proliferation; polyphialides not observed. Conidia aseptate, ovoid or fusoid, with truncate base at both ends, hyaline, thin-, smooth-walled, 3.5–6.5 × 1.7–2.8 μm, eguttulate, arranged long conidial chains. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 15–17 mm diam, flat, dusty, white, margin entire, reverse white; On MEA reaching 16–17 mm diam, flat, felty, rosy buff at centre, dirty white at periphery, margin filiform, reverse pale orange; On PDA reaching 16–18 mm diam, flat, radially rugose, felty, rosy buff, margin dendritic, reverse concolourous; On SNA reaching 6 mm diam, flat, felty, white, margin dendritic, reverse concolourous.
Typus: Netherlands, Horst, Forestry Dept., Frimochalk, unknown date, F.P. Geels (holotype CBS H-24605, ex-type culture CBS 102462).
Notes: Waltergamsia catenata formed a distinct branch, being distant from other species in Waltergamsia. Waltergamsia catenata is morphologically different from W. moroccensis and W. zeylanica in its ovoid or fusoid conidia arranged in long chains, while that of W. moroccensis are ellipsoid arranged in slimy heads (Fig. 63), and W. zeylanica produces lanceolate or narrow-ovoid conidia arranged in chains (Fig. 65).
Fig. 63.

Waltergamsia moroccensis (ex-type culture CBS 512.82). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidial heads. F–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Fig. 65.

Waltergamsia zeylanica (ex-type culture CBS 746.73). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidial chains. E–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Waltergamsia citrina (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845883.
Basionym: Acremonium citrinum A. Giraldo et al., Mycologia 106: 334. 2014.
Description & illustration: Giraldo et al. (2014).
Typus: Papua New Guinea, Madang, Jais Aben, from decaying fruit along the coast, Nov. 1995, A. Aptroot, isol. Nov. 1995 by A. van Iperen, No. A 145 (holotype CBS H-21330, ex-type cultures CBS 384.96 = FMR 11427).
Additional material examined: Netherlands, from human sputum, unknown collection date and collector, dep. A. Kikstra, Academic Hospital Groningen, culture CBS 758.69.
Notes: This fungus was introduced based on the collection of decaying fruit in Madang, Papua New Guinea (Giraldo et al. 2014). It is morphologically characterised by the production of diffusible light yellow pigment and restricted growth on PDA media in which a diameter of 4–5 mm is attained after 14 d (Giraldo et al. 2014). Based on our phylogeny, the ex-type culture of A. citrinum nestled with full support in the Waltergamsia clade, and therefore the new combination W. citrina is introduced here.
Waltergamsia dimorphospora (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845884.
Basionym: Acremonium dimorphosporum A. Giraldo et al., Mycol. Progr. 16: 356. 2017.
Description & illustration: Giraldo et al. (2017).
Typus: USA, Texas, from human bronchoalveolar lavage fluid, 15 Oct. 2008, D.A. Sutton (holotype CBS H-22021, ex-type culture CBS 139050 = UTHSC 08-3639 = FMR 10548).
Notes: Waltergamsia dimorphospora is characterised by producing dimorphic conidia arranged in slimy heads and is morphologically similar to Bulbithecium borodinense (previously Acremonium borodinense; Ito et al. 2000, Giraldo et al. 2017). However, the ex-type culture of A. dimorphospora phylogenetically clustered within the genus Waltergamsia, which is distant from Bu. borodinense (Fig. 2).
Waltergamsia epimycota (Samuels) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845885.
Basionym: Nectriopsis epimycota Samuels, Mem. New York Bot. Gard. 48: 64. 1988.
Description & illustration: Samuels (1988).
Typus: French Guiana, Vic. Saül, alt. 200 m, on Kretzschmaria sp. (Xylariales), Feb. 1986, G.J. Samuels (holotype Samuels 3463 in NY).
Additional materials examined: Germany, former West-Germany, from root of Triticum aestivum (Poaceae), unknown collection date and collector, isol. A. Walz, No. W2111, culture CBS 562.86; near Braunschweig, from agricultural löss soil, unknown collection date and collector, isol. H. Nirenberg, BBA, Inst.f. Mikrobiol., Berlin, No. 308, CBS H-24600, culture CBS 265.89. Puerto Rico, from Pyrenomycete in forest, unknown collection date, G.J. Samuels, G.J.S. 95-94, BPI 737695, culture CBS 127459.
Waltergamsia fusidioides (Nicot) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845886.
Basionym: Paecilomyces fusidioides Nicot, Cah. Maboké 6: 18. 1968.
Synonym: Acremonium fusidioides (Nicot) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 70. 1971.
Description & illustration: Gams (1971).
Typus: Central African Republic, from dung of antelope, unknown collection date and collector, isol. R. Cailleux, CBS H-24601 (holotype of Paecilomyces fusidioides Culture no. 1964 in Mycothèque L.C. Paris, ex-type culture CBS 840.68 = IAM 14648 = LCP 66.1964).
Additional materials examined: France, Toulouse, unknown substrate, unknown collection date, W. Gams, “Bouteille”, No. 50, CBS H-8187, culture CBS 705.86. Italy, Padova, Botanical Garden, from decaying leaf of Canna indica (Cannaceae), Dec. 1968, L. de Zoller, isol. Dec. 1968 by W. Gams, CBS H-8239, culture CBS 113.69.
Notes: According to Gams (1971), all cultures of this species were observed to produce dimorphic conidia: i.e., globose and spindle-shaped. According to our phylogenetic inference, the ex-type culture of Paecilomyces fusidioides (CBS 840.68 falls in a fully supported clade (BPP/MLBS = 1/100 %) in Waltergamsia, therefore the new combination W. fusidioides is proposed here.
Waltergamsia hennebertii (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845887. Fig. 62.
Fig. 62.

Waltergamsia hennebertii (ex-type culture CBS 768.69). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
Basionym: Acremonium hennebertii W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 79. 1971.
Description & illustration: Gams (1971).
Typus: Zaire, Kimuenza, Lovanium University Campus, from leaf rachis of Elaeis guineensis (Arecaceae), 20 Mar. 1968, G.L. Hennebert (holotype CBS H-6614, isotypes CBS H-6615 & CBS H-6616, ex-type culture CBS 768.69 = MUCL 11580 = MUCL 28812).
Notes: Waltergamsia hennebertii was originally described as Acremonium hennebertii from a leaf rachis of Elaeis guineensis, which was treated as one of the species of Acremonium section Acremonium (Gams 1971). Based on the multi-locus phylogenetic analysis, the ex-type culture of A. hennebertii (CBS 768.69) clustered in the clade representing the genus Waltergamsia (Fig. 2). Morphologically, W. hennebertii differs from W. alkalina in the production of inverted ovoid conidia, while that of W. alkaline are cylindrical with rounded base at both ends; W. hennebertii differs from W. obpyriformis by the absent of polyphialides (Gams 1971; Fig. 2). Acremonium hennebertii is therefore transferred to Waltergamsia, and a new combination is proposed.
Waltergamsia moroccensis L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845888. Fig. 63.
Etymology: Referring to Morocco, the country where this fungus was collected.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.3–2.5 μm wide. Conidiophores solitary or aggregated, erect, straight or curved, arising directly from aerial or substratal mycelium, usually reduced to single phialides, unbranched or basitonously branched, bearing 1–2 levels with 2–4 divergent phialides per node, up to 23.5 μm long, 1.3–1.9 μm wide at base, hyaline, smooth-walled, with a single basal septum, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate or ampulliform, slightly swollen and flat at lower part, narrowed at base, hyaline, thin-, smooth-walled, (3.5–)10.5–21.5 μm long, 1–2.5(–3.2) μm wide at base, with inconspicuous periclinal thickening at conidiogenous loci; polyphialides with up to four conidiogenous loci occasionally present. Conidia aseptate, ovoid, ellipsoid, both ends rounded, hyaline, thin-, smooth-walled, 2.8–4 × 1.7–2.4 μm, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 20–24 mm diam, flat, thinly felty, slightly floccose, rosy buff at centre, white at periphery, margin entire, reverse creamy white; On MEA reaching 18–20 mm diam, raised, radially folded, felty, buff, margin crenate, reverse straw, pale ochreous at centre, with buff radial lines; On PDA reaching 19–20 mm diam, flat, short hairy and dirty white at centre, thinly felty and white at periphery, margin entire, reverse buff.
Typus: Morocco, from fragmenting human nail, unknown collection date and collector (holotype CBS H-24602, ex-type culture CBS 512.82).
Notes: Based on the multi-gene phylogenetic analysis, Waltergamsia moroccensis forms an independent branch, clearly separated from other species in Waltergamsia (Fig. 2). Morphologically, W. moroccensis differs from its most closely related species, W. obpyriformis, by producing shorter phialides [10.5–21.5 μm vs 16.8–36(–49.5) μm] and conidia with rounded ends, arranged in slimy heads, while W. obpyriformis has conidia with elongated hilum at basal ends, arranged in chains.
Waltergamsia obpyriformis L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845889. Fig. 64.
Fig. 64.
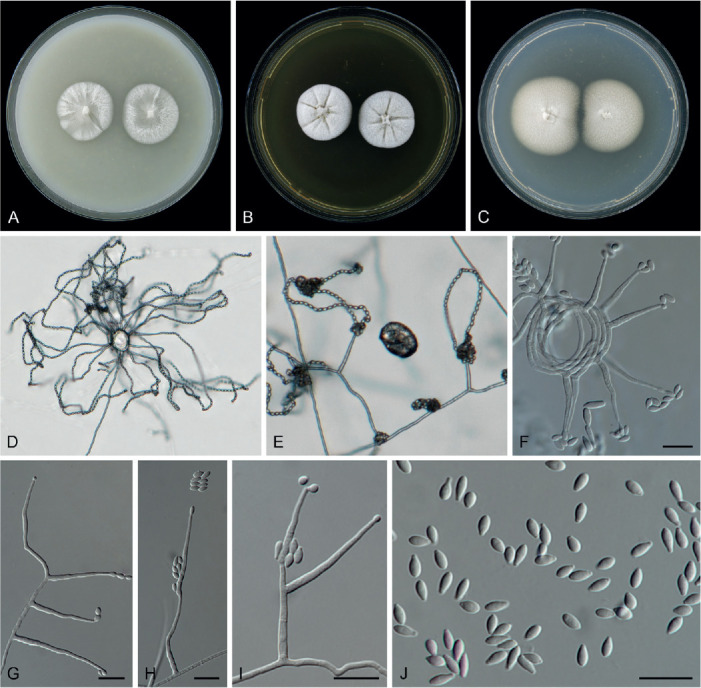
Waltergamsia obpyriformis (ex-type culture CBS 595.73). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, F. Conidiophores radiating out from coils formed by the mycelia. E. Conidiophores with conidial chains and heads. G. Unbranched conidiophores. H. Conidiophores with terminally proliferating phialides. I. Conidiophores with terminally proliferating phialides and polyphialides. J. Conidia. Scale bars = 10 μm.
Etymology: Referring to the shape of conidia produced by this species.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.3–1.8 μm wide, abundant mycelial ropes and coils present. Sporulation abundant, phalacrogenous, plectonematogenous, rarely nematogenous. Conidiophores solitary or aggregated, (sub-)erect, straight or slightly curved, arising directly from submerged or superficial hyphae, sometimes radiating out from coils or ropes formed by the mycelium, usually reduced to single phialides, unbranched or branched, bearing 1–2 levels with 1–2 phialides per node, 17.5–57(–64.5) μm long, 1.7–2.7 μm wide at base, with 1–4 inconspicuous or conspicuous septa, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal, lateral, subulate, hyaline, thin-, smooth-walled, 16.8–36(–49.5) μm long, 1–2.2 μm wide at base, with inconspicuous periclinal thickening and conspicuous cylindrical collarette at conidiogenous loci, occasionally with a percurrent or subterminal proliferation; polyphialides with two conidiogenous loci occasionally present. Conidia aseptate, obpyriform or obovoid with elongated apiculate bases and obtuse apices, hyaline, thin-, smooth-walled, 3–5 × 1.7–2.4 μm, arranged dry chains, often collapsing soon in dry heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 25–28 mm diam, flat, dusty at centre, hairy at periphery, white, margin entire, reverse buff; On MEA reaching 27 mm diam, flat, radially folded, felty, dirty white, margin entire, reverse saffron, with radial buff lines; On PDA reaching 35–38 mm diam, flat, felty, dirty white at centre, creamy white at periphery, margin entire, reverse pale saffron at centre, pale rosy buff at periphery; On SNA reaching 23 mm diam, flat, dusty, white, margin entire, reverse concolourous.
Typus: India, Bangalore, Forestry Dept., from decaying pod of Delonix regia (Fabaceae), Jan. 1973, W. Gams (holotype CBS H-24604, ex-type culture CBS 595.73).
Additional material examined: Colombia, Caldas, Chinchina, from coffee berry pulp (Rubiaceae), unknown collection date and collector, isol. 25 Mar. 2004 by F. Posada, CF 2-1, dep. F. Vega, CBS H-24687, culture CBS 115960.
Notes: Phylogenetically, Waltergamsia obpyriformis is closely related to W. hennebertii and W. moroccensis (Fig. 2). However, it differs from W. hennebertii by producing branched, multi-septate conidiophores and longer phialides [16.8–36(–49.5) μm vs 18–20 μm], while conidiophores of W. hennebertii are unbranched (Gams 1971). Conidia of W. obpyriformis are wider (1.7–2.4 μm), obpyriform or ovoid with an elongated hilum at basal end, while those of W. hennebertii are narrower (1.3–1.5 μm), narrowly obovoid (Gams 1971). Waltergamsia obpyriformis can be distinguished from W. moroccensis by its longer phialides [16.8–36(–49.5) μm vs 10.5–21.5 μm] and obpyriform or ovoid conidia with elongated apiculate bases and obtuse apices, while conidia of W. moroccensis are ovoid or ellipsoid with both ends rounded.
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hit using the LSU sequence is the type culture of A. hennebertii [currently W. hennebertii; culture CBS 768.69; GenBank MH871191.1; Identity = 774/777 (99 %), no gaps] and closest hit using the ITS sequence is also the type culture of A. hennebertii [currently W. hennebertii; culture CBS 768.69; GenBank MH859420.1; identity = 463/489 (95 %), 4 gaps (0 %)].
Waltergamsia parva (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845891.
Basionym: Acremonium parvum A. Giraldo et al., Mycologia 106: 332. 2014.
Description & illustration: Giraldo et al. (2014).
Typus: Netherlands, North Holland Province, Texel, from Tubercularia vulgaris (Nectriaceae), unknown collection date and collector, isol. 1968 by W. Gams, No. 1452 (holotype CBS H-21329, ex-type culture CBS 381.70A = VKM F-2845 = FMR 12358).
Additional material examined: Netherlands, Flevoland Province, Zuidelijk Flevoland, reserve Oostvaardersplassen, from old stem of Cirsium arvense (Asteraceae), 30 Apr. 1997, W. Gams, culture CBS 831.97.
Waltergamsia pilosa (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845890.
Basionym: Acremonium pilosum A. Giraldo et al., Mycologia 106: 332. 2014.
Description & illustration: Giraldo et al. (2014).
Typus: Netherlands, Flevoland Province, Oostelijk Flevoland, from agricultural soil, unknown collection date, J.H. van Emden, isol. Sep. 1969 by J.W. Veenbaas-Rijks, 03.625, No. 652 (holotype CBS H-8173, ex-type cultures CBS 124.70 = FMR 11415).
Additional materials examined: India, Bangalore, Mysore, mycoparasitic Periconia cookei (Pleosporales) on Dendrocalamus sp. (Poaceae), Jan. 1973, W. Gams, CBS H-9319, culture CBS 390.73 = IMI 185375. Netherlands, Flevoland Province, Oostelijk Flevoland, from agricultural soil, unknown collection date, J.H. van Emden, isol. 29 Sep. 1969 by J.W. Veenbaas-Rijks, No. 543, CBS H-8174, culture CBS 125.70; from agricultural soil, unknown collection date and collector, isol. J.H. van Emden, No. 690312/122, CBS H-8177, culture CBS 410.70; Noordoostpolder, Nagele, from agricultural soil, unknown collection date and collector, isol. H. Nylander, No. 771, culture CBS 511.82.
Waltergamsia zeylanica (Petch) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845892. Fig. 65.
Basionym: Cephalosporium zeylanicum Petch, Trans. Brit. Mycol. Soc. 16: 236. 1932 (1931).
Synonym: Acremonium zeylanicum (Petch) W. Gams & H.C. Evans, Trans. Brit. Mycol. Soc. 64: 393. 1975.
Description based on culture CBS 746.73: Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–2.2 μm wide. Conidiophores solitary or aggregated, (sub-)erect, straight or slightly curved, arising directly from submerged or superficial hyphae, sometimes radiating out from sterile coils or ropes formed by the mycelium, usually reduced to single phialides, unbranched, poorly branched, bearing 1–2 phialides per node, 15–33(–45) μm long, 1.5–2.8 μm wide at base, 1–3-septate at base or middle part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides terminal, lateral, subulate, hyaline, thick-, smooth-walled, 10–39 μm long, 1.5–2.8 μm wide at base, with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, slightly lanceolate or narrow-ovoid, both ends apiculate, hyaline, thick-, smooth-walled, (4.2–)4.5–6.3 × 1.6–2 μm, guttulate or eguttulate, arranged in dry long chains. Chlamydospores and sexual morph not observed (also see Petch 1932).
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 55 mm diam, flat, dusty, white at centre, creamy white at periphery, margin entire, reverse buff; On MEA reaching 57 mm diam, flat, radially folded, felty, creamy white at centre, white at periphery, margin entire, reverse luteous, pale luteous at periphery, with radial buff lines; On PDA flat, felty, white at centre, dirty white or pale grey at periphery, spreading through the plate, margin entire, reverse straw.
Typus: Sri Lanka, Nuwara Eliya, on leaf hopper, 24 Oct. 1926, unknown collector (holotype K(M) 156581).
Materials examined: Ghana, Tafo, from Coccidae, on cocoa leaf, unknown collection date and collector, isol. H.C. Evans, No. 24, CBS H-8485, culture CBS 746.73 = ATCC 32188; Kukurantumi, from Coccidae, unknown collection date and collector, isol. H.C. Evans, No. 81, culture CBS 734.73; Tafo, from Araneida, on cocoa leaf, unknown collection date and collector, isol. H.C. Evans, No. 16, culture CBS 736.73B; ibid. No. 75, culture CBS 735.73A; ibid. No. 76, culture CBS 735.73B. South America, from Theobroma gileri (Malvaceae), unknown collection date and collector, culture CBS 111659.
Notes: Cephalosporium zeylanicum was described by Petch (1932) and isolated from a leafhopper collected in Sri Lanka. Gams (1971) examined the type material of this fungus on its natural substrate and concluded that C. zeylanicum was a synonym of Acremonium griseum, based on conidia with sharply pointed ends and arranged in chains, that agree with A. griseum. As a result, A. griseum was transferred to Verticillium as V. griseum (Gams 1971). Subsequently, several cultures of this species were collected from insects and spiders in Ghana, indicating that although C. zeylanicum usually has a few mononematous aerial hyphae, it cannot be accommodated in Verticillium. Ultimately, C. zeylanicum was redisposed into the genus Acremonium based on its growth habit (Gams 1975).
Five strains identified as A. zeylanicum are examined in this study. Based on our phylogenetic analysis, they form a full-supported clade in Waltergamsia (Fig. 2), therefore a new combination is proposed as W. zeylanica. This species is apparently a truly entomogenous taxon among acremonium-like species (Gams 1975). The culture CBS 746.73 was isolated from the same host as the type material. However, their locations are different, therefore we will not designate it as ex-epitype culture, but consider it as a representative culture for A. zeylanicum.
Clade O34
Bulbithecium Udagawa & T. Muroi, Bull. Natl. Sci. Mus., Tokyo, B 16: 13. 1990.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae or tough with chondroid hyphae. Sexual morph: Ascomata cleistothecial, scattered, solitary or confluent, semi-immersed to immersed, non-stromatic, non-ostiolate, initially bulbil-like or irregularly coiled, globose to sub-globose, orange, dark orange-brown, transparent, glabrous or covered with hyphal-like hairs. Cleistothecial wall dark brown, dextrinoid, membranaceous, thick, composed of two layers of angular or rounded cells. Paraphyses lacking. Asci irregularly disposed, or uniformly distributed in the centrum, non-catenulate, unitunicate, 8-spored, clavate, ellipsoid, obovoid or pyriform, evanescent, deep yellow in Melzer’s reagent. Ascospores one-celled, ellipsoid, hyaline to slightly yellow, smooth-, thick-walled, without germ pores or slits, surrounded by gelatinous sheath: young ascospores yellow in Melzer’s reagent, or without gelatinous layers. Asexual morph: Conidiophores hyaline, smooth-walled, solitary or in pairs, delicate, (sub-)erect, or irregularly curved, arising directly from submerged or superficial hyphae, or coil formed by mycelium, mostly unbranched, reduced to single phialide, septate at base and middle, colourless, with a cell wall usually thicker than those of vegetative hyphae. Phialides orthotropic, erect, or irregularly bent, unbranched or poorly branched, lateral or terminal, cylindrical, acicular or subulate, hyaline, thick-, smooth-walled, with conspicuous or inconspicuous periclinal thickening and collarette at conidiogenous loci; adelophialides and polyphialides absent. Conidia aseptate, variable in shape, (broadly) ellipsoid, cylindrical, ovoid, elongate-phaseoliform, (sub-)globose or broadly ovoid, with ends symmetrically rounded, broadly truncate or sharply pointed, hyaline, thin- or thick-, smooth-walled; or thick-, rough-walled, finely warty, with two kinds of conidia on same colony, type I: ellipsoidal to cylindrical, type II: ellipsoidal to ovoid, eguttulate or guttulate, arranged in chains and slimy heads on same colony. Chlamydospores absent (emended from Udagawa & Muroi 1990).
Type: Bulbithecium hyalosporum Udagawa & T. Muroi
Other accepted species with available sequences: Bulbithecium ammophilae L.W. Hou, L. Cai & Crous, Bu. arxii (Malloch) L.W. Hou, L. Cai & Crous, Bu. borodinense (Tad. Ito, et al.) L.W. Hou, L. Cai & Crous, Bu. ellipsoideum L.W. Hou, L. Cai & Crous, Bu. pinkertoniae (W. Gams) L.W. Hou, L. Cai & Crous, Bu. spinosum (Negroni) L.W. Hou, L. Cai & Crous, Bu. truncatum L.W. Hou, L. Cai & Crous
Notes: Bulbithecium refers to its characteristics, producing bulbil-like ascomata with dextrinoid, membranous peridium, yellowish colour reaction in Melzer’s reagent in the asci and young ascospores, and acremonium-like asexual morph (Udagawa & Muroi 1990). It was introduced as a monotypic genus, containing the single species B. hyalosporum, and originally assigned to Hypocreales, but it has not yet been placed with certainty into any family (Udagawa & Muroi 1990). In the present study, additional Acremonium species clustered together with the ex-type culture of Bu. hyalosporum and formed a fully supported clade in Bionectriaceae. Species in the genus are known from soil, dung or skin of humans, and few are associated with plants.
Bulbithecium ammophilae L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845893. Fig. 66.
Fig. 66.

Bulbithecium ammophilae (ex-type culture CBS 178.78). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads. E–G, J. Conidiophores with cylindrical conidia. H. Conidiophore with ellipsoidal conidium. I. Conidia. Scale bars = 10 μm.
Etymology: Name reflects Ammophila, the host genus from which it was collected.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 0.9–1.9 μm wide. Sporulation sparse, phalacrogenous, nematogenous. Conidiophores solitary, (sub-)erect, straight or curved, arising directly from submerged or superficial hyphae, unbranched, thin, long, 35.6–86.5(–127) μm long, 1–1.5 μm wide at base, 1–4-septate at base and middle, hyaline, smooth-walled, with a cell wall usually thicker than those of vegetative hyphae. Phialides lateral, rarely terminal, cylindrical, acicular or subulate, hyaline, thin, long, thick-, smooth-walled, 27.7–65 μm long, 0.8–1.3 μm wide at base, with inconspicuous periclinal thickening and collarette at conidiogenous loci; polyphialides not observed. Conidia two types, conidia of type 1: aseptate, (sub-)globose or ellipsoidal, straight, smooth-, thin-walled, hyaline, eguttulate, singly, 4.9–8.3 × 2.7–3.9 μm; Conidia of type 2: aseptate, cylindrical, straight, with elongated apiculate bases and obtuse apices, hyaline, thin-, smooth-walled, 4.3–7.7 × 1.7–2.4 μm, with two small-sized guttules, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 27 mm diam, flat, membranous with sparse aerial mycelium, white, margin entire, reverse concolourous; On MEA reaching 24–25 mm diam, flat, short hairy, buff to dirty white, margin entire, reverse saffron; On PDA reaching 27 mm diam, flat, spirally folded, short hairy at centre, thinly felty at periphery, pale rosy buff, margin entire, reverse buff; On SNA reaching 21 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse white. Without odour on all media.
Typus: Netherlands, South Holland Province, Katwijk, from sand 40 cm depth under Ammophila arenaria (Sphecidae, insect), Jan. 1978, isol. Feb 1978, coll. and isol. W. Gams, blanco 60=1/95 26 (holotype CBS H-1034, ex-type culture CBS 178.78).
Notes: Bulbithecium ammophilae was isolated from sand under Ammophila arenaria, and possibly was an entomogenous species. Morphologically Bu. ammophilae is similar to Bu. ellipsoideum in its thin, long conidiophores, but can be distinguished by the production of dimorphic conidia that are longer than Bu. ellipsoideum [(sub-)globose or ellipsoidal, 4.9–8.3 × 2.7–3.9 μm, or cylindrical, 4.3–7.7 × 1.7–2.4 μm vs 2.7–5.8(–6.1) × 2.2–3.6 μm], while conidia of Bu. ellipsoideum are broadly ellipsoid with apiculate base.
Bulbithecium arxii (Malloch) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845894.
Basionym: Leucosphaerina arxii Malloch, Stud. Mycol. 31: 107. 1989.
Description & illustration: Malloch (1989).
Typus: USA, North Carolina, Carteret County, Shackleford Bank, from dung of horse collected on open sand in a dune area, 25 Oct. 1982, isol. 24 Nov. 1982, coll. and isol. D.W. Malloch, No. M.134g (holotype in TRTC, ex-type culture CBS 737.84 = UAMH 6113).
Bulbithecium borodinense (Tad. Ito, et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845895.
Basionym: Acremonium borodinense Tad. Ito, et al., Mycol. Res. 104: 77. 2000.
Description & illustration: Ito et al. (2000).
Typus: Japan, Okinawa Pref., South Borgino island, Minami Daito-Mura, Siojiri-gun, from soil in sugarcane field, 1996, isol. 25 Sep. 1996, coll. and isol. T. Ito, No. H8-16-2, CBS H-6555 (holotype IFO H-12224, ex-type culture CBS 101148 = IFO 33057).
Notes: On the phylogenetic tree, this species shows affinity to two species that were originally accepted as Acremonium, A. pinkertoniae and A. spinosum. They are morphologically comparable by producing rough-walled conidia but differ from each other in the shape and size of their conidia (Gams 1971, Ito et al. 2000). In the present study, all three species fall into the Bulbithecium clade and form a fully supported sub-clade (Fig. 2).
Bulbithecium ellipsoideum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845896. Fig. 67.
Fig. 67.

Bulbithecium ellipsoideum (ex-type culture CBS 993.69). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores and conidial heads. F. Conidial heads. G. Conidia. H, I. Conidiophores. Scale bars = 10 μm.
Etymology: Referring to the ellipsoid conidia produced by this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–1.8 μm wide, mycelial coils present. Sporulation sparse, phalacrogenous. Conidiophores solitary or aggregate, (sub-)erect, straight or curved, arising directly from submerged or superficial hyphae, mostly unbranched, reduced to single phialides, or poorly branched, bearing 1–2 phialides per node, 21–100(–145) μm long, 1.1–1.7 μm wide at base, 1–2-septate at base and middle, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, rarely terminal, cylindrical, acicular, hyaline, thin and long, thick-, smooth-walled, (19–)23.5–87(–103) μm long, 1–1.5 μm wide at base, with inconspicuous periclinal thickening and collarette at conidiogenous loci. Conidia aseptate, ellipsoidal with apiculate base, straight, hyaline, thick-, smooth-walled, 2.7–5.8(–6.1) × 2.2–3.6 μm, with one medium-sized guttule, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 26–27 mm diam, flat, membranous without aerial mycelium, dirty white at centre, white at periphery, margin entire, with crystals and pigment, reverse concolourous; On MEA reaching 20–21 mm diam, flat, felty, short hairy, moist, rosy buff at centre, white at periphery, margin entire, reverse pale orange; On PDA reaching 25–26 mm diam, flat, rugose, felty, short hairy, moist, creamy white, margin entire, reverse pale rosy buff; On SNA reaching 23–26 mm diam, flat, membranous without aerial mycelium, white, margin lobate, reverse white. Without odour on all media.
Typus: Netherlands, from human skin, unknown collection date and collector, isol. Apr. 1969 by Geleen hospital (holotype CBS H-8062, ex-type culture CBS 993.69).
Notes: Bulbithecium ellipsoideum is characterised by thin, long conidiophores and phialides with globose conidial droplets. Although it morphologically resembles its closely related species Bu. ammophilae, Bu. ellipsoideum differs from the latter by the longer phialides and conidiophores [conidiophores: 21–100(–145) μm vs 35.6–86.5(–127) μm, phialides: (19–)23.5– 87(–103) μm vs 27.7–65 μm long], and shorter, broadly ellipsoid conidia [2.7–5.8(–6.1) × 2.2–3.6 μm], while conidia of Bu. ammophilae are dimorphic, (sub-)globose or ellipsoidal, 4.9–8.3 × 2.7–3.9 μm or cylindrical, 4.3–7.7 × 1.7–2.4 μm.
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hit using the LSU sequences is Leucosphaerina arxii [currently Bu. arxii, culture CBS 737.84; GenBank NG_057892.1; Identity = 770/775 (99 %), no gaps]; and the closest hit using the ITS sequences also is Leucosphaerina arxii [currently Bu. arxii, culture CBS 737.84; GenBank MH861832.1; Identity = 442/474 (93 %), 12 gaps (2 %)].
Bulbithecium hyalosporum Udagawa & T. Muroi, Bull. Natl. Sci. Mus., Tokyo, B 16: 14. 1990.
Description & illustration: Udagawa & Muroi (1990).
Typus: Peru, Dept. Cuzco, Quispicanchis Province, Marcapata, Ceja de Selva zone, alt. 2 630 m, from dung of horse, 13 Sep. 1984, K. Yokoyama, CBS H-24681 (holotype NHL 2983, ex-type culture CBS 318.91 = NHL 2983a).
Bulbithecium pinkertoniae (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845897.
Basionym: Acremonium pinkertoniae W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 48. 1971.
Description & illustration: Gams (1971).
Typus: Netherlands, Utrecht Province, Baarn, soil from tropical greenhouse, unknown collection date, W. Gams, isol. 1966 by J. Broer (isotype CBS H-6653, ex-type culture CBS 157.70 = MUCL 8529).
Material examined: Italy, Torino, soil, unknown collection date and collector, isol. A. Fontana, No. b2/35, CBS H-8311, culture CBS 158.70.
Bulbithecium spinosum (Negroni) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845898.
Basionym: Cephalosporium spinosum Negroni, Revta Soc. Argent. Biolog. 9: 16. 1933.
Synonyms: Hyalopus spinosus (Negroni) M.A.J. Barbosa [as ‘spinosum’], Subsidios para o Estudo Parasitologico do Genero Hyalopus Corda, 1838: 28. 1941.
Acremonium spinosum (Negroni) W. Gams, Cephalosporium-artige Schimmelpilze: 78. 1971.
Lasionectriopsis spinosa (Negroni) Lechat & P.-A. Moreau, Ascomycete.org 11: 4. 2019.
Description & illustration: Gams (1971).
Typus: Argentina, from human toe nail with onychomycosis, unknown collection date and collector, dep. P. Negroni, No. 144.1, CBS H-24683 (ex-type culture of Cephalosporium spinosum CBS 136.33 = ATCC 9471).
Materials examined: Brazil, from air, unknown collection date and collector, dep. A.C. Batista, CBS H-8390 & CBS H-8391, culture CBS 391.66 = IMUFPe 1542. Germany, Edersee, from root of Picea abies (Pinaceae), attacked by Heterobasidion annosum (Bondarzewiaceae), unknown collection date and collector, isol. E. Falk, No. B134, culture CBS 915.85.
Notes: This species was recently recombined in the genus Lasionectriopsis as L. spinosa (Lechat et al. 2019). Sequences of the ex-type culture of Cephalosporium spinosum (CBS 136.33; GenBank: HE608637, HE608655) generated by Giraldo et al. (2012) and cited by Lechat et al. (2019) are identical to the culture CBS 784.69 that clustered within the Lasionectriopsis pteridii clade (Lechat et al. 2019). However, they are different from the DNA barcode sequences of CBS 136.33 provided by the CBS culture collection and those we sequenced in the current study. Culture CBS 136.33 is also morphologically different from L. pteridii (Gams 1971): culture CBS 136.33 produces warty conidia, while conidia of L. pteridii are smooth-walled. In the present study, culture CBS 136.33 is distant from type of Lasionectriopsis but clusters within the Bulbithecium clades (Fig. 2). Therefore, we consider that the sequences in the previous studies and GenBank may be incorrect, and this species is transferred to Bulbithecium based on the corrected sequences of CBS 136.33. Corrected sequences were uploaded to GenBank (Supplementary Table S1).
Bulbithecium truncatum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845899. Fig. 68.
Fig. 68.

Bulbithecium truncatum (ex-type culture CBS 113718). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads and conidial chains. E–H. Conidiophores. I. Conidiophores radiating out from coils formed by the mycelium. J. Conidia. Scale bars = 10 μm.
Etymology: Referring to the broadly truncate conidia produced by the ex-type strain.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.3–2.4 μm wide, mycelial coils and ropes present. Sporulation sparse, phalacrogenous. Conidiophores solitary, (sub-)erect, straight or irregularly curved, flexuous, arising directly from submerged or superficial hyphae, or from coil formed by mycelium, mostly unbranched, reduced to single phialides, occasionally swollen at base, (25–)32–63.5(–74.5) μm long, 1.6–2.7 μm wide at base, 1–2(–5)-septate at base and middle, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, rarely terminal, cylindrical or subulate, irregularly curved, hyaline, thick-, smooth-walled, (12.8–)20–42(–54) μm long, 1.6–2.2 μm wide at base, with conspicuous periclinal thickening and minute collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, sub-globose or broadly ovoid, straight, with broadly truncate and elongated bases and obtuse apices, hyaline, thin-, smooth-walled, 2.6–4.1 × 1.8–2.8 μm, eguttulate or with one medium-sized guttule, arranged in chains and slimy heads at same colony. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 23 cm diam, flat, membranous without aerial mycelium, white, margin entire, reverse concolourous; On MEA reaching 20–21 mm diam, flat, radially fold, moderate aerial mycelium, felty, white at centre, vinaceous in middle with dirty white edge, margin entire, reverse cinnamon with radially white lines, and pale saffron edge; On PDA reaching 22–23 mm diam, flat, floccose, creamy white, pale fawn at centre, margin entire, reverse concolourous; On SNA reaching 16–17 mm diam, flat, membranous without aerial mycelium, white, margin lobate, reverse white. Without any odour in all media.
Typus: Iran, Bandar Abbas-Roudan, from Vachellia nubica (Fabaceae), 2002, Z. Ghanbari (holotype CBS H-24682, ex-type culture CBS 113718).
Notes: Bulbithecium truncatum is phylogenetically close to two species that originated from horse dung, Bu. arxii and Bu. hyalosporum (Fig. 2). However, our culture was from Vachellia nubica and is clearly different from the last two species by producing sub-globose conidia with broad-truncate hilum (2.6–4.1 × 1.8–2.8 μm), arranged either in chains or slimy heads, while conidia of B. hyalosporum are ellipsoid or allantoid (3–8 × 1–2 μm) and arranged in slimy heads, and conidia of Bu. arxii are ellipsoid to elongate-phaseoliform (3.9–6.2 × 1.0–1.7 μm,) and arranged also in heads (Malloch 1989, Udagawa & Muroi 1990). Besides, conidiophores of Bu. truncatum are occasionally swollen near the base, which is rare in acremonium-like species.
Clade O35
Ovicillium Zare & W. Gams, Mycol. Progr. 15: 1020. 2016.
Colonies on PDA, depending on the species, slow- to fast-growing, white, yellowish white to greyish yellow, radially folded, buff to dark buff, light brown to pale pink, light honey to hazel, ochraceous to pale ochraceous, light hazel to buff, with or without pale orange margin, compact, velvety, cottony, brownish grey, brown, olive pigment present in some species. Reverse white, greyish white to cream-coloured, yellowish or amber yellow, light pink to light brown, honey, ochraceous, light to dark buff, light hazel to very light smoke-grey. Conidiophores erect, septate, solitary, often branched, mostly with secondary branches, bearing verticillate phialides of 4–6 in a whorl, strongly cyanophilic, smooth or rough near the base, gradually tapering towards the apex, phialide tip undulate in some species. Phialides terminal or lateral, straight, acicular or aculeate, attenuated from the middle, mostly undulated near the tip (visible under high magnification), rather cyanophilic, adelophialides were observed on OA in some species. Conidia aseptate, globose, subglobose, ovoid, napiform to cylindrical, more or less apiculate at the base, thick- and smooth-walled, hyaline to subhyaline, chromophilic, produced in large globose heads, cyanophilic. Sessile conidia present in some species, directly growing on vegetative hyphae, cylindrical or ellipsoidal. Chlamydospores present in some species, globose to ovoid. Crystals absent. Sexual morph not observed. (emended from Giraldo et al. 2012, Zare & Gams 2016).
Type: Ovicillium attenuatum Zare & W. Gams
Other accepted species with available sequences: Ovicillium asperulatum (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, O. oosporum Zare & W. Gams, O. subglobosum Zare & W. Gams, O. variecolor (A. Giraldo et al.) L.W. Hou, L. Cai & Crous
Ovicillium asperulatum (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845900.
Basionym: Acremonium asperulatum A. Giraldo et al., Mycologia 104: 1463. 2012.
Synonym: Ovicillium napiforme Zare & W. Gams, Mycol. Progr. 15: 1022. 2016.
Descriptions & illustrations: Giraldo et al. (2012), Zare & Gams (2016).
Typus: Spain, Aragon, San Nicolás de Bujaruelo, Ordesa y Monte Perdido National Park, from soil, unknown collection date, M. Hernández-Restrepo & J. Cano, isol. A. Giraldo (holotype IMI 500816, ex-type culture CBS 130362 = FMR 11065 = MUCL 53781).
Additional material examined: Germany, near Bonn, from wood of Sorbus aria (Rosaceae), unknown collection date, isol. Feb. 1995, coll. and isol. K. Weise, CX 2.6 (holotype of Ovicillium napiforme CBS H-22410, ex-type culture CBS 426.95).
Notes: Ovicillium napiforme was originally described from wood of Sorbus aria in Germany (Zare & Gams 2016). When the authors described this species based on phylogeny and morphology, they were presumably unaware of Giraldo et al. (2012), as the ITS and LSU sequences are identical to those of A. asperulatum. Our phylogenetic analysis shows that the ITS, LSU, rpb2 and tub2 sequences of the ex-type strains of O. napiforme are identical to those for corresponding loci of O. asperulatum (basionym: A. asperulatum; Fig. 2). The two entities are morphologically similar in producing globose conidia and sub-globose chlamydospores. Therefore, O. napiforme is synonymised with A. asperulatum.
Ovicillium attenuatum Zare & W. Gams, Mycol. Progr. 15: 1021. 2016.
Description & illustration: Zare & Gams (2016).
Typus: Cuba, Pinar del Rio, Soroa, from dead mite on Auricularia sp. (Auriculariaceae), unknown collection date and collector, isol. 6 Jan. 1986 by R.F. Castañeda (holotype CBS H-22409, ex-type culture CBS 399.86 = INIFAT C86/59-3).
Additional material examined: Papua New Guinea, Madang Province, foothills of Finisterre range, 40.8 km along road Madang-Lae, alt. 200 m, from branch with stroma, 2 Nov. 1995, A. Aptroot, isol. Nov 1995 by A. van Iperen, No. A 134, CBS H-6434, culture CBS 158.96.
Ovicillium subglobosum Zare & W. Gams, Mycol. Progr. 15: 1023. 2016.
Description & illustration: Zare & Gams (2016).
Typus: China, Hong Kong, from soil, 1998, A. Aptroot, isol. Mar. 1999 by W. Gams, No. W 12 (holotype CBS H-22412, culture CBS 101963).
Ovicillium variecolor (A. Giraldo et al.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845901.
Basionym: Acremonium variecolor A. Giraldo et al., Mycologia 104: 1463. 2012.
Description & illustration: Giraldo et al. (2012).
Typus: Spain, Escuain, Ordesa y Monte Perdido National Park, from Aragon Forest soil, unknown collection date, M. Hernández-Restrepo & J. Cano, isol. A. Giraldo (holotype IMI 500815, ex-type culture CBS 130360 = FMR 11140 = MUCL 53779).
Notes: Ovicillium variecolor is phylogenetically allied to O. oosporum isolated from Theobroma gileri in South America (Fig. 2; Zare & Gams 2016). They are morphologically comparable by producing verticillate conidiophores with up to five phialides per node (Giraldo et al. 2012, Zare & Gams 2016). However, O. oosporum and O. variecolor differ in the shape and size of conidia and conidiophores: conidia of O. variecolor are sub-globose or ovoid, measuring 3–4(–5) × 2–4 um, slightly apiculate base, while conidia of O. oosporum are subglobose, ovoid to broadly ovoid 4–6 × 2.5–4 μm; conidiophores are branched, up to 290 μm in O. variecolor and 20–50 × 1.2–2.2 μm in O. oosporum. Conidia of some strains identified as O. oosporum had a basal protrusion, but this was not observed in A. variecolor (Giraldo et al. 2012, Zare & Gams 2016).
Clade O36
Proxiovicillium L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845902.
Etymology: Named after its phylogenetically similarity to the genus Ovicillium.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores (sub-)erect, unbranched or poorly branched, arising directly from submerged or superficial hyphae, sometimes radiating out from sterile coils formed by the mycelium, 1–3-septate. Conidiogenous cells enteroblastic, monophialidic, terminal, lateral, acicular, cylindrical or subulate, hyaline, thick- and smooth-walled, with inconspicuous collarette and periclinal thickening at conidiogenous loci; adelophialides and polyphialides present in some species. Conidia aseptate, sub-globose, broadly ovoid or ellipsoid, tapering to apiculate base, or with apiculate bases and obtuse apices, hyaline, thin-, smooth-walled, arranged in long chains. Chlamydospores and sexual morph unknown.
Type: Proxiovicillium blochii (Matr.) L.W. Hou, L. Cai & Crous
Other accepted species with available sequences: Proxiovicillium lepidopterorum L.W. Hou, L. Cai & Crous.
Notes: Proxiovicillium is phylogenetically close to Ovicillium, but morphologically different by producing conidia arranged in long chains, while most species in Ovicillium produce conidia aggregated in large globose to sub-globose conidial heads. Two species are accommodated in this genus.
Proxiovicillium blochii (Matr.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845903. Fig. 69.
Fig. 69.

Proxiovicillium blochii (ex-neotype culture CBS 427.93). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–F. Conidiophores. G. Conidia. Scale bars = 10 μm.
Basionym: Mastigocladium blochii Matr., Compt. Rend. Hebd. Séances Acad. Sci. Paris. 152: 325. 1911.
Synonyms: Scopulariopsis blochii (Matr.) Vuill., Bull. Soc. Mycol. France. 27: 148. 1911.
Acremonium blochii (Matr.) W. Gams, Cephalosporium-artige Schimmelpilze: 78. 1971.
Description based on the ex-neotype culture CBS 427.93: Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–1.5 μm wide. Conidiophores solitary, (sub-)erect, straight or serrated, arising directly from submerged or superficial hyphae, sometimes radiating out from sterile coils formed by the mycelium, unbranched, reduced to single phialides, rarely branched, 10–27 μm long, 0.9–2.1 μm wide at base, with single inconspicuous septum at base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, cylindrical or subulate, serrated at base, hyaline, thick-, smooth-walled, 9.5–26.5 μm long, 0.8–1.8 μm wide at base, with inconspicuous periclinal thickening and collarette at conidiogenous loci; polyphialides with two conidiogenous loci are occasionally present; adelophialides 11.4–21 × 1.5–2 μm. Conidia aseptate, subglobose or broadly ovoid, straight, apiculate at both ends, or with apiculate bases and obtuse apices, hyaline, thin-, smooth-walled, eguttulate, 3–4 × 1.5–2.5 μm, arranged in chains. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 20–22 mm diam, flat, sparse aerial mycelium, dusty, white, margin entire, reverse creamy white; On MEA reaching 22–24 mm diam, raised, moderate aerial mycelium, felty, short hairy, rosy buff, margin entire, reverse pale luteous; On PDA reaching 22–24 mm diam, flat, rugose, floccose, white, margin entire, reverse buff, with pale brown pigment.
Typus: Germany, Hamburg, from human skin, unknown collection date, Universitäts-Krankenhaus Eppendorf, Hautklinik, Hamburg, No. 240485 (neotype ofMastigocladium blochii designated here CBS 427.93, preserved as metabolically inactive culture MBT10009869, ex-neotype culture CBS 427.93).
Additional materials examined: Netherlands, from human patient, unknown collection date and collector, isol. Dec. 1932 by H.A.P.C. Oomen, GLA-M, identified by F.H. van Beyma as Scopulariopsis blochii, CBS H-8061, culture CBS 324.33 = MUCL 9013.
Notes: Mastigocladium blochii (syn. Acremonium blochii) was originally described from verrucose cankers on human hands and elbows in France (Matruchot 1911). Gams (1971) examined two cultures, CBS 324.33 and CBS 993.69, with possible human pathogenicity as representatives of A. blochii. According to our phylogenetic inference, CBS 993.69 clustered within Bulbithecium, and has broadly ellipsoid conidia arranged in slimy heads, which are morphologically incompatible with the original description of A. blochii, while culture CBS 324.33 falls into a fully supported clade (BPP/MLBS = 1/100 %), together with another culture (CBS 427.93) from human skin, that is representative of a novel genus in the Bionectriaceae close to Ovicillium. Morphologically, cultures CBS 427.93 and CBS 324.33 agree well with the original description of A. blochii as they produce thin, delicate hyphae, and ovoid conidia arranged in chains. We therefore designate CBS 427.93 as neotype of M. blochii to stabilise the application of the generic type.
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hits using the ITS sequence had the highest similarity to Acremonium blochii [culture CBS 993.69, GenBank HE608636.1; identities = 417/477 (87 %), 22 gaps (4 %)]; A. pinkertoniae [culture CBS 157.70, GenBank NR_159611.1; identities = 417/482 (87 %), 16 gaps (3 %)]. The closest hits using LSU sequence had the highest similarity to A. blochii [culture CBS 324.33, GenBank MH866909.1; identities = 774/774 (100 %), no gaps] and “Verticillium insectorum” [culture CBS 101239, GenBank HQ248107.1; Identities = 768/775 (99 %), 1 gap (0 %)].
Proxiovicillium lepidopterorum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845904. Fig. 70.
Fig. 70.

Proxiovicillium lepidopterorum (ex-type culture CBS 101239). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–I. Conidiophores. J. Conidiophores radiating out from coils formed by the mycelium. K. Conidia. Scale bars =10 μm.
Etymology: The species epithet refers to a species of the host order, Lepidoptera.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 0.9–2.2 μm wide. Sporulation abundant, phalacrogenous, nematogenous. Conidiophores solitary, (sub-)erect, straight, arising directly from submerged or superficial hyphae, sometimes radiating out from sterile coils formed by mycelium, unbranched and reduced to single phialides, poorly branched, with 1–2 phialides per node, 37.5–117.5 μm long, 1.1–1.9 μm wide at base, 1–3-septate, hyaline, smooth-walled. Phialides lateral or terminal, cylindrical, acicular, or subulate, hyaline, thick-, smooth-walled, (16–)35.5–61.5 μm long, 1–1.6 μm wide at base, with periclinal thickening and inconspicuous collarette at conidiogenous loci. Conidia aseptate, ovoid, ellipsoid, straight, tapering to apiculate at both ends, hyaline, thin-, smooth-walled, 3–4.7 × 1.4–2.1 μm, eguttulate, arranged in chains, later collapsing soon as conidial heads. Prismatic crystals present on MEA plate. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 23–24 mm diam, flat, membranous, dirty white at centre, white at periphery, margin entire, reverse concolourous; On MEA reaching 25–27 mm diam, raised, radially folded, rugose, with moderate aerial mycelium, moist, felty or hairy, straw at centre, white at periphery, margin entire, reverse amber, abundant crystals formed on surface of plate; On PDA reaching 26–27 mm diam, flat, radially folded, rugose, with moderate aerial mycelium, felty, floccose at centre, creamy white, margin entire, reverse pale straw. Lacking odour on all media.
Typus: Malaysia, from Lymantriidae (insect, Lepidoptera), unknown collection date, isol. 1982, coll. and isol. C.L. Bong (holotype CBS H-24606, ex-type culture CBS 101239 = IMI 264729).
Notes: The type culture of Proxiovicillium lepidopterorum (CBS 101239) was received as “Verticillium insectorum”. Although no type specimen of Vm. insectorum was available, we consider this culture to represent a different species, because they differ from the original host, location, and also morphological characters: Vm. insectorum was described on a spider or pupae in Nuwara Eliya, central Sri Lanka (Petch 1931, Gams 1971), while CBS 101239 was collected on moths in the Lymantriidae subfamily in Malaysia. The colony of Vm. insectorum on OA was woolly, while that of Pr. lepidopterorum was flat and dusty. In addition, its conidiophores are longer than Vm. insectorum (37.5–117.5 μm long vs 12–28 μm long), and conidia are wider than those of Vm. insectorum (1.5–2.1 μm vs 0.75–1 μm). Proxiovicillium lepidopterorum is resolved in a separate branch close to Pr. blochii, and is significantly different from the latter in morphology: phialides are 9.5–26.5 × 0.8–1.8 μm in Pr. blochii, while those of Pr. lepidopterorum are (16–)35.5–61.5 × 1–1.6 μm; conidia are oval or sub-globose, measuring 2.6–3.7 × 1.9–2.6 μm in Pr. blochii, and ovoid or ellipsoid, 3–4.7 × 1.4–2.1 μm in Pr. lepidopterorum.
Clade O37
Hapsidospora Malloch & Cain, Canad. J. Bot. 48: 1819. 1970.
Synonyms: Nigrosabulum Malloch & Cain, Canad. J. Bot. 48: 1822. 1970.
Mycoarachis Malloch & Cain, Canad. J. Bot. 48: 1820. 1970.
Mycelium consisting of branched, remotely septate, hyaline, smooth-, thin-walled hyphae. Sexual morph: Ascomata cleisthotecial or perithecial, superficial, initially produced as distinct cylindrical coils, later becoming sub-globose to globose, ostiolate or non-ostiolate, (metallic) black by reflected light, dark olivaceous green to black by transmitted light, smooth, glabrous, non-stromatic. Ascomatal wall consisting of dark cells of layer and a hyaline inner layer. Asci irregularly disposed, unitunicate, 8-spored, evanescent, sub-globose to globose, non-stipitate. Ascospores 0–1-septate, globose or cylindrical to broadly ellipsoidal, hyaline or dark olive green to brown, reticulate or smooth, without germ pores, with small particles adhering to the wall in some species. Asexual morph: Conidiophores solitary or aggregated, (sub-)erect or curved, arising directly from submerged or superficial hyphae, or ropes formed by mycelium, usually reduced to single phialides, mostly unbranched, occasionally basitonously branched, septate, bearing 1–2 phialides per node, hyaline, smooth-walled, sometimes with chromophilic warty incrustation at base, with cell walls usually thicker than those of vegetative hyphae. Phialides mono- or polyphialidic, terminal or lateral, subulate, subcylindrical, tapering from base to apex, hyaline, thin- or thick-, smooth-walled, chromophilic with age, with inconspicuous or conspicuous periclinal thickening and collarette at conidiogenous loci. Conidia aseptate, variable in shape and size, ellipsoid, somewhat allantoid, fusoid, ovoid, cylindrical, oblong, lunate with both ends rounded, occasionally with truncate or apiculate base at one end, hyaline, thin- or thick-, smooth-walled, straight or slightly curved, eguttulate or guttulate, arranged in chains or in heads, pink to orange. Chlamydospores can be present (emended from Malloch & Cain 1970).
Type: Hapsidospora irregularis Malloch & Cain
Other accepted species with available sequences: H. chrysogena (Thirum. & Sukapure) L.W. Hou, L. Cai & Crous, H. flava (W. Gams) L.W. Hou, L. Cai & Crous, H. globosa (Malloch & Cain) L.W. Hou, L. Cai & Crous, H. inversa (Malloch & Cain) L.W. Hou, L. Cai & Crous, H. stercoraria L.W. Hou, L. Cai & Crous, H. variabilis L.W. Hou, L. Cai & Crous
Notes: Hapsidospora was introduced by Malloch & Cain (1970) based on its type H. irregularis, which is characterised by producing sub-globose and globose ascomata, globose and dark reticulate ascospores. It was initially placed in Pseudeurotiaceae (Malloch & Cain 1970). Later, a study from Locquin (1984) established a new family, Hapsidosporaceae, to accommodate this genus. However, the family turned out to be invalid because it was published without a Latin description or diagnosis or by a reference to a previously and effectively published Latin description or diagnosis [nom. inval., Art. 39.1 (Melbourne)].
In the present study, Hapsidospora together with the other two genera, Nigrosabulum and Mycoarachis that were described in the same article, are compared based on both phylogeny and morphology: phylogenetically, all three genera clustered in Bionectriaceae and formed a well-supported clade (Fig. 2). Morphologically, they are similar in producing irregularly disposed, sub-globose to globose ascomata. Therefore, we suggest they are congeneric, and Hapsidospora is chosen over the other two genera (Malloch & Cain 1970). Hapsidospora is morphologically characterised by the production of long phialides and variably shaped and sized conidia as well as globose non-stipitate asci and globose to cylindrical or broadly ellipsoidal ascospores.
Hapsidospora chrysogena (Thirum. & Sukapure) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845905.
Basionym: Cephalosporium chrysogenum Thirum. & Sukapure, Mycologia 55: 565. 1963.
Synonym: Acremonium chrysogenum (Thirum. & Sukapure) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 109. 1971.
Description & illustration: Gams (1971).
Typus: India, Maharashtra, Pimpri-Poona, from soil, unknown collection date and collector, isol. 10 Jan. 1975 by M.J. Thirumalachar, CBS H-24694 (holotype of Cephalosporium chrysogenum HACC 113, ex-type culture CBS 144.62 = ATCC 14615 = CECT 2718 = HACC 113 = IFO 30055 = IMI 091579 = MUCL 9718).
Additional materials examined: Egypt, Cairo, unknown substrate, collection date and collector, isol. Saadia M. Essa, (255)3, culture CBS 899.85. Italy, Sardinia, Sassari Province, from sea water, unknown collection date and collector, isol. G. Brotzu, NRDC 8650, CBS H-8132, culture CBS 779.69 = ATCC 11550 = ATCC 14553 = CECT 2723 = DSM 880 = IAM 14645 = IMI 049137 = MUCL 16146 = MUCL 18474. Unknown, unknown substrate, collection date and collector, dep. M.A. Pisano, CBS H-8131, culture CBS 401.65.
Notes: This fungus was isolated from soil samples collected at Pimpri in India, and has the ability to produce the antibiotic cephalosporin-N, which is active against both gram-positive and gram-negative bacteria (Sukapure & Thirumalachar 1963). Gams (1971) transferred this fungus to Acremonium as A. chrysogenum. However, in the present study, the ex-type culture of A. chrysogenum (CBS 144.62) clustered in the Hapsidospora clade (Fig. 2).
Hapsidospora flava (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845907.
Basionym: Acremonium flavum W. Gams, Trans. Brit. Mycol. Soc. 59: 521. 1972.
Description & illustration: Gams & Lacey (1972).
Typus: Germany, Braunschweig-Völkenrode, from agricultural soil, unknown collection date and collector, isol. K.H. Domsch, No. VF 1014, dep. W. Gams (holotype CBS 596.70 preserved as metabolically inactive culture, ex-type culture CBS 596.70 = ATCC 24621).
Additional materials examined: Germany, Braunschweig-Völkenrode, from agricultural soil, unknown collection date and collector, isol. K.H. Domsch, No. D30, dep. W. Gams, culture CBS 597.70; former West-Germany, from domestic waste, unknown collection date, A. von Klopotek, No. A 91, culture CBS 963.87; former West-Germany, from heated municipal compost, unknown collection date and collector, isol. D.O. Knösel, No. BM MP 11, culture CBS 316.72. Netherlands, Gelderland Province, Wageningen, from agricultural soil, unknown collection date and collector, isol. J.H. van Emden, culture CBS 142.71.
Notes: Acremonium flavum was originally described from agricultural soil in the Netherlands and was introduced as one of the species from Acremonium section Nectrioidea (Gams & Lacey 1972). Six strains labelled A. flavum are included in this study. They form a fully supported clade in the genus Hapsidospora including the ex-type (CBS 596.70) and therefore are recombined as H. flava (Fig. 2). Morphologically, the pigmentation is similar to its phylogenetic neighbour H. irregularis, but differs from the latter in the production of longer phialides (40–70 × 2–2.7 μm vs 20–50 × 1.5–2 μm) and conidia (5–6.5 × 1.7–2.5 μm vs 3.5–5.5 × 2.0–3.0 μm). In addition, H. flava could be clearly distinguished from H. irregularis in its abundant chlamydospores, thermotolerant character, and lack of yeast-like growth (Gams & Lacey 1972, Malloch & Cain 1970)
Hapsidospora globosa (Malloch & Cain) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845908.
Basionym: Nigrosabulum globosum Malloch & Cain, Canad. J. Bot. 48: 1823. 1970.
Description & illustration: Malloch & Cain (1970).
Typus: USA, Wyoming, Laramie County, E of Cheyenne, from dung of cow, 16 Aug. 1964, D.W. Malloch (holotype TRTC 43288, ex-isotype culture CBS 512.70 = ATCC 22102 = IFO 31743 = IFO 31783 = IMI 148372).
Additional materials examined: Australia, Queensland Simpson desert, from dung of Macropus rufus (Macropodidae), Jan. 2001, A. Bell, No. A283, culture CBS 110041. Namibia, Gamsberg Pass, 95 km W of Rehoboth, from dung of baboon, 24 Apr. 1963, Nordenstam, isol. D.W. Malloch, TRTC 45651 & CBS H-15163, culture CBS 513.70 = ATCC 22103. Netherlands, Gelderland Province, Wageningen, from soil, unknown collection date and collector, isol. J.H. van Emden, CBS H-24617, culture CBS 416.73. Tanzania, Mt. Kilimanjaro, W of Loitokitok, from dung of cow, 17 Aug. 1966, R.F. Cain, D. Griffin & J.C. Krug, isol. D.W. Malloch, TRTC 66.1741 & CBS H-15164, culture CBS 514.70 = ATCC 22104 = IMI 148370. USA, Montana, Fallon, unknown date, from dung of horse, 4 Sep. 1957, R.F. Cain, isol. D.W. Malloch, TRTC 35756 & CBS H-15162, culture CBS 511.70 = ATCC 22101 = IMI 148373.
Notes: Hapsidospora globosa is a cleistothecial species reported on dung from Africa, Australia, Europe, and North America (Malloch & Cain 1970). It is characterised by the production of sub-globose to globose ascomata and globose ascospores with small particle-like attachments. This species was originally described as type of Nigrosabulum in the family Pseudeurotiaceae (Malloch & Cain 1970). Nigrosabulum is similar to Mycoarachis and Pseudeurotium, but distinct from these genera in the production of globose ascospores, which is an unusual feature in Hapsidospora. Recent molecular phylogenetic analyses and morphological studies showed that the family Pseudeurotiaceae was heterogeneous and that the included taxa were allied to both apothecial and perithecial lineages (Plishka et al. 2009). In the present study, two perithecial genera, Mycoarachis and Nigrosabulum, are synonymised with Hapsidospora based on multi-locus phylogenetic analysis and morphological characters.
Hapsidospora inversa (Malloch & Cain) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845906.
Basionym: Mycoarachis inversa Malloch & Cain, Canad. J. Bot. 48: 1822. 1970.
Description & illustration: Malloch & Cain (1970).
Typus: Uganda, Queen Elisabeth National Park, Mweya Lodge, from dung of Loxodonta africana (Elephantidae), 27 Jul. 1966, R.F. Cain, D. M. Griffin & J.C. Krug, isol. D.W. Malloch, CBS H-7382 (holotype TRTC 662166f, ex-isotype culture CBS 517.70 = ATCC 22107 = IMI 148374).
Notes: The monotypic genus Mycoarachis was established for M. inversa isolated from elephant dung from Uganda (Malloch & Cain 1970). The species is characterised by the production of globose ascomata of which the peridium appears to be reversed in structure. The peridium is made up of pale and dark layers of cells, but the pale-coloured layer is exterior to the dark layer. Hapsidospora inversa is characterised by the production of 2-celled hyaline ascospores and can thus easily be distinguished from the closely related species, Hapsidospora globosa (Malloch & Cain 1970, as Nigrosabulum globosum). In the present study, M. inversa nests within the Hapsidospora clade, closely related to H. stercoraria but forming a separate lineage (Fig. 2). The generic name Mycoarachis is now regarded as a synonym of Hapsidospora.
Hapsidospora irregularis Malloch & Cain, Canad. J. Bot. 48: 1819. 1970.
Description & illustration: Malloch & Cain (1970).
Typus: Canada, Ontario, York County, Toronto, from lawn grass compost heap, Jul. 1966, W.F. Collins, isol. D.W. Malloch (holotype TRTC 44852, ex-type culture CBS 510.70 = ATCC 22087 = IFO 31742 = IMI 148377).
Hapsidospora stercoraria L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845909. Fig. 71.
Fig. 71.

Hapsidospora stercoraria (A–G from ex-type culture CBS 516.70, H–K from culture CBS 217.84). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Epithet based on the adjective stercorarius (growing on dung), referring to the substrate from which the ex-type strain was collected.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, up to 1.9 μm wide. Conidiophores solitary or aggregated, (sub-)erect, straight, arising directly from submerged or superficial hyphae, or ropes formed by mycelium, mostly unbranched, poorly branched, with 1–2 distinct septa at base, (26.7–) 32–77 μm long, hyaline, smooth-walled, clearly chromophilic with age, with large orange guttules, cell walls usually thicker than those of vegetative hyphae. Phialides lateral, rarely terminal, subulate, subcylindrical, hyaline, thick-, smooth-walled, 20–77 μm long, 1.5–3.3 μm wide at base, with inconspicuous periclinal thickening and collarette at conidiogenous loci, chromophilic with age; polyphialides not observed. Conidia variable in shape and size, aseptate, lunate, cylindrical, or vesicular distended, straight or slightly curved, chromophilic cells observed in old cultures, hyaline, thin-, smooth-walled, 3.8–9.5 × 1.5–2.4 μm, with several minute guttules, and pale orange large guttules with age, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 11 mm diam, flat, with sparse aerial mycelium, dusty, salmon at periphery, white at centre, margin entire, reverse concolourous; On MEA reaching 8–10 mm diam, raised and rugose, cerebriform, membranous with sparse aerial mycelium, buff, margin undulate, reverse umber; On PDA reaching 17–20 mm diam, flat, slightly raised and cerebriform at centre, thinly felty at periphery, with sparse aerial mycelium, felty, salmon, margin entire, reverse saffron; On SNA reaching 12 mm diam, flat, membranous with sparse aerial mycelium, whitish, margin fimbriate, reverse concolourous.
Typus: Tanzania, Mt. Kilimanjaro, N of Lyamungu, from dung of herbivore, 11 Aug. 1966, R.F. Cain, D.M. Griffin & J.C. Krug (holotype CBS H-24619, ex-type culture CBS 516.70 = ATCC 22106 = IMI 148375 = TRTC 661707a).
Additional materials examined: Unknown, contaminant of Oospora halophila culture CBS 232.32 (current name of culture Aspergillus baarnensis; Aspergillaceae), unknown collection date and collector, isol. W. Gams, CBS H-24620, culture CBS 217.84.
Notes: Two strains of Hapsidospora stercoraria formed a sister clade to H. inversa (Fig. 2), another species from the dung of Loxodonta africana in Uganda (Malloch & Cain 1970; 94 % sequence similarity in ITS, 89 % on rpb2 and 96 % on tef-1α). However, they can be morphologically distinguished by the differences in the length of their conidiophores [(26.7–)32–77 μm vs 14–35 μm in H. inversa], and in the shape of conidia (lunate, cylindrical or vesicular distended in H. stercoraria, ellipsoid, fusoid, ovoid, cylindrical or allantoid in H. inversa).
Hapsidospora variabilis L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845910. Fig. 72.
Fig. 72.

Hapsidospora variabilis (ex-type culture CBS 100549). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophore with conidial head. E. Unbranched conidiophores. F, G. Branched conidiophores. H. Branched conidiophores with short outgrowth. I. Conidia. Scale bars = 10 μm.
Etymology: From Latin variabilis, due to the variable shape and size of the conidia.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.3–2.7 μm wide, mycelial ropes formed. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, (sub-)erect, straight or to flexuous, mostly curved, irregularly wavy at base, thick and long, arising directly from submerged or superficial hyphae, or from the ropes formed by mycelium, mostly with 1–2 irregularly basitonous side branches, bearing 1–3 levels with 1–2 phialides per node, or unbranched and reduced to single phialides, often with short sterile outgrowths, 1–2(–4)-septate at base and middle, (18–)45–105.5(–127) μm long, 2.2–4.5 μm wide at base, hyaline, smooth-walled, rough- and thick-walled at basal part, cell walls usually thicker than those of vegetative hyphae. Phialides lateral, cylindrical or subulate, hyaline, thick-, smooth-walled, (19.5–)36.5–90.5 μm long, 2–3.8 μm wide at base, with conspicuous periclinal thickening and collarette at conidiogenous loci; polyphialides not observed. Conidia variable in shape and size, aseptate, cylindrical, fusoid, oblong or ovoid, straight or slightly curved, with a slightly apiculate base, hyaline, thick-, smooth-walled, (4.8–)5–13.5(–15.5) × 2.3–3.7 μm, with 2–3 large guttules, not chromophilic, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 20–21 mm diam, flat, with sparse aerial mycelium, dusty, hairy, mycelial ropes present, salmon at centre, dirty white at periphery, margin entire, reverse concolourous; On MEA reaching 19–21 mm diam, raised, abundant aerial mycelium, hairy, moist, flesh at centre, dirty white at periphery, margin entire, reverse orange; On PDA reaching 19–20 mm diam, flat, sparse aerial mycelium, thinly felty, rosy buff, margin entire, reverse concolourous; Colonies on SNA reaching 18 mm diam, flat, membranous sparse aerial mycelium, white, margin entire, reverse concolourous.
Typus: Denmark, from compost of Miscanthus (Poaceae) and pig slurry, unknown collection date, C. Jakobsen (holotype CBS H-24696, ex-type culture CBS 100549).
Notes: Phylogenetically, Hapsidospora variabilis forms a distinct lineage basal to the clade containing H. chrysogena, H. flava and H. irregularis (Fig. 2). Morphologically, H. variabilis differs from the closely related species in its production of irregularly curved, basitonously branched conidiophores and phialides with short sterile outgrowths. Hapsidospora variabilis could also be distinguished from other species in this genus by producing larger (4.8–)5–13.5(–15.5) × 2.3–3.7 μm and variably sized conidia.
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hit using the LSU sequence is Nigrosabulum globosum (currently H. globosa, culture CBS 268.91; GenBank MH873934.1; Identity = 99.36%), and the closest hit using the ITS sequence is also Nigrosabulum globosum (currently H. globosa, culture CBS 416.73; GenBank MH860723.1, Identity = 94.17%).
Clade O38
Alloacremonium L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845911.
Etymology: From Greek allos, different; in reference to the morphological resemblance to acremonium-like conidia, but phylogenetically different.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores hyaline, smooth-walled, solitary, straight, (sub-)erect or curved, arising directly from submerged or superficial hyphae, unbranched, or poorly branched, occasionally proliferating sympodially, bearing 1–3 levels with 1–2 phialides per node, 1–2-septate at base. Conidiogenous cells enteroblastic, mono- and polyphialidic, lateral or terminal, subulate, hyaline, thick-, smooth-walled, with periclinal thickening and collarette at conidiogenous loci. Conidia aseptate, ellipsoid, oblong to short cylindrical, symmetrically obtuse at both ends, hyaline, thin-, smooth-walled, straight, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph absent.
Type: Alloacremonium humicola L.W. Hou, L. Cai & Crous
Other accepted species with available sequences: Alloacremonium ferrugineum L.W. Hou, L. Cai & Crous
Notes: Two cultures from agricultural soil and a clinical sample of a human toenail form a clade with 100 % bootstrap support on the LSU, ITS, rpb2, and tef-1α tree (Fig. 2), representing two new species with oblong to short cylindrical conidia arranged in slimy heads. Since two species herein clearly form an independent lineage and are phylogenetically segregated from other genera, we introduce Alloacremonium as a new genus to accommodate these species.
Alloacremonium ferrugineum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845912. Fig. 73.
Fig. 73.

Alloacremonium ferrugineum (ex-type culture CBS 102877). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidial heads. E–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: From Latin ferrugineus, rust colour. Referring to the pigment colour on PDA media.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.3–2.3 μm wide. Conidiophores solitary, (sub-)erect, straight, arising directly from submerged or superficial hyphae, unbranched, poorly branched, bearing 1–2 phialides per node, 23.5–56 μm long, (1–)1.5–2.5 μm wide at base, 1–2-septate at base or middle, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate, hyaline, thick-, smooth-walled, 20–50.5 μm long, (1–)1.5–2.5 μm wide at base, with conspicuous periclinal thickening and inconspicuous collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, ellipsoidal, oblong to short cylindrical, symmetrically rounded, straight, hyaline, thin-, smooth-walled, (2.3–)2.5–4.5(–6) × 1.4–1.8 μm, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 21–22 mm diam, flat, membranous without aerial mycelium, buff to salmon at centre, dirty white at periphery, margin entire, reverse pale rosy buff; On MEA reaching 24 mm diam, flat, slightly radially folded, felty, creamy white, margin entire, reverse pale orange; On PDA reaching 25–27 mm diam, flat, dusty, creamy white, with pale rust pigment, margin filiform, reverse buff. Lacking odour on all media.
Typus: China, Guizhou Province, from human toenail, apparently etiologic, unknown collection date and collector, isol. 1999, dep. G. Bulmer (holotype CBS H-24607, ex-type culture CBS 102877).
Notes: Alloacremonium ferrugineum is based on CBS 102877 received as Acremonium persicinum (currently Verruciconidia persicina), but collected from a different host and locality than the holotype of A. persicinum (basionym: Paecilomyces persicinus, Nicot 1958). Based on our phylogenetic analysis, Al. ferrugineum forms a separate lineage in the genus Alloacremonium, close to Al. humicola and distant from A. persicinum (Fig. 2). Morphologically, Al. ferrugineum shares some characters with Al. humicola in the shape and size of conidia, but differs in its rarely branched, straight, shorter conidiophores, measuring 23.5–56 μm in length, while those of Al. humicola are commonly branched, flexuose and up to 92 μm long.
The late Glenn Bulmer informed one of us (RCS), in a personal communication connected to culture deposition, that, in a dermatologic mycology workshop in 1998, a sponsoring official had donated sufficient material from his hyperkeratotic great toenail that all class members could perform microscopy and culture. Direct microscopy was positive for fungal filaments, and this species was consistently and exclusively isolated in culture by all students, making over 30 isolations in total. One of these cultures was submitted as CBS 102877.
Based on a blastn search of NCBIs GenBank nucleotide database, the closest hit using the ITS sequence is A. egyptiacum (culture CBS 127849; GenBank MH864730; Identity = 87 %), and the closest hit using the LSU sequence is Geosmithia microcorthyli (culture CCF 3861; GenBank NG_067560.1; Identity = 96 %), the closest hit using the rpb2 sequence is G. putterillii (culture CBS 342.52; GenBank LR535706.1; Identity = 74 %), the closest hit using the tef-1α sequence is Amphichorda guana (culture LC5815; GenBank KX855211.1; Identity = 95 %).
Alloacremonium humicola L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845913. Fig. 74.
Fig. 74.

Alloacremonium humicola (ex-type culture CBS 613.82). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidial heads. E. Conidiophores with mono-, polyphialides, and phialides with proliferation (arrows). F–I. Conidiophores. J. Conidia. Scale bars = 10 μm.
Etymology: Name derived from the substrate this species was collected from, soil.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–2 μm wide. Conidiophores solitary, (sub-)erect, straight to flexuose, curved, arising directly from submerged or superficial hyphae, unbranched or branched, proliferating sympodially, bearing 1–3 levels with 1–2 phialides per node, up to ca. 92 μm long, 1.6–2.3 μm wide at base, 1–2-septate at base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate, hyaline, thick-, smooth-walled, 21.5–42(–49) μm long, 1–2 μm wide at base, with conspicuous periclinal thickening and collarette at conidiogenous loci, commonly with a percurrent or subterminal proliferation; polyphialides with up to two conidiogenous loci commonly present. Conidia aseptate, ellipsoid, oblong to short cylindrical, symmetrically rounded, hyaline, thin-, smooth-walled, straight, 3.5–4.5 × 1.7–2.4 μm, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 24–26 mm diam, flat, sparse aerial mycelium, dusty, white, margin crenate, reverse concolourous; On MEA reaching 18–20 mm diam, raised, radially folded, with moderate aerial mycelium, hairy at centre, without aerial mycelium at periphery, rosy buff, margin crenate, reverse pale ochreous; On PDA reaching 25–30 mm diam, flat, with sparse aerial mycelium, dusty, white, margin entire, reverse buff at centre, dirty white at periphery.
Typus: Netherlands, Flevoland Province, Noordoostpolder, Nagele, from agricultural soil, unknown collection date and collector, isol. H. Nielander, No. 919 (holotype CBS H-24688, ex-type culture CBS 613.82).
Notes: Alloacremonium humicola is sister to Al. ferrugineum in the multi-locus phylogenetic analysis with a fully supported bootstrap value (Fig. 2; BPP/MLBS = 1/100 %). These two species are similar in having oblong to short cylindrical conidia that are arranged in slimy heads (Figs 73, 74). However, Al. humicola is distinguished in having more branched, longer, flexuose conidiophores (up to ca. 92 μm long) and polyphialides, as well as by its wider conidia (1.7–2.4 μm vs 1.4–1.8 μm), and its lack of rust-coloured pigment on PDA medium, while conidiophores of Al. ferrugineum are poorly branched and shorter (23.5–56 μm long), polyphialides absent, and rust-coloured pigment present on PDA medium.
Clade T
Myrotheciomycetaceae Crous, Persoonia 40: 351. 2018.
Classification: Hypocreales, Sordariomycetes.
Type genus: Myrotheciomyces Crous
Trichothecium Link : Fr., Mag. Gesell. Naturf. Freunde, Berlin 3: 18. 1809.
Type: Trichothecium roseum (Pers.) Link
Other accepted species with available sequences: Trichothecium hongkongense L.W. Hou, L. Cai & Crous, T. indicum (Arx, Mukerji & N. Singh) Summerb., Seifert & Schroers, T. crotocinigenum (Schol-Schwarz) Summerb.
Notes: Trichothecium was revised by Summerbell et al. (2011), comprising five species with three different asexual forms, contemporarily classified as Acremonium (phialoconidia), Spicellum (sympodial blastoconidia), and Trichothecium (retrogressive blastoconidia). In the present study, species of Trichothecium form a fully supported clade together with Stanjemonium spectabile (phialoconidia) and T. hongkongense (phialoconidia) described below. Myrotheciomyces corymbiae also clusters within the Trichothecium clade based on its ITS and LSU sequences, but with low support value, suggesting it is not congeneric with Trichothecium. Morphologically, Myrotheciomyces differs from Trichothecium species in the production of sporodochial conidiomata, which were not observed in Trichothecium.
Trichothecium hongkongense L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845915. Fig. 75, 76.
Fig. 75.

Trichothecium hongkongense (ex-type culture CBS 101444). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Ascomata on OA. E, F. Ascomata. G–I. Asci. J. Ascospores. K, L, N. Conidiophores. M. Chlamydospore. O. Conidia. Scale bars: E, F = 100 μm; G–O = 10 μm.
Fig. 76.

Trichothecium hongkongense (culture CBS 117586). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Sporodochium-like structures on OA. E–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Named after the location where the fungus was collected, Hong Kong.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.3–2.5 μm wide. Sexual morph: Ascomata perithecial, superficial, solitary, gregarious or scattered, globose, sub-globose or broadly pyriform, with pale brown, to dark brown papilla, uniloculate, ostiolate, with abundant white or pale brown, cylindrical, septate and unbranched hyphae covered, 215–300 × 204–288 μm. Ostiole single, central, or with two ostioles, papilla, or elongated to long necks, up to 158 μm. Ascomatal wall 13–30 μm wide, composed of several layers of thick-walled, hyaline to pale brown, cylindrical cells forming textura angularis. Paraphyses not observed. Asci unitunicate, 8-spored, cylindrical, apex simple, 48.8–70.5 × 6.5–8.5 μm. Ascospores uniseriate, with overlapping ends, cylindrical, symmetrically rounded at both ends, 1-septate, not constricted at septa, hyaline, thick-walled, faintly striate, 9–11.8 × 3.7–4.5 μm, eguttulate, without mucilaginous sheath. Asexual morph: Sporulation abundant, mostly nematogenous and plectonematogenous. Conidiophores solitary or aggregated, (sub-)erect, arising directly from submerged or superficial hyphae, irregularly basitonously branched, bearing 1–3 (–5) levels with 1–2 phialides per node, or unbranched, or repeatedly branched and arranged as sporodochia-like structure in some strains, with short sterile outgrowths, 22.8–72.8(–90.82) μm long, 1–2.5 μm wide at base, 1–3-septate at base and middle, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, cylindrical or subulate, occasionally swollen in the lower part, hyaline, thick-, smooth-walled, (8.5–)24–44.5 μm long, 1–2 μm wide at base, with inconspicuous periclinal thickening and collarette at conidiogenous loci, or with conspicuous cylindrical collarette; polyphialides with up to two conidiogenous loci can present. Conidia aseptate, cylindrical, oblong or ovoid, rounded at both ends, straight or slightly curved, hyaline, thin-, smooth-walled, 4.1–6.8 × 2.1–2.6 μm, eguttulate, arranged in slimy heads. Chlamydospores occasionally present in some strains, laterally on short stalks, single, globose or sub-globose, hyaline, guttulate, smooth-, thick-walled, 4.4–6.3 × 2.3–4.3. Sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 80 mm diam, flat, felty, granulose and hairy, dirty white at centre, white at periphery, margin entire, reverse buff, without odour; On MEA reaching 80 mm diam, flat, abundant aerial mycelium, floccose, granulose, white, margin entire, reverse luteous, without odour; On PDA reaching 80 mm diam, flat, moderate aerial mycelium, felty, granulose, white, creamy white, margin entire, reverse dirty white; On SNA reaching 80 mm diam, flat, membranous without aerial mycelium, white, margin dendritic, reverse white.
Typus: China, Hong Kong, from living leaf, Jun. 1998, isol. Aug. 1998, coll. and isol. A. Aptroot, No. 43374 (holotype CBS H-24632, ex-type culture CBS 101444).
Additional materials examined: Dominica, Lauro Club Reef, from Ectyplasia sp. (sponge), unknown collection date, E. Eguereva, CBS H-24633, culture CBS 117586. Ecuador, from basidium of Marasmius sp. (Marasmiaceae) in tropical rain forest, 1988, unknown collector, isol. A. Ainsworth, H.J.S. No. N9683-02 & No. 0326, CBS H-24704, culture CBS 102186.
Notes: Three cultures from different substrates form a fully supported clade, representing a new species of Trichothecium. Morphologically, this species differs from known Trichothecium species in the production of larger ascospores (9–11.8 × 3.7–4.5 μm) and the absence of a mucilaginous sheath (Summerbell et al. 2011). The strain from sponge, CBS 117586, clustered slightly apart from the ex-type of T. hongkongense based on 57 bp changes over the sequences of four genes (ITS, LSU, tef-1α and rpb2). The culture is morphologically similar to the ex-type of T. hongkongense (CBS 101444), but slightly differs from CBS 101444 in its conidiophores with less septa, more branches and sporodochia-like structures (Fig. 76), which reflect intraspecific variability.
Clade U
Valsonectriaceae L.W. Hou, L. Cai & Crous, fam. nov. MycoBank MB 845916.
Classification: Hypocreales, Sordariomycetes.
Stroma immersed in the substratum, becoming partially erumpent, pale yellow, pseudoparenchymatous. Ascomata immersed in valsoid, lightly pigmented stroma, globose to sub-globose, yellow, KOH-, ostiolate. Asci clavate to cylindrical. Ascospores narrowly ellipsoid to fusoid, equally 1-septate, hyaline or yellow-brown, smooth or coarsely striate. Asexual morph: acremonium-like.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores erect or slightly curved, repeatedly basitonously branched, often proliferating sympodially, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, or unbranched, septate or aseptate at base, middle and apex, swollen at lower part, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Conidiogenous cell enteroblastic, mono- or polyphialidic, lateral or terminal, cylindrical or subulate, inflated at base, tapering gradually towards apex, hyaline, thin- or thick-, smooth-walled, often proliferating terminally and subterminally, inflated at lower part. Conidia aseptate, cylindrical, (sub-)globose, reniform, broadly fusoid, ovoid, with apiculate ends, with hilar extensions at one end or both ends, hyaline, thin- or thick-, smooth-walled, straight, eguttulate, arranged in long chains. Chlamydospores not observed.
Type genus: Valsonectria Speg.
Notes: Valsonectriaceae, erected for Valsonectria, is based on V. pulchella, a species originally reported on rotten branches of Melia azedarach in Buenos Aires, Argentina (Spegazzini 1881). Phylogenetic analysis shows that the type of Valsonectria, V. pulchella, together with V. portsmouthensis, V. simpsonii, and several Acremonium strains identified A. inflatum and A. roseolum (basionym: Paecilomyces roseolus) formed a distinct lineage close to the familial clades of Sarocladiaceae and Myrotheciomycetaceae in Hypocreales (Fig. 1). In our analysis, we confirm the relationship of the Valsonectria species and the Acremonium strains in the same genus, and the introduction of new family Valsonectriaceae.
Valsonectria Speg., Anales Soc. Ci. Argent. 12: 211. 1881.
Sexual morph: Stroma immersed in the substratum, becoming partially erumpent, pale yellow, pseudoparenchymatous. Ascomata immersed in valsoid, lightly pigmented stroma, globose to sub-globose, yellow, KOH-, ostiolate. Asci clavate to cylindrical. Ascospores narrowly ellipsoid to fusoid, equally 1-septate, hyaline or yellow-brown, smooth or coarsely striate. Asexual morph: acremonium-like. Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores solitary or aggregated, erect or curved, arising directly from submerged or superficial hyphae, or from ropes formed by mycelium, unbranched, repeatedly basitonously branched, often proliferating sympodially, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, with 0–3-septa in basal, medial or apical regions, swollen near base, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Conidiogenous cell monophialidic or polyphialidic, lateral or terminal, cylindrical or subulate, ampulliform, inflated at base, occasionally inflated into a (sub-)globose base, tapering gradually towards apex, hyaline, thin- or thick-, smooth-walled, with inconspicuous or conspicuous periclinal thickening and collarette at conidiogenous loci, often terminally or subterminally proliferating; polyphialides with up to three conidiogenous loci occasionally present; adelophialides occasionally present. Conidia aseptate, cylindrical, (sub-)globose, ellipsoid, allantoid, reniform, broadly fusoid, fusoid-ellipsoid, ovoid, with both ends apiculate, or with apiculate bases and obtuse apices, hyaline, olivaceous green or grey green, thin- or thick-, smooth-walled, straight, eguttulate, arranged in slimy heads or in long chains, collapsing into conidial heads or not. Chlamydospores absent (revised from Rossman et al., 1999).
Type: Valsonectria pulchella Speg.
Other accepted species with available sequences: Valsonectria crystalligena L.W. Hou, L. Cai & Crous, V. hilaris L.W. Hou, L. Cai & Crous, V. inflata (C.H. Dickinson) L.W. Hou, L. Cai & Crous, V. portsmouthensis Crous & Jurjević, V. roseola (G. Sm.) L.W. Hou, L. Cai & Crous, V. simpsonii Samuels & Seifert, V. soli L.W. Hou, L. Cai & Crous
Notes: The genus Valsonectria is characterised by having nectria-like ascomata immersed in a valsoid, lightly pigmented stroma, 1-septate ascospores, and acremonium-like asexual morphs (Spegazzini 1881, Rossman et al. 1999). It was placed in the order Hypocreales, Bionectriaceae based on the examination of the type specimen of V. pulchella (Petrak & Sydow 1936, Rossman et al. 1999). Valsonectria pulchella together with 16 cultures labelled Acremonium s. lat. species were examined and formed a fully supported, independent clade in Hypocreales (Fig. 1), representing a new family. Most acremonium-like cultures in this clade were identified as A. inflatum and A. roseolum, sharing generic diagnostic features such as sympodially proliferating conidiophores and the phialides inflating in the basal part (Fig. 1).
Valsonectria crystalligena L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845917. Fig. 77.
Fig. 77.

Valsonectria crystalligena (ex-type culture CBS 126650). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial chains. E–G. Conidiophores. H, I. Conidiophores with sympodially proliferated phialides. J. Crystals. K. Conidia. Scale bars = 10 μm.
Etymology: Named after the crystals produced by this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.3–2.7 μm wide. Sporulation moderate, phala-crogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight or slightly bent, arising directly from submerged or superficial hyphae, or from ropes formed by mycelium, unbranched, often proliferating sympodially, showing phialides as short lateral, cylindrical asymmetrical projections, with 1–3-septa at base or near apex, 21.5–55 μm long, 2–4.5 μm wide at base, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral, rarely terminal, subulate, inflated at lower part or dilated at basal part, tapering gradually towards apex, occasionally with a short spiculate tip, hyaline, thick-, smooth-walled, 7.2–39.5(–48.5) μm long, 1.4–3.7 μm wide at base, with inconspicuous periclinal thickening and minute collarette at conidiogenous loci, occasionally as lateral branches; polyphialides with up to two conidiogenous loci occasionally present. Conidia aseptate, fusoid-ellipsoid, with an elongated apiculate bases and obtuse apices, straight, hyaline, thick-, smooth-walled, eguttulate, 5.5–11 × 2.2–3.5 μm, arranged in long chains. Brown crystals present, globose. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 30–32 mm diam, with sparse aerial mycelium, dusty, dirty white, margin entire, reverse concolourous; On MEA reaching 37–39 mm diam, flat, radially folded, felty, buff at centre, white at periphery, margin filiform, reverse orange, saffron at periphery, with buff radial lines; On PDA reaching 33–34 mm diam, flat, with moderate aerial mycelium, dusty, white, margin filiform, reverse buff at centre, dirty white at periphery; On SNA reaching 17 mm diam, flat, with sparse aerial mycelium, membranous, white, margin dendritic, reverse colourless. With strong pungent odour on PDA and MEA media, with light geosmin odour on OA and SNA media.
Typus: Italy, Viterbo Province, Lago di Vico, NW side, from decaying grass leaves with Uredinales, 20 Nov. 2009, W. Gams (holotype CBS H-24642, ex-type culture CBS 126650).
Notes: The ex-type strain of Valsonectria crystalligena (CBS 126650) was initially identified as Acremonium zeylanicum (currently Waltergamsia zeylanica), but observations of the conidiogenous structures in culture revealed this species to have basally inflated phialides as well as polyphialides with lateral branches. Valsonectria crystalligena is different from W. zeylanica in producing globose brown crystals. Valsonectria crystalligena is placed in an independent branch that is close to the V. roseola clade (Fig. 1). Morphologically, V. crystalligena can be distinguished from V. roseola by its production of brown crystals, and by its longer, narrower fusoid-ellipsoid conidia (5.5–11 × 2.2–3.5 μm vs 4.8–7.5 × 2.7–4.5 μm), while that of V. roseola are ovoid. In addition, V. crystalligena differs from V. roseola by lack of adelophialides.
Valsonectria hilaris L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845918. Fig. 78.
Fig. 78.

Valsonectria hilaris (ex-type culture CBS 381.73). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–F. Branched conidiophores with mono- and polyphialides. G, H. Branched conidiophores with monophialides I. Unbranched conidiophore. J. Conidia. Scale bars = 10 μm.
Etymology: Named for conidia with a hilum at both ends.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.6–2.7 μm wide, mycelial ropes and coils formed. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight or slightly, irregularly curved, arising directly from submerged or superficial hyphae, mostly basitonously branched, often repeatedly proliferating sympodially, phialides as short lateral, cylindrical, asymmetrical projections, forming sporodochia-like structure, rarely unbranched and reduced to single phialides, 1–2-septate at base and middle, up to 110 μm long, 1–3.3 μm wide at base, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate, straight or slightly curved, terminally and subterminally proliferating, hyaline, thick-, smooth-walled, (13–) 21–62.5 μm long, 1–2.8 μm wide at base, with conspicuous periclinal thickening and apiculate cylindrical collarette at conidiogenous loci; polyphialides with up to three conidiogenous loci occasionally present. Conidia aseptate, fusoid or ovoid, with an elongated hilum at basal end, and a pointed apical end, straight, hyaline, thick-, smooth-walled, 4–7.4 × 1.8–2.7 μm, eguttulate, arranged in long chains. Crystals not observed. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 40–42 mm diam, flat, with sparse aerial mycelium, felty, with some membranous zones, creamy white, margin entire, with abundant mycelial ropes, reverse pale olivaceous buff; On MEA reaching 41 mm diam, flat, abundant aerial mycelium, felty, dirty white, margin entire, with abundant mycelial ropes, reverse luteous, with buff radial lines; On PDA reaching 30–38 mm diam, flat, sparse aerial mycelium, thinly felty, salmon, with moderate mycelial ropes, margin crenate, reverse pale luteous at centre, buff at periphery; On SNA reaching 26 mm diam, flat, with sparse aerial mycelium, dusty, colourless, margin entire, reverse colourless. Without odour on all media.
Typus: India, Bangalore, Hortus Lal Bagh, from dead stem of bamboo, Jan. 1973, W. Gams (holotype CBS H-24641, ex-type culture CBS 381.73).
Notes: Although initially identified as Acremonium implicatum, CBS 381.73 is not conspecific with this species. Phylogenetic analysis placed this strain in an independent lineage in the Valsonectria clade, described here as V. hilaris (Fig. 1). Morphologically, V. hilaris differs from its most closely related species V. crystalligena by producing longer phialides [(13–)21–62.5 μm vs 7.2–39.5(–48.5) μm] with repeatedly branches; phialides of V. crystalligena have fewer branches. In addition, V. hilaris can be distinguished from V. crystalligena by producing significantly shorter conidia (4–7.4 × 1.8–2.7 μm vs 5.5–11 × 2.2–3.5 μm) and by lacking brown crystals.
Valsonectria inflata (C.H. Dickinson) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845919. Fig. 79.
Fig. 79.

Valsonectria inflata (ex-type culture CBS 212.69). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidial heads. E. Conidiophores with conidial heads. F–I. Conidiophores. J. Conidia. Scale bars =10 μm.
Basionym: Gliomastix inflata C.H. Dickinson, Mycol. Pap. 115: 20. 1968.
Synonym: Acremonium inflatum (C.H. Dickinson) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 88. 1971.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.1–2.5 μm wide. Conidiophores aggregated or solitary, erect, mostly irregularly curved, or straight, arising directly from submerged or superficial hyphae, or the ropes formed by the mycelium, unbranched, occasionally with 1–2 irregularly basitonous side branches, aseptate or with a single septum at base, 9.5–32 μm long, 1–3.1 μm wide at base, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, cylindrical, subulate, ampulliform, mostly inflated or swollen at base, into a (sub-)globose base, hyaline, thin-, smooth-walled, 9–23 μm long, 1–3.3 μm wide at base, 1.5–4.2 μm at widest part, with conspicuous periclinal thickening and dark olivaceous flared collarette at conidiogenous loci; polyphialides with two conidiogenous loci occasionally present. Conidia variable in shape and size, aseptate, (sub-)globose, ellipsoid, allantoid, cylindrical or ovoid, straight or curved, hyaline at beginning, becoming olivaceous green or grey green with age, thin-, smooth-walled, 2.5–4.6(–5.2) × 1.8–3.1 μm, eguttulate, arranged in slimy heads. Crystals not observed. Chlamydospores and sexual morph not observed (revised from Dickinson 1968).
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 19–20 mm diam, flat, membranous with sparse aerial mycelium, olivaceous, margin entire, reverse concolourous; On MEA reaching 21 mm diam, flat, with moderate aerial mycelium, hairy, moist, rosy buff, margin crenate, reverse saffron, orange at centre, with buff radial lines; On PDA reaching 21–22 mm diam, flat, wrinkly fold at centre, dusty, white, with pale olivaceous edge, margin entire, reverse buff; On SNA reaching 10 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse colourless. With geosmin odour on OA and MEA media, without odour on PDA and SNA media.
Typus: UK, Lincolnshire, Gibraltar Point, from intertidal salt marsh mud, 1962, C.H. Dickinson, No. G13, CBS H-6645 (holotype IMI 100877, ex-isotype culture CBS 212.69 = IMI 100877 = VKM F-1544).
Additional materials examined: Germany, Giessen, soil, unknown collection date and collector, isol. A. von Klopotek, No. G 11, culture CBS 604.68. Netherlands, Flevoland Province, Oostelijk Flevoland, agricultural soil, unknown collection date and collector, isol. Mar. 1969 by J.H. van Emden, No. 307, culture CBS 403.70; Gelderland Province, Wageningen, sandy soil under permanent wheat, unknown collection date and collector, isol. 30 Jan. 1970 by J.H. van Emden, No. Rb 4/15, culture CBS 439.70; Noordoost Polder, Nagele, agricultural soil, unknown collection date and collector, isol. H. Nylander, H.N. 1146, culture CBS 497.82. UK, Rothamsted, cereal stem, unknown collection date and collector, isol. B. Fitt, CBS H-8200, culture CBS 305.74.
Notes: When this species was originally described, the production of hyaline mycelium, the regularly orthotropic phialides, mostly pronounced in a synnematogenous arrangement and the green to grey-green pigmented conidia led it to be identified as a member of Acremonium series Murorum (Gams 1971). Our study agrees with the result of previous phylogenetic analysis based on LSU and SSU sequences, which places this species in an independent clade close to the Stachybotrys clade (Summerbell et al. 2011), distant from Acremonium s. str. and the Bionectriaceae but clustering within the Valsonectria clade (Fig. 1). This species is hereby transferred to the genus Valsonectria. The description of the observed cultures is relatively consistent with that of A. inflatum (Dickinson 1968; Gams 1971). Morphologically, V. inflata differs from other species in Valsonectria by the production of (sub-)globose, green to grey-green conidia arranged in conidial heads (Fig. 79), while those of other species have fusoid, broadly fusoid, or ovoid conidia with a hilum at both ends, and are hyaline and arranged in long chains. Considering the unique characteristics of V. inflata, it may not be congeneric with the clade contained V. crystalligena, V. hilaris, V. portsmouthensis, V. roseola, and V. soli. However, we prefer to retain them in one genus until more material and molecular data are available.
Valsonectria roseola (G. Sm.) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845920. Fig. 80.
Fig. 80.

Valsonectria roseola (ex-type culture CBS 289.62). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial heads and chains. F. Conidiophores with proliferated phialides and adelophialides. G, H. Conidiophores with proliferated phialides and polyphialides. I, J. Branched conidiophores. K. Conidia. Scale bars =10 μm.
Basionym: Paecilomyces roseolus G. Sm., Trans. Brit. Mycol. Soc. 45: 388. 1962.
Synonyms: Acremonium roseolum (G. Sm.) W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 68. 1971.
Sagrahamala roseola (G. Sm.) Subram., Curr. Sci. 41: 49. 1972.
Description based on the ex-isotype culture CBS 289.62: Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–2 μm wide. Conidiophores solitary or aggregated, erect, straight or slightly curved, arising directly from submerged or superficial hyphae, or from ropes formed by mycelium, repeatedly basitonously branched, often proliferating sympodially, showing phialides as short lateral, cylindrical asymmetrical projections, bearing 1–5 levels with 1–4 phialides per node, or unbranched, swollen at lower part, 0–2(–3)-septate in base and middle, 15–90.5 μm long, 2.5–3.8 μm wide at base, hyaline, smooth-walled, cell walls usually thicker than those of vegetative hyphae. Phialides lateral, rarely terminal, subulate, ampulliform, inflated in lower part, hyaline, thick-, smooth-walled, (5.5–)7.5–37 μm long, 0.9–3.5 μm wide at base, with inconspicuous periclinal thickening and collarette at conidiogenous loci, often terminally and sub-terminally proliferating; polyphialides with two conidiogenous loci commonly present; adelophialides present, subulate, 12.5–22.5 × 2.8–3.8 μm. Conidia aseptate, ovoid, lemon-shaped, with apiculate hila at both ends, straight, hyaline, thick-, smooth-walled, 4.8–7.5 × 2.7–4.5 μm, eguttulate, arranged in long chains, soon collapsing into conidial heads. Crystals not observed. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 48–50 mm diam, flat, dusty, dirty white, margin entire, reverse saffron; On MEA reaching 45–50 mm diam, flat, spirally folded, felty, dirty white, margin entire, reverse orange, with buff radial lines; On PDA reaching 50–52 mm diam, flat, slightly folded at centre, dusty, creamy white or buff, margin entire, reverse concolourous; On SNA reaching 35–37 mm diam, flat, dusty, white, margin entire, reverse concolourous. Without odour on all media (description revised from Smith 1962).
Typus: UK, England, Sheffield, from dead stems of Dactylis glomerata (Poaceae), unknown collection date, isol. Sep. 1955, coll. and isol. J. Webster, No. Pa. 66, CBS H-6666 (holotype of Paecilomyces roseolus IMI 094090, ex-isotype culture CBS 289.62 = ATCC 18508 = IAM 14660 = IMI 094090 = LSHB Pa66).
Additional materials examined: Brazil, State Ceará, Pacajus county, from nut of Anacardium occidentale (Anacardiaceae), unknown collection date, isol. 1996, coll. and isol. F. Freire, CBS H-24640, culture CBS 371.97. UK, Manchester, from Dianthus caryophyllus (Caryophyllaceae), unknown collection date and collector, isol. G.S. Taylor, culture CBS 416.81.
Notes: Valsonectria roseola is based on Paecilomyces roseolus from dead stems of Dactylis glomerata in the UK (Smith 1962, Gams 1971). It was subsequently transferred to the genus Sagrahamala (Subramanian 1972). Morphologically, this species is characterised in its subulate phialides that are typically inflated in the lower part. Valsonectria roseola could be differentiated from V. crystalligena in the lack of crystals, ovoid conidia with apiculate ends, while V. crystalligena abundantly produces brown crystals, fusoid-ellipsoid conidia with hilum at one end. Phylogenetic analyses in this study show that this species clusters within Valsonectria (Fig. 1). When Smith (1962) described the species Pae. roseolus, he mentioned the phialides “tapering from about half the length to a slender tip”. Later, Gams (1971) provided a detailed line drawing when he re-examined the type culture and illustrated the slightly swollen phialides, a finding confirmed in our study as a typical character of Valsonectria.
Valsonectria soli L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 848118.
Etymology: Named after the substrate it was isolated from, soil.
Sterile in culture/in vitro. Valsonectria soli differs from its closest phylogenetic neighbour V. roseola (CBS 289.62) by unique alleles in four loci based on alignments deposited in Figshare (doi: 10.6084/m9.figshare.22258765): ITS position 235(C), 273(A, insertion), 396(gap), 736(T), 737(gap), 837 (C), 838 (T), 1 012 (C, insertion), 1 013(A, insertion); LSU position 1 472(C), 1 473(T); rpb2 position 1 892(A), 1 898(T), 1 916(C), 1 922(C), 1 928(T), 1 931(A), 1 934(G), 1 937(T), 1 943(G), 1 946(T), 1 958(T), 1 961(G), 1 967(A), 1 976(G), 1 979(C), 1 994(C), 2 006(C), 2 009(G), 2 027(C), 2 054(G), 2 087(C), 2 090(C), 2 093(C), 2 096(T), 2 102(C), 2 117(C), 2 129(C), 2 132(C), 2 151(T), 2 170(C), 2 173(T), 2 179(C), 2 185(T), 2 194(A), 2 209(G), 2 224(T), 2 227(T), 2 228(C), 2 251(C), 2 272(T), 2 275(G), 2 281(G), 2 284(C), 2 287(C), 2 329(T), 2 338(C), 2 342(T), 2 359(C), 2 368(T), 2 380(A), 2 395(A), 2 410(T), 2 440 (C), 2 446(A), 2 461(T), 2 464(C), 2 474(T), 2 476(G), 2 491(C), 2 497(A), 2 503(C), 2 506(C), 2 518(A), 2 533(T), 2 542(T), 2 545(A), 2 599(G), 2 629(T), 2 635(A), 2 666(T), 2 669(T), 2 676(T), 2 698(C), 2 710(G), 2 734(C), 2 737(T), 2 743(C), 2 752(C), 2 758(T), 2 764(T), 2 770(C).
Culture characteristics after 14 d at ca. 25 °C: Colonies on OA reaching 25 mm diam, flat, membranous without aerial mycelium, pale saffron, margin entire, reverse concolourous; On MEA reaching 36 mm diam, raised, spirally folded, moist, short hairy, pale saffron, margin entire, reverse luteous; On PDA reaching 33 mm diam, flat, membranous without aerial mycelium, pale rosy buff, margin fimbriate, reverse concolourous; On SNA reaching 20 mm diam, flat, membranous without aerial mycelium, colourless, margin fimbriate, reverse colourless.
Typus: France, Grignon, from agricultural soil, unknown date, isol. J. Guillemat & J. Montégut, (holotype CBS H-8195, culture ex-type CBS 770.69).
Additional materials examined: Netherlands, Oostelijk Flevoland, from agricultural soil, 1969, Veenbaas-Rijks, specimen CBS H-8359, CBS H-8361, culture CBS 106.70; Texel, Den Hoorn, from dead leaf of Cladium mariscus (Cyperaceae), isol. 2 Jun. 1968 by W. Gams, culture CBS 446.68; Zuidelijk Flevoland, from agricultural soil, unknown date, isol. J.W. Veenbaas-Rijks, CBS 888.72; unknown substrate, isol. 7 Oct. 1969 by J.W. Veenbaas-Rijks, specimen CBS H-8360, CBS H-8362, culture CBS 267.70.
Notes: Valsonectria soli is represented by five cultures from Europe. The cultures formed a fully supported clade close to V. roseola, but phylogenetically distinct, which supports this species as unique. Unfortunately, all cultures remained sterile in all culture media tested in this study.
Clade V
Sarocladiaceae L. Lombard, Persoonia 41: 343. 2018.
Classification: Hypocreales, Sordariomycetes.
Type genus: Sarocladium W. Gams & D. Hawksw.
Clade V1
Sarocladium W. Gams & D. Hawksw., Kavaka 3: 57. 1976 (1975).
Colonies on MEA slimy-glabrous to moderately floccose, cottony, to deeply dusty, sometimes ropy; terminology according to (Gams 1971): phalacrogenous, nematogenous, to plectonematogenous conidiation; whitish to pinkish to salmonaceous or, when conidia are formed in chains, sometimes acquiring vivid conidial mass colouration such as ochraceous or greenish glaucous; reverse pale to pinkish orange to pale grey brown, rarely greenish blue. Mycelium septate, hyaline, becoming pale brown with age, smooth- and thin-walled, sometimes bound together into ropes, could produce hyphal coils and nets. Conidiogenous erect, arising directly from vegetative hyphae or from ropes or coils of hyphae, straight or slightly bent, hyaline to subhyaline, smooth-walled, septate, apparatus ranging from adelophialides, solitary orthotropic phialides to conidiophore structures with one or a few branches, or with cymose branching or occasionally with one or two ranks of loosely structured verticils, sometimes repeatedly branching, basitonously or verticillately branched and arising in whorls, occasionally proliferating sympodially. Conidiogenous cell mono- or polyphialidic, lateral, terminal or intercalary, subcylindrical, subulate, acicular, aculeate to acerose, straight or slightly curved, or undulate, hyaline, thin- or thick- and smooth-walled, 15–60(–76) μm long, with minute collarette, with distinct or inconspicuous apical periclinal thickening and collarette, proliferating percurrently in some species. Conidia borne in mucoid heads or dry chains, or born in chains at first, and collapsing into heads, notably longer than broad, l/w mostly 2.2–7.0, bacilliform, oblong, cylindrical, bacilliform, ellipsoidal, ovoid, spindle-shaped, fusoid, limoniform to subglobose, or irregular, aseptate, hyaline or subhyaline, thin-, smooth-walled, straight or slightly curved, with rounded, pointed or tapered-truncate ends, 3.5–8(–14) × 0.5–2 μm. Crystals can present in some species, rust, brown, globose or irregular-shaped. Chlamydospores present or absent, when present relatively thick-walled, smooth or slightly roughened, globose to ellipsoidal, intercalary or terminal, mostly solitary, occasionally in short chains (emended from Summerbell et al. 2011).
Type: Sarocladium oryzae (Sawada) W. Gams & D. Hawksw.
Other accepted species with available sequences: Sarocladium agarici L.W. Hou, L. Cai & Crous, S. attenuatum W. Gams & D. Hawksw., S. bacillisporum (Onions & G.L. Barron) Summerb., S. bactrocephalum (W. Gams) Summerb., S. bifurcatum A. Giraldo, Gené & Deanna A. Sutton, S. brachiariae X.B. Liu, G.X. Huang & Z.K. Guo, S. citri L.W. Hou, L. Cai & Crous, S. dejongiae L. Lombard, S. ferrugineum L.W. Hou, L. Cai & Crous, S. fuscum L.W. Hou, L. Cai & Crous, S. gamsii A. Giraldo, Gené & Guarro, S. hominis A. Giraldo, Gené & Deanna A. Sutton, S. implicatum (J.C. Gilman & E.V. Abbott) A. Giraldo, Gené & Guarro, S. junci Crous & Osieck, S. kiliense (Grütz) Summerb., S. glaucum (W. Gams) Summerb., S. ochraceum (Onions & G.L. Barron) Summerb., S. pseudostrictum A. Giraldo, Gené & Deanna A. Sutton, S. spinificis Y.H. Ye & R. Kirschner, S. strictum (W. Gams) Summerb., S. subulatum A. Giraldo, Gené & Guarro, S. summerbellii A. Giraldo, Gené & Guarro, S. terricola (J.H. Mill., Giddens & A.A. Foster) A. Giraldo, Gené & Guarro, S. theobromae L.W. Hou, L. Cai & Crous, S. zeae (W. Gams & D.R. Sumner) Summerb.
Sarocladium agarici L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845934. Fig. 81.
Fig. 81.

Sarocladium agarici (ex-type culture CBS 113717). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores arised from rope of mycelium. E. Conidiophores radiating out from mycelial ropes. F. Conidial heads. G–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the host genus from which it was isolated, Agaricus.
Mycelium consisting of branched, septate, hyaline, becoming pale brown with age, smooth-, thin-walled hyphae, ropes of mycelium or coiled hyphae present, 1–2 μm wide. Sporulation moderate, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, arising directly from aerial or substratal mycelium, or from ropes or coils formed by the mycelium, unbranched or poorly basitonously branched, bearing 1–3 phialides per node, up to 99 μm long, 1.5–2.5 μm wide at base, with 1–2 septa at base or middle, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate, acicular, or cylindrical, hyaline, thick-, smooth-walled, 31–73 μm long, 1.2–2.5 μm wide at base, commonly with inconspicuous periclinal thickening and collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, cylindrical, with rounded ends, straight or curved, hyaline, thin-, smooth-walled, 3–6.9 × 1.3–2 μm, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 35–36 mm diam, flat, dusty, creamy white, pale hazel at centre, margin entire, reverse concolourous; On MEA reaching 31–34 mm diam, raised, woolly, white, margin fimbriate, reverse pale orange, with buff radial lines; On PDA reaching 35–37 mm diam, flat, hairy, white, with dirty white and fimbriate margin, reverse buff; On SNA reaching 42–43 diam, flat, membranous, white, margin entire, reverse concolourous. Abundant mycelial ropes present on all media
Typus: Iran, Tehran, Shahriar, from Agaricus bisporus (Agaricaceae), 2002, H. Khabbaz (holotype CBS H-24650, ex-type culture CBS 113717).
Additional material examined: USA, from soil, unknown collection date and collector, culture CBS 126941.
Notes: Sarocladium agarici is represented by two cultures, one from Agaricus bisporus and one from soil, forming a distinct clade close to S. spinificis (Fig. 3). However, S. agarici differs from S. spinificis in its longer phialides [31–73 μm vs 20–37.5(−42) μm] and shorter conidia [3–6.9 μm vs 7–9(–13) μm; Yeh & Kirschner 2014]. Also, adelophialides are not observed in S. agarici, while they are present in S. spinificis (Yeh & Kirschner 2014).
Sarocladium citri L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845935. Fig. 82.
Fig. 82.

Sarocladium citri (ex-type culture CBS 145044). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the host genus from which it was isolated, Citrus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, with brown guttules in older cultures, 1.3–2.5 μm wide, chondroid with aging. Sporulation abundant, phalacrogenous, nematogenous. Conidiophores solitary or aggregated, erect, straight to flexuous, curved, irregularly bent, arising directly from aerial or substratal mycelium, unbranched or repeatedly basitonously branched, bearing 1–4 levels with 1–2 phialides per node, occasionally proliferating sympodially, showing conidiogenous cells as short, lateral, cylindrical asymmetrical projections, up to 125 μm long, 1–2.8 μm wide at base, with 1–3 septa in base, middle or the apical part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae, chondroid at basal part. Phialides lateral or terminal, subulate or cylindrical, hyaline, thick-, smooth-walled, (12–)22–50(–76) μm long, 1.2–2.3 μm wide at base, commonly with inconspicuous periclinal thickening and collarette at conidiogenous loci, commonly percurrent or subterminal proliferating; polyphialides not observed. Conidia aseptate, cylindrical or bacilliform, straight, with obtuse apices and bases, occasionally with slightly apiculate bases, hyaline, thin-, smooth-walled, 3–7.8 × 1.5–2 μm, eguttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristic after 14 d at 25 °C: Colonies on OA reaching 26–28 mm diam, flat, with sparse aerial mycelium, dusty, creamy white, rosy buff in old cultures, margin filiform, reverse buff. On MEA reaching 24–26 mm diam, flat, radially folded, with moderate aerial mycelium and abundant mycelial ropes, felty, rosy buff, margin entire, reverse saffron, with buff radial lines. On PDA reaching 16–20 mm diam, flat, rugose at centre, with sparse aerial mycelium and mycelial ropes, dusty, creamy white, margin filiform, reverse buff. On SNA reaching 27–28 mm diam, flat, with sparse aerial mycelium, dusty, colourless, margin filiform, reverse colourless.
Typus: Italy, Noto, Siracusa, from twigs of Citrus sinensis (Rutaceae), 4 Jul. 2016, V. Guarnaccia (holotype CBS H-24718, ex-type culture CBS 145044 = CPC 31198).
Notes: Sarocladium citri forms a distinct branch showing a close phylogenetic affinity to S. dejongiae (Fig. 3). Sarocladium citri can be clearly distinguished from S. dejongiae in its repeatedly basitonously branched and longer conidiophores (up to 125 μm long), while conidiophores of S. dejongiae are unbranched or rarely branched, measuring up to 35 μm long (Crous et al. 2018a). Sarocladium citri also clearly differs from S. dejongiae in producing creamy white colony on OA and PDA, while that of S. dejongiae are pale saffron, salmon or rosy buff. Conidia of S. dejongiae have multiple shapes including cylindrical, ellipsoid, ovoid and irregular, while the conidial shapes of S. citri are relatively constantly short-cylindrical or bacilliform. In addition, S. citri differs in lacking chlamydospores, while intercalary chlamydospores are formed in S. dejongiae (Crous et al. 2018a).
Sarocladium ferrugineum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845936. Fig. 83.
Fig. 83.

Sarocladium ferrugineum (ex-type culture CBS 102673). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial heads. F. Conidial heads. G–J. Conidiophores. K. Rusty crystal. L. Conidia. Scale bars = 10 μm.
Etymology: Latin ferrugineo, rusty, referring to the production of rusty coloured crystals.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–2.4 μm wide. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight, arising directly from aerial or substratal mycelium, or from mycelial ropes, unbranched, basitonously or verticillately branched, bearing 1–3 phialides per node, up to 60.5 μm long, 1.6–3 μm wide at base, with 1–2 septa in base, middle or the apical part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, rarely terminal, subulate, acicular, hyaline, thick-, smooth-walled, (11.2–)16.7–43.8(–58.8) μm long, 1.2–2.5 μm wide at base, commonly with conspicuous periclinal thickening and flared collarette at conidiogenous loci. Conidia aseptate, cylindrical, straight, with truncate bases at both ends, hyaline, thin-, smooth-walled, 3.7–6.5(–7.7) × 1.1–1.7 μm, eguttulate, arranged in slimy heads. Crystals present, rust in colour, irregular-shaped. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 31 mm diam, flat, dusty, yellow at centre, white at periphery, with rusty crystals, margin entire, reverse concolourous; On MEA reaching 38 mm diam, flat, radially folded, floccose, creamy white, margin crenate, reverse pale luteous, with buff radial lines; On PDA reaching 46 mm diam, flat, raised at centre, felty, hairy at centre, white, dirty white at periphery, margin entire, reverse pale scarlet at centre, buff to dirty white at periphery; On SNA reaching 56 mm diam, flat, without aerial mycelium, colourless, margin filiform, reverse colourless. Lacking odour on all media.
Typus: Russia, unknown substrate, collection date and collector, isol. B.A. Borisov (holotype CBS H-24651, ex-type culture CBS 102673 = VKM F-3436).
Notes: In the phylogenetic tree, Sarocladium ferrugineum is represented by a single culture, CBS 102673, which is clearly distinguished from other species in the genus Sarocladium (Fig. 3). Morphologically, S. ferrugineum can be differentiated from its closest neighbour S. zeae in its longer conidia 3.7–6.5(–7.7) × 1.1–1.7 μm, with slightly truncate ends, while S. zeae has shorter conidia 3.5–5.8 × 1.2–1.9 μm, with symmetrically rounded ends (Gams 1971). In addition, Sarocladium ferrugineum differs from S. zeae in the production of rusty crystals on OA media (Gams 1971).
Sarocladium fuscum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845937. Fig. 84.
Fig. 84.

Sarocladium fuscum (ex-type culture CBS 334.80). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores radiating out from mycelial coil. E, F. Conidiophores with conidial heads. G–I. Conidiophores. J. Crystals. K. Conidia. Scale bars = 10 μm.
Etymology: From Latin fuscus, meaning brownish. Referring to the production of brownish pigment crystals.
Mycelium consisting of branched, septate, smooth-, thin-walled hyphae, hyaline at first, yellowish in old cultures, 1.7–2 μm wide, mycelial ropes and coils commonly present. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight or irregularly curved and flexuose, arising directly from aerial or substratal mycelium, or radiating out from the mycelial coils, unbranched or poorly branched, bearing 1–2 phialides per node, up to 73 μm long, 1.6–2.2 μm wide at base, with 1–2-septa in base or middle, hyaline, smooth-walled, rough-walled at basal part, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, cylindrical, acicular, or subulate, occasionally curved, tapering to apex, flexuose, hyaline, thick-, smooth-walled, 23–55.5 μm long, 1–2 μm wide at base, commonly with inconspicuous periclinal thickening and collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, short cylindrical or bacilliform, straight, with truncate bases, and rounded apices, hyaline, thin-, smooth-walled, 3–5.9 × 1.1–1.7 μm, eguttulate, arranged in slimy heads. Abundant globose or irregular-shaped brownish crystals. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 20–22 mm diam, flat, dusty, pale luteous to luteous, with dirty white edge, with abundant irregular shaped or globose crystals, margin entire, abundant orange pigment and crystals in old cultures, reverse concolourous; On MEA reaching 24–26 mm diam, flat, radially folded, rugose, with sparse aerial mycelium, rosy buff, margin filiform, reverse saffron, with buff radial lines; On PDA reaching 18–20 mm diam, flat, short hairy, pale orange at centre, with buff ropes formed by the mycelium, with peach and crenate margin, abundant orange pigment in old cultures, reverse apricot, with buff radial lines; On SNA reaching 18–20 mm diam, flat, membranous, white to pale luteous, margin entire, reverse concolourous.
Typus: Colombia, road Bogotá-Fómeque, alt. ca. 1 800 m, from dead stem of Bambusa sp. (Poaceae), Dec. 1979, W. Gams, Col. 160 (holotype CBS H-24717, ex-type culture CBS 334.80).
Notes: Sarocladium fuscum is phylogenetically allied to S. gamsii and S. theobromae with a moderate support (PP/MLBS = 1/96 %, Fig. 3). Morphologically, S. fuscum can be differentiated from S. gamsii and S. theobromae mainly by the production of abundant brown crystals on OA plates. It is also distinct in its colony colour on OA media: S. fuscum is pale luteous, while S. gamsii and S. theobromae are white (Giraldo et al. 2015). In addition, these species differ in the shape and size of their conidia: S. fuscum produces short cylindrical or bacilliform conidia arranged only in slimy heads (3–5.9 × 1.1–1.7 μm), while conidia of S. gamsii are fusoid, measuring as 3–5 × 1–2 μm and arranged in both slimy heads and conidial chains, and conidia of S. theobromae are short cylindrical and arranged in slimy heads, measuring as 2.8–4.4 × 1.3–1.8 μm. Sarocladium fuscum also differs from S. theobromae in the production of unbranched or poorly branched conidiophores, while those of S. theobromae are mostly repeatedly branched or verticillate (Fig. 85).
Fig. 85.

Sarocladium theobromae (ex-type culture CBS 113440). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial heads. F–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Sarocladium theobromae L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845938. Fig. 85.
Etymology: Referring to the host genus from which it was isolated, Theobroma.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 0.8–1.9 μm wide. Sporulation moderate, phalacrogenous, nematogenous, plectonematogenous, or synnematogenous. Conidiophores solitary or closely aggregated, erect, arising directly from aerial or substratal mycelium, or synnematogenous, occasionally basitonously or verticillately branched, then bearing 1–3 levels with 2–3 phialides per node, or unbranched, up to 112 μm long, 1.2–2.2 μm wide at base, with 1–3(–4)-septa at base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, rarely terminal, cylindrical, acicular, straight or slightly curved, hyaline, thick-, smooth-walled, (21–)26.8–63 μm long, 1–1.6 μm wide at base, commonly with conspicuous periclinal thickening and cylindrical or slightly flared collarette at conidiogenous loci; occasionally with short sterile outgrowths; polyphialides with 2 conidiogenous loci occasionally present. Conidia aseptate, short cylindrical, rounded at both ends, straight, hyaline, thin-, smooth-walled, 2.8–4.4 × 1.3–1.8 μm, 1–2-guttulate, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 30–32 mm diam, flat, dusty, white, margin entire, without odour, reverse buff; On MEA reaching 36 mm diam, flat, radially and densely folded, rugose, felty, moist, dirty white, margin filiform, reverse orange at centre, saffron at periphery, with buff radial lines; On PDA reaching 37–39 mm diam, flat, white and hairy at centre, dirty white and dusty at periphery, margin entire, reverse dirty white, with white radial lines; On SNA reaching 38–40 mm diam, flat, membranous, white, margin entire, reverse concolourous.
Typus: Ecuador, from Theobroma gileri (Malvaceae), unknown collection date, H.C. Evans & K.A. Holmes (holotype CBS H-24652, ex-type culture CBS 113440).
Notes: Thus far, no species of Sarocladium has been described on Theobroma. Phylogenetically, S. theobromae is placed in an independent branch in this genus, proximal to S. fuscum (Fig. 3). Sarocladium theobromae can be distinguished from S. fuscum by the following set of characters: white or dirty white colonies, longer, synnematogenous conidiophores (up to 112 μm long vs up to 73 μm long), shorter conidia (2.8–4.4 × 1.3–1.8 μm vs 3–5.9 × 1.1–1.7 μm) and lack of brownish crystals.
Clade V2
Parasarocladium Summerb. et al., Microorganisms 6: 17. 2018.
Mycelium consisting of hyaline, septate, branched hyphae, occasionally inflated with brown guttules. Colonies smooth to thinly floccose, with salmonaceous, orange, yellow, or greenish-black reverse colouration. Conidiophores solitary or aggregated, arising laterally from vegetative hyphae, erect, cylindrical to subcylindrical, unbranched or basitonously branched, or proliferating sympodially, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, septate, smooth, hyaline. Conidiogenous cells enteroblastic, monophialidic or polyphialidic, arising laterally from hyphae, in terminal pairs, verticils of three, or small monopodially branched tufts of up to four from conidiophores, hyaline, smooth, up to two-septate, elongate-ampulliform to subcylindrical, subulate, acicular, with inconspicuous or conspicuous periclinal thickening and collarettes, occasionally with percurrent, terminal or sub-terminal proliferation. Conidia aseptate, smooth-walled or with chromophilic roughening, allantoid, oblong, cylindrical, ellipsoid, ovoid, bacilliform or fusoid, with both ends rounded, sometimes with a slightly apiculate base, straight, sometimes slightly curved, eguttulate, or with hyaline or pale brown guttules, forming slimy heads on phialides. Chlamydospores absent but hyphal swellings may be present. Sexual morph absent (emended from Summerbell et al. 2018).
Type: Parasarocladium radiatum (Sukapure & Thirum.) Summerb. et al.
Other accepted species with available sequences: Par. aestuarinum M. Gonçalves, T. Vicente & A. Alves, Par. alavariense M. Gonçalves, T. Vicente & A. Alves, Par. breve (Sukapure & Thirum.) Summerb., J.A. Scott, Guarro & Crous, P. chondroidum L.W. Hou, L. Cai & Crous, Par. debruynii L. Lombard, Par. funiculosum (Sukapure & Thirum.) L.W. Hou, L. Cai & Crous, Par. fusiforme M. Gonçalves, T. Vicente & A. Alves, Par. gamsii (Tichelaar) Summerb., J.A. Scott, Guarro & Crous
Notes: The genus Parasarocladium was originally introduced to accommodate three soilborne acremonium-like species of the breve/radiatum complex (Summerbell et al. 2018). Later another two soilborne species, one plant pathogen and three species associated with macroalgae in an estuarine environment were described (Crous et al. 2018a, 2020, 2021a, Gonçalves 2020). Currently, a total of 10 species are recognised in Parasarocladium. Two additional species, one soilborne and the other plant endophytic, are included in the present study (Fig. 3).
Parasarocladium chondroidum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845928. Fig. 86.
Fig. 86.

Parasarocladium chondroidum (ex-type culture CBS 652.93). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–F, J, K. Branched conidiophores. G. Hyphae inflated with brown guttules. H. Phialides with a percurrent proliferation (arrow). I. Polyphialides (arrow). L. Unbranched conidiophores. M, N. Periclinal thickening and flared collarette at the conidiogenous locus. O. Conidia. Scale bars = 10 μm.
Etymology: Referring to the chondroid conidiophores produced by this fungus.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, occasionally inflated, chondroid, with abundant brown guttules, 1.3–2.6(–3.2) μm wide. Conidiophores solitary or aggregated, erect, straight or irregularly curved, arising directly from aerial or substratal mycelium, unbranched or repeatedly basitonously branched, bearing 2–5(–7) levels with 2–4 phialides per node, commonly proliferating sympodially, showing conidiogenous cells as short, lateral, cylindrical asymmetrical projections, up to 125 μm long, 1.3–3.2 μm wide at base, 1–2-septate at base or middle, constrict, chondroid, hyaline, with brown guttules at base or middle, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate or subcylindrical, tapering to the apex, occasionally inflated at lower part, straight or irregularly curved, hyaline, thin-, smooth-walled, with brown guttules with age, 13.8–38.5 μm long, 1.2–2.7 μm wide at base, commonly with conspicuous periclinal thickening and flared collarette at conidiogenous loci, occasionally with a percurrent, terminal or subterminal proliferation; polyphialides with up to two conidiogenous loci occasionally present; adelophialides not observed. Conidia aseptate, cylindrical or ellipsoidal, curved, with both ends rounded, sometimes with a slightly apiculate base, hyaline, thin-, smooth-walled, with several brown guttules, 3.4–8 × 1.2–2.5 μm, arranged in slimy heads. Chlamydospores not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 32–35 mm diam, flat, thinly felty, granulate, white at periphery, buff at centre, margin entire, reverse concolourous; On MEA reaching 32–33 mm diam, flat, finely radially folded, rugose, thinly felty and pale salmon at centre, membranous without aerial mycelium and rosy buff at periphery, moist, margin entire, reverse saffron, with white radially lines; On PDA reaching 28–29 mm diam, flat, radially folded and rugose at centre, dusty, filiform strings through colony, pale buff to pale yellow, margin filiform, reverse concolourous. Without odour on all media.
Typus: New Zealand, endophyte in Gramineae, unknown collection date, M. di Menna, New Zealand Pastoral Agriculture Research Inst., Ruakura, No. 3a (holotype CBS H-24648, ex-type culture CBS 652.93).
Notes: Although represented by a single culture, Parasarocladium chondroidum is distinct from its closely related species, P. debruynii and Par. gamsii, based on the multi-locus tree (Fig. 3). Morphologically, it is characterised by the production of large brown guttules not only in hyphae, but also in conidiophores and conidia, which are not observed in other species in this family. Mycelium is occasionally thickened and inflated with brown guttules in older cultures. Otherwise, it mostly produces extensively branched conidiophores that are irregularly curved at the apex and have a chondroid base, while those of Par. debruynii are straight, rarely branched and without a chondroid base, and those of Par. gamsii consists of solitary orthophialides without a chondroid base (Tichelaar 1972, Crous et al. 2018a, Summerbell et al. 2018).
Parasarocladium funiculosum (Sukapure & Thirum.) L.W. Hou, L. Cai & Crous, comb. et stat nov. MycoBank MB 845929. Fig. 87.
Fig. 87.

Parasarocladium funiculosum (ex-type culture CBS 141.62). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial heads. F, G. Unbranched conidiophores. H–J. Branched conidiophores with mono- and polyphialides and proliferated phialides. K. Conidia. Scale bars = 10 μm.
Basionym: Cephalosporium acremonium var. funiculosum Sukapure & Thirum., Bull. Torrey Bot. Club. 93: 306. 1966.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.3–2.5 μm wide. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight, arising directly from aerial or substratal mycelium, or from ropes formed by mycelium, unbranched or basitonously branched, bearing 1–4 levels with 1–3(–4) phialides per node, or repeatedly proliferating sympodially, showing conidiogenous cells as lateral, cylindrical, asymmetrical projections, 36–170.5(–200) μm long, 1.2–2.9 μm wide at base, with 1–2(–3)-septa at base, middle or apex, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate or cylindrical, hyaline, thick-, smooth-walled, 30.5–70.5 μm long, 0.8–2 μm wide at base, commonly with inconspicuous periclinal thickening and collarette at conidiogenous loci, occasionally with percurrent, terminal or subterminal proliferation; polyphialides with up to three conidiogenous loci occasionally present. Conidia aseptate, oblong, cylindrical, with both ends rounded, sometimes with a slightly apiculate base, hyaline, thin-, smooth-walled, 3–5.6 × 1.6–2 μm, eguttulate, arranged in slimy heads. Chlamydospores not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 55–58 mm diam, flat, moderate aerial mycelium, thinly felty, granulose, rosy buff at centre, white at periphery, with olivaceous buff pigment at periphery, margin entire, reverse rosy buff at centre, dirty white at periphery;. On MEA reaching 65–67 mm diam, flat, radially folded, sparse aerial mycelium, membranous, vinaceous buff, rosy buff at periphery, margin filiform, reverse ochreous with orange centre, with buff radial lines. On PDA reaching 60–63 mm diam, flat, sparse aerial mycelium, membranous with few granulose zones, a few shallow striations over the colony surface, buff, margin fimbriate, reverse pale olivaceous buff; On SNA reaching 67–70 mm diam, flat, sparse aerial mycelium, slightly dusty, concolourous, margin fimbriate, reverse concolourous (emended from Sukapure & Thirumalachar 1966).
Typus: India, Maharashtra, Poona, Pimpri, from soil, 5 Sep. 1957, M.J. Thirumalachar, CBS H-24647 (holotype of Cephalosporium acremonium var. funiculosum HACC 102, ex-type culture CBS 141.62 = ATCC 14608 = IMI 091572).
Notes: The type of the basionym of Parasarocladium funiculosum, Cephalosporium acremonium var. funiculosum, was isolated from a soil sample collected in Pimpri, India (Sukapure & Thirumalachar 1966). Based on the multi-locus phylogenetic analysis, the ex-type culture of C. acremonium var. funiculosum formed a distinct branch in the genus Parasarocladium (Fig. 3). In the protologue, it was described and illustrated with branched and variable-length conidiophores (26–66 × 1.5–2.5 μm), and oblong conidia (2.7–3.7 × 1.2–1.5 μm) (Sukapure & Thirumalachar 1966). However, in the present study, it is observed to produce comparably shaped but longer conidiophores [36–170.5(–200) long] and larger conidia on OA (3–5.6 × 1.6–2 μm) (Fig. 88).
Fig. 88.

Chlamydocillium acaciae (ex-type culture CBS 523.72). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidial heads. E, F. Unbranched conidiophores with monophialides. G, I. Conidiophores with polyphialides. H. Monophialides and adelophialides. J. Conidia. Scale bars = 10 μm.
Clade V3
Chlamydocillium Zare & W. Gams, Mycol. Progr. 15: 1018. 2016.
Synonym: Kiflimonium Summerb. et al., Microorganisms 6: 17. 2018.
Saprotrophic, mostly soilborne phialidic hyphomycetes. Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores solitary or aggregated, erect or slightly curved, arising directly from aerial or substratal mycelium, or from ropes of hyphae, unbranched or basitonously branched, producing solitary and/or verticillate phialides up to three per node, septate at base or middle part, occasionally chondroid at base. Conidiogenous cells enteroblastic, mono- and polyphialidic, lateral or terminal, subulate, acicular, (sub-)cylindrical, hyaline, thin- or thick-, smooth-walled, commonly with inconspicuous or conspicuous periclinal thickening and flared or cylindrical collarette at conidiogenous loci; adelophialides can be present in some species. Conidia aseptate, cylindrical, bacilliform to ellipsoid, short reniform, or allantoid,with obtuse apices and bases, or slightly apiculate at bases, mostly curved, or straight, hyaline, thin- and smooth-walled, eguttulate or guttulate, arranged in slimy heads. Crystals absent. Chlamydospores lateral or intercalary, solitary, arranged in single, pairs or in short chains, (sub-)globose, ovoid, limoniform, ampulliform, dolliform, oblong to ellipsoid with truncated ends, hyaline, thick-walled. Sexual morph absent.
Type: Chlamydocillium cyanophilum Zare & W. Gams
Other accepted species with available sequences: Chlamydocillium acaciae L.W. Hou, L. Cai & Crous, C. antarcticum L.W. Hou, L. Cai & Crous, C. curvulum (W. Gams) L.W. Hou, L. Cai & Crous, C. guttulatum L.W. Hou, L. Cai & Crous, C. lolii L.W. Hou, L. Cai & Crous, C. terrestre L.W. Hou, L. Cai & Crous, C. soli L.W. Hou, L. Cai & Crous
Notes: The genus Chlamydocillium was originally proposed to accommodate a single species C. cyanophilum isolated from wheat-field soil and had a verticillium-like asexual morph with abundant intercalary chlamydospores (Zare & Gams 2016). The taxonomic relationship of the genus Chlamydocillium with the Bionectriaceae was uncertain at that time, and it was treated as belonging to an undefined family (Zare & Gams 2016). In our present study, a number of cultures labelled Acremonium spp. were examined, which nest with Chlamydocillium and form a fully supported branch in Sarocladiaceae (Figs 1, 3). Most of the cultures were identified as A. curvulum based on the acremonium-like morphological characters and the production of curved conidia. However, these cultures are phylogenetically heterogeneous and scattered across nine clades that are close to C. cyanophilum, and match the characters of Chlamydocillium and are thus congeneric.
Chlamydocillium acaciae L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845921. Fig. 88
Etymology: Referring to the common name of the host genus it was isolated from, “acacia”.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, up to 2 μm wide. Sporulation moderate, phalacrogenous, rarely nematogenous. Conidiophores solitary or aggregated, erect, arising directly from aerial or substratal mycelium, unbranched, rarely branched at lower part, with 2–3 phialides per node, occasionally chondroid at base, up to 39 μm long, 2–4 μm wide at base, aseptate or with a single septum in basal part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate, hyaline, thick-, smooth-walled, 11–31 μm long, 1.5–3 μm wide at base, commonly with inconspicuous periclinal thickening and collarette at conidiogenous loci; polyphialides with two conidiogenous loci are occasionally present; adelophialides present, 7–22.3 × 1.2–2.7 μm. Conidia aseptate, cylindrical, bacilliform or allantoid, curved, rarely straight, with both ends rounded, occasionally with truncate bases and obtuse apices, hyaline, thin-, smooth-walled, 3.4–8.9 × 1.4–2.1 μm, eguttulate or with two large guttules at end, arranged in slimy heads. Chlamydospores not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 31 mm diam, flat, entire margin, dusty, buff at centre, creamy white at periphery, margin entire, reverse concolourous; On MEA reaching 26 mm diam, raised, rugose and cerebriform at centre, radially folded and membranous without aerial mycelium towards periphery, rosy buff, margin entire, reverse umber, with buff radial lines; On PDA reaching 29 mm diam, slightly rugose and radially folded at centre, flat towards periphery, thinly felty, creamy white, margin filiform, reverse concolourous, with buff radial lines.
Typus: South Africa, from leaf litter of Vachellia karroo (= Acacia karroo) (Fabaceae), unknown collection date and collector, isol. M.C. Papendorf, M.C.P. 1177 (holotype CBS H-24712, ex-type culture CBS 523.72).
Notes: Chlamydocillium acaciae is a distinct lineage phylogenetically allied to C. guttulatum (Fig. 3). Morphologically, C. acaciae can be differentiated from the latter in the production of shorter conidiophores and phialides [conidiophores: up to 39 μm vs up to 84 μm; phialides: 11–31 × 1.5–3 μm vs 10–61.5(–78.7) × 1.3–3 μm ]. In addition, adelophialides are present in C. acaciae, and were not observed in C. guttulatum.
Chlamydocillium antarcticum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845922.
Etymology: Named after the location where the fungus was collected, Antarctica.
Sterile in culture/in vitro. Chlamydocillium antarcticum differs from its closest phylogenetic neighbour C. cyanophilum by unique alleles in four loci based on alignments deposited in Figshare (doi: 10.6084/m9.figshare.22258765): ITS position 39(C), 40(A), 63(T), 67(G), 68(T), 69(gap), 74(gap), 76 (C), 81 (C), 89(G), 94(G), 95(T, insertion), 124(C), 129(C), 132(G), 142(G), 143(A), 153(T), 157(G), 158(C), 161(C), 165(C), 166-172(gap), 177(A), 191(A), 192(G), 207(gap), 308(T), 343(T), 383(gap), 431(G), 432(C), 441(T), 442(C), 462(C), 466(T), 471(C), 472(G), 474(T), 481(T), 483(A), 490(T), 495(T), 515(C), 532(A), 546(C), 555(gap), 556(T), 564(A), 573(A), 574(A, insertion); LSU position 635(T), 656(T), 672(C), 675(C), 692(C), 699(G), 734(C), 740(C), 941 (G), 945 (A), 948 (T), 957 (T), 958 (G), 970 (C), 971 (A), 983 (A), 986(gap), 987(C), 989(C), 1 001 (A), 1 005 (T), 1 041(C), 1 042(C), 1 043 (A), 1 044(C, insertion), 1 045(G, insertion), 1 079 (T), 1 086 (T), 1 100 (T), 1 101 (A), 1 102 (T, insertion), 1 202 (T), 1 217(C), 1 257 (C); rpb2 position 1 385 (A), 1 400 (T), 1 403 (T), 1 410 (C), 1 412 (T), 1 415 (T), 1 418 (T), 1 421 (G), 1 430 (G), 1 433 (T), 1 436 (A), 1 439 (C), 1 442 (G), 1 464 (C), 1 478 (C), 1 481 (C), 1 493 (T), 1 496 (G), 1 499 (T), 1 502 (C), 1 505 (G), 1 526 (C), 1 530 (C), 1 532 (T), 1 535 (T), 1 541 (G), 1 563 (A), 1 580 (G), 1 586 (G), 1 589 (A), 1 598 (T), 1 604 (T), 1 607 (T), 1 616 (T), 1 619 (T), 1 622 (G), 1 643 (C), 1 646 (T), 1 652 (A), 1 656 (G), 1 661 (G), 1 664 (A), 1 667 (T), 1682 (T), 1 685 (T), 1 688 (T), 1 691 (G), 1 694 (A), 1 700 (G), 1 703 (C), 1 709 (T), 1 718 (T), 1 739 (C), 1 742 (T), 1 745 (C), 1 751 (T), 1 758 (A), 1 760 (G), 1 763 (A), 1 772 (G), 1 775 (C), 1 781 (A), 1 787 (A), 1 791 (G), 1 796 (A), 1 799 (G), 1 808 (A), 1 823 (T), 1 826 (C), 1 837 (T), 1 838 (G), 1 841 (G), 1 850 (G), 1 862 (T), 1 864 (C), 1 867 (A), 1 871 (C), 1 873 (A), 1 874 (C), 1 876 (A), 1 877 (T), 1 882 (A), 1 883 (G), 1 884 (A), 1 886 (C), 1 890 (C), 1 891 (G), 1 892 (C), 1 896 (T), 1 903 (A), 1 904 (G), 1 905 (G), 1 913 (G), 1 914 (G), 1 915 (A), 1 916 (G), 1 919 (G), 1 922 (T), 1 928 (T), 1 937 (C), 1 946 (G), 1 949 (G), 1 952 (G), 1 977 (A), 1 980 (A), 1 989 (G), 2 001 (T), 2 002 (C), 2 008 (A), 2 016 (A), 2 019 (A), 2 021 (C), 2 022 (T), 2 023 (G), 2 024 (C), 2 031 (C), 2 037 (A), 2 041 (T), 2 042 (T), 2 043 (G), 2 049 (C), 2 055 (G), 2 067 (C), 2 073 (C), 2 078 (T), 2 079 (G), 2 082 (T), 2 088 (A), 2 097 (G), 2 100 (T), 2 103 (G), 2 106 (T), 2 121 (A), 2 131 (C), 2 139 (T); tef-1α position 2 186 (C), 2 332 (C), 2 353 (C), 2 354 (A), 2 386 (T), 2 402 (T), 2 403 (C), 2 437 (T), 2 458 (C), 2 485 (A), 2 486 (G), 2 529 (A), 2 549 (C), 2 585 (T), 2 592 (A), 2 593 (A), 2 594 (C), 2 595 (T), 2 596 (G), 2 597 (C), 2 636 (T), 2 642 (T), 2 660 (T), 2 663 (T), 2 703 (A), 2 753 (T), 2 792 (T), 2 816 (T), 2 819 (T), 2 828 (C), 2 837 (G), 2 849 (C), 2 852 (T), 2 855 (C), 2 861 (C), 2 894 (T), 2 909 (C), 2 927 (T), 2 933 (G), 2 945 (T), 2 969 (G), 2 978 (T), 2 987 (G), 3 044 (C), 3 050 (T), 3 053 (T), 3 062 (T).
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 26 mm diam, flat, membranous, white, margin entire, reverse concolourous; On MEA reaching 27–29 mm diam, flat, felty, moist, radially folded, rosy buff, margin fimbriate, reverse saffron, with pale umber radial lines; On PDA reaching 18 mm diam, flat, sparse aerial mycelium, rugose, membranous, creamy white, margin fimbriate, reverse buff.
Typus: Antarctica, Victoria Land, Dry Valleys, Don Juan Pond, unknown substrate, unknown collection date, L. Connell, No. 03-143 (holotype CBS H-24710, ex-type culture CBS 120502).
Notes: The culture used in this study forms a unique lineage in Chlamydocillium (Fig. 3) and is positioned on a very long branch (Fig. 3). No Chlamydocillium species is currently known from Antarctica. Unfortunately, this culture is sterile. The molecular differences are based on the sequence data provided.
Based on a blastn comparison against the alignment, C. antarcticum CBS 120502 shares 91 % (310/342, 8 gaps) similarity with “Kiflimonium curvulum” culture CBS 101442 (GenBank MH424701.1) based on ITS; 95 % (750/783, 5 gaps) similarity with “K. curvulum” culture CBS 384.70C (currently C. guttulatum) based on LSU; 77 % (592/765) similarity with “K. curvulum” culture CBS 400.52 (GenBank KM232425.1) based on rpb2, and 93 % (749/808) similarity with Parasarocladium wereldwijsianum culture NL19094011 (GenBank MW890112.1) based on tef-1α.
Chlamydocillium curvulum (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845923. Fig. 89.
Fig. 89.

Chlamydocillium curvulum (ex-type culture CBS 430.66). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads. E. Conidial heads. F–H, K–M. Conidiophores. I, J. Collarettes. N. Conidia. Scale bars = 10 μm.
Basionym: Acremonium curvulum W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 57. 1971.
Synonym: Kiflimonium curvulum (W. Gams) Summerb. et al., Microorganisms 6: 18. 2018.
Description: Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–2.3 μm wide. Sporulation abundant, phalacrogenous, nematogenous, and plectonematogenous. Conidiophores solitary or aggregated, erect, straight or waved, arising directly from aerial and substratal mycelium, or from ropes of hyphae, unbranched or basitonously branched, with 1–2 phialides per node, 40.5–119(–150) μm long, 1.1–4 μm wide at base, with 1–2(–4) septa at base or lower part, hyaline, smooth-walled, occasionally rough at lower part, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, rarely terminal, subulate, acicular, or subcylindrical, hyaline, thick-, smooth-walled, (25–)38–106.5 μm long, 1.2–3.6 μm wide at base, commonly with conspicuous periclinal thickening and inconspicuous collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, cylindrical, ellipsoid, or short reniform, straight or curved, with both ends rounded, slightly apiculate at the base, hyaline, thin-, smooth-walled, 3.2–8.2 × 2–2.6 μm, eguttulate, arranged in slimy heads. Chlamydospores not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 40–42 mm diam, flat, dusty, creamy white, margin entire, reverse buff; On MEA reaching 43–44 mm diam, flat, abundant aerial mycelium, felty, white to buff, margin entire, reverse pale orange; On PDA reaching 50–58 mm diam, flat, moderate aerial mycelium, felty, white, creamy white at periphery, margin entire, reverse concolourous; On SNA reaching 50–53 mm diam, flat, membranous without aerial mycelium, white, margin entire, reverse white. Pleasant smell, mushroom-like.
Typus: Germany, Kiel-Kitzeberg, from wheat field soil, unknown collection date, isol. 1964, coll. and isol. W. Gams, No. C 343, CBS H-6809 (holotype of Acremonium curvulum CBS 430.66 preserved as metabolically inactive culture, ex-type culture CBS 430.66 = DSM 1549).
Additional material examined: Canada, Ontario, Dolman, from nursery soil, unknown collection date and collector, isol. Jul. 1974 by O. Vaartaja, 9802B & No. 26, culture CBS 229.75.
Notes: The cultures labelled Acremonium curvulum examined in this study are phylogenetically heterogeneous and distributed in different clades along the tree (Fig. 3). Most of them, including the ex-type culture CBS 430.66, cluster in Chlamydocillium. However, they formed four clearly different branches that are distant from each other. Acremonium curvulum is therefore transferred to Chlamydocillium as C. curvulum, based on the phylogenetic analysis, while the others are described as new species and are discussed separately. Morphologically, characters of the ex-type culture are similar to the description available in the literature (Gams 1971), except for the production of slightly longer and thick-walled phialides [(25–)38–106.5 μm vs 25–60 μm, up to 100 μm] and larger conidia (3.2–8.2 × 2–2.6 μm vs 4.0–6.7 × 1.4–2.1 μm), which could result from different media used for cultures.
Acremonium curvulum has been isolated on rare occasions from arable and meadow soils. It differs from the similar A. recifei (currently Xenoacremonium recifei) by mostly lacking branches on the conidiophores, as well as by the lacking chlamydospores (Gams 1971).
Chlamydocillium cyanophilum Zare & W. Gams, Mycol. Progr. 15: 1020. 2016.
Description and illustration: Zare & Gams (2016).
Typus: Germany, Kiel-Kitzeberg, from wheat-field soil, unknown collection date, W. Gams, W.G. C1027 (holotype CBS H-22405, ex-type culture CBS 246.74A).
Additional materials examined: Canada, Manitoba Winnipeg, from soil, unknown collection date and collector, isol. J. Reid, No. UM 190, culture CBS 632.73. Germany, Berlin, from stem of Helianthus annuus (Asteraceae), unknown collection date and collector, isol. H.I. Nirenberg, culture CBS 599.93. Netherlands, from Beta vulgaris (Amaranthaceae), unknown collection date, isol. 20 Dec. 1978, coll. and isol. J.W. Veenbaas-Rijks, No. 2, culture CBS 102681 = IPO 1450 = V-KN 37-1; Utrecht Province, Maartensdijk, from dead leaf sheath of Musa sapientum (Musaceae) in greenhouse, unknown collection date, isol. Jul. 1973, coll. and isol. W. Gams, culture CBS 699.73 = IAM 14653; unknown locality, from Daucus carota (Apiaceae), unknown collection date, isol. 16 Nov. 1979, coll. and isol. J.W. Veenbaas-Rijks, culture CBS 102685 = IPO 1555.
Chlamydocillium guttulatum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845924. Fig. 90.
Fig. 90.

Chlamydocillium guttulatum (ex-type culture CBS 104.78). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads. E, F. Conidiophores and phialides with cylindrical collarette. G–J. Conidiophores. K. Conidia. Scale bars = 10 μm.
Etymology: Name derived from its guttulate conidia.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.3–2.6 μm wide. Sporulation moderate, phala-crogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight or occasionally zigzag and irregularly curved in lower part, arising directly from aerial or substratal mycelium, or from mycelial ropes, unbranched or basitonously branched, with 2–3 phialides per node, occasionally chondroid at base, up to 84 μm long, 1.4–3.3 μm wide at base, with a single septum in lower part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate, cylindrical, hyaline, thick-, smooth-walled, 10–61.5(–78.7) μm long, 1.3–3 μm wide at base, commonly with conspicuous periclinal thickening and a cylindrical collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, cylindrical, bacilliform or allantoid, curved, rarely straight, rounded at both ends, hyaline, thin-, smooth-walled, (3.3–)4–8.5(–10.3) × 1.3–2.1 μm, with two large guttules at ends, arranged in slimy heads. Chlamydospores not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 46 mm diam, flat, membranous with sparse aerial mycelium, creamy white, becoming rosy buff on older cultures, margin entire, reverse concolourous; On MEA reaching 45 mm diam, flat, slightly raised at centre, radially folded, felty, mycelial ropes present, pale rosy buff, margin entire, reverse pale ochreous, with buff radial lines; On PDA reaching 48 mm diam, flat, felty, creamy white at centre, olivaceous buff at periphery, margin entire, reverse primrose, with olivaceous buff pigment; On SNA reaching 42 mm diam, flat, membranous, white, margin filiform, reverse concolourous. Without odour on all media.
Typus: Germany, Göttingen, from culm base of Triticum aestivum (Poaceae), unknown collection date and collector, isol. 1977 by P. Reinecke (holotype CBS H-24643, ex-type culture CBS 104.78).
Additional material examined: Netherlands, from agricultural soil, unknown collection date and collector, isol. 5 May 1969 by J.W. Veenbaas-Rijks, CBS H-24711, culture CBS 384.70C.
Notes: Nine cultures examined in this study were labelled as Acremonium curvulum. All the cultures produce curved conidia that matched the description of A. curvulum. However, they are phylogenetically heterogeneous distributed in different clades among Sarocladiaceae (Fig. 3). Two of them (CBS 104.78 and CBS 384.70C) form an independent branch in Chlamydocillium, situated relatively distantly from the rest of the cultures, most notably the ex-type culture of A. curvulum (CBS 430.66) (Fig. 3). Cultures CBS 104.78 and CBS 384.70C are morphologically similar to the related species Chlamydocillium lolii, but differ by the production of phialides with conspicuous periclinal thickening and cylindrical collarette at the conidiogenous loci, while those of C. lolii are inconspicuous. In addition, colonies on MEA media of C. lolii are covered with radially arranged mycelial ropes, while those of the two C. guttulatum cultures are felty and radially folded. Morphologically, C. guttulatum differs from its closely related species C. acaciae in production of longer conidiophores (up to 84 μm long vs up to 39 μm long), phialides [10–61.5(–78.7) μm long vs 11–31 μm long], and in the lacking of polyphialides and adelophialides. Therefore, a new species, C. guttulatum, is introduced here to accommodate these cultures.
Chlamydocillium lolii L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845925. Fig. 91.
Fig. 91.

Chlamydocillium lolii (ex-type culture CBS 214.70). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads. E–G. Conidiophores. H. Conidia. Scale bars = 10 μm.
Etymology: Referring to the host genus Lolium, from which this species was isolated.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–2.6(–3.2) μm wide. Conidiophores solitary or aggregated, erect, straight, occasionally curved, arising directly from aerial or substratal mycelium, unbranched, branched at lower part or basitonously branched, bearing 1–2 phialides per node, up to 98.5 μm long, 1–3.5 μm wide at base, with 1–2(–5) septa at base or middle, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, acicular, subcylindrical, hyaline, thin-, smooth-walled, 37–70 μm long, 1.8–2.5 μm wide at base, commonly with inconspicuous periclinal thickening and collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, short cylindrical, bacilliform or allantoid, with obtuse apices and bases, mostly curved, hyaline, thin-, smooth-walled, with two large guttules at both ends, 3.5–11.9 × 1.5–2.9 μm, arranged in slimy heads, confluent in older culture. Chlamydospores not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 47–50 mm diam, flat, dusty, white at centre, creamy white at periphery, margin entire, reverse buff; On MEA reaching 47–48 mm diam, flat, densely and radially covered thick, mycelial ropes, felty, moist, creamy white at centre, buff at periphery, margin filiform, reverse apricot at centre, saffron at periphery, with apricot radial lines; On PDA reaching 46–48 mm diam, radially folded at centre, flat towards periphery, felty or dusty, white at centre, creamy white at periphery, with few shallow striations over the colony surface, margin filiform, reverse pale olivaceous buff, with olivaceous buff pigment, with buff radial lines. Without odour on all media.
Typus: Germany, Kr. Plön, Kasseteich, from rust-infected leaf of Lolium sp. (Poaceae), unknown collection date and collector, isol. Oct. 1966 by W. Gams, No. 642 (holotype CBS H-24644, ex-type culture CBS 214.70).
Notes: Similar to the cultures of Chlamydocillium guttulatum, C. lolii based on culture CBS 214.70, also originally labelled as Acremonium curvulum, forms a separate lineage (Fig. 3). Morphologically, C. lolii differs from C. acaciae in its longer conidiophores, phialides and conidia (conidiophores: up to 98.5 μm long vs up to 39 μm long; phialides: 37–70 μm vs 11–31 μm long; conidia: 3.5–11.9 × 1.5–2.9 μm vs 3.4–8.9 × 1.4–2.1 μm). In addition, C. lolii could be differentiated from C. acaciae by lack of adelophialides and polyphialides.
Chlamydocillium soli L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845927. Fig. 92.
Fig. 92.

Chlamydocillium soli (ex-type culture CBS 347.76). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Rope of hyphae and conidiophores. E–G. Conidiophores and conidial heads. H–J. Conidiophores. K. Chlamydospores. L. Conidia. Scale bars = 10 μm.
Etymology: Name derived from the substrate from which the holotype was collected, soil.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 2–2.8 μm wide. Sporulation moderate, phalacrogenous, nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight to flexuous, arising directly from aerial or substratal mycelium, or from ropes formed by mycelium, unbranched, poorly basitonously branched, with 1–2 phialides per node, up to 98 μm long, 1.5–3.3 μm wide at base, with a single septum in lower part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate, acicular, or subcylindrical, hyaline, thick-, smooth-walled, 42.5–87.5 μm long, 1.5–2.8 μm wide at base, commonly with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci; polyphialides not observed. Conidia aseptate, cylindrical, allantoid, oblong, with obtuse apices and bases, straight or slightly curved, hyaline, thin-, smooth-walled, 4–7.8(–10.3) × 1.6–2.4 μm, eguttulate, arranged in slimy heads. Chlamydospores lateral or intercalary, or surrounded in the mycelial ropes, mostly in single or unbranched chains, (sub-)globose, ovoid or limoniform, hyaline, smooth-, thick-walled, 4.8–10.9 × 4–7.3 μm.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 45–48 mm diam, flat, felty at centre, membranous at periphery, creamy white, margin entire, reverse pale olivaceous buff; On MEA reaching 44–46 mm diam, flat, membranous at centre, felty at periphery, creamy white, margin filiform, reverse pale ochreous, with buff radial lines; On PDA reaching 42–45 mm diam, flat, felty or dusty, dirty white, margin filiform, reverse pale olivaceous buff; On SNA reaching 30–35 mm diam, flat, membranous, dirty white, margin dendritic, reverse concolourous.
Typus: China, Taiwan, from soil under Saccharum officinarum (Poaceae), unknown collection date and collector, isol. T. Watanabe, X-67 (holotype CBS H-24645, ex-type culture CBS 347.76).
Notes: Chlamydocillium soli morphologically resembles C. curvulum in the shape and size of conidiophores and conidia. However, the globose or ellipsoid, catenulate chlamydospores with irregularly shaped guttules form a conspicuous feature unique to C. soli. Chlamydocillium soli is phylogenetically distinct and shares low sequence similarity with C. curvulum (88 % on ITS, 97 % on LSU).
Chlamydocillium terrestre L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845926. Fig. 93.
Fig. 93.

Chlamydocillium terrestre (ex-type culture CBS 110514). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores. F. Chlamydospores. G. Conidia. Scale bars = 10 μm.
Etymology: Referring to soil, the substrate from which this fungus was isolated.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, mycelial coils present, 1.3–2.6 μm wide. Sporulation spare, phalacrogenous nematogenous, plectonematogenous. Conidiophores solitary or aggregated, erect, straight to flexuous, arising directly from aerial or substratal mycelium, or from mycelial coils, unbranched, up to 67 μm long, 1.5–3.2 μm wide at base, with 1–2 septa at base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate or cylindrical, hyaline, thick-, smooth-walled, 25–60.5 μm long, 1.5–2.6 μm wide at base, commonly with inconspicuous periclinal thickening and collarette at conidiogenous loci. Conidia aseptate, cylindrical or bacilliform, with obtuse apices and bases, straight or slightly curved, hyaline, thin-, smooth-walled, with several minute guttules, 4.3–8.4 × 1.4–2.1 μm, arranged in slimy heads. Chlamydospores lateral or intercalary, mostly single or in chains, subglobose, ellipsoid, ampulliform or dolliform, hyaline, smooth-, thick-walled, 4.5–10.5 × 3.8–4.5 μm.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 32–35 mm diam, flat, thinly felty and buff at centre, membranous and creamy white at periphery, pale orange in older cultures, margin entire, reverse olivaceous buff; On MEA reaching 30–35 mm diam, raised at centre, flat at periphery, radially folded, rugose, with sparse aerial mycelium, hairy, thinly felty, rosy buff, margin fimbriate, reverse apricot, with buff radial lines; On PDA reaching 37–38 mm diam, flat, with sparse aerial mycelium, dusty, creamy white to buff, margin fimbriate, reverse pale olivaceous buff, with buff radial lines; On SNA reaching 23–25 mm diam, flat, membranous without aerial mycelium, white, margin filiform, reverse concolourous. Without odour on all media.
Typus: India, Maharashtra, Mumbai, Borivli, Sanjay Gandhi National Park, Kanheri, from soil in cave, Jul. 1997, H.B. Lee & C.-J. Kim (holotype CBS H-24646, ex-type culture CBS 110514).
Notes: Chlamydocillium terrestre is represented by a culture from soil collected in an Indian cave. According to phylogenetic inference from the ITS, LSU, rpb2, and tef-1α loci (Fig. 3), C. terrestre occupies a branch that is clearly different from other species in Chlamydocillium. Morphologically, C. terrestre differs from other species in having ellipsoid or ampulliform chlamydospores.
Clade V4
Polyphialocladium L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845932.
Etymology: Referring to the polyphialides produced by the type.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores solitary or aggregated, straight or curved, arising directly from aerial or substratal mycelium, unbranched or basitonously branched, commonly repeatedly sympodially proliferating, with conidiogenous cells short lateral, cylindrical asymmetrical projections, occasionally chondroid at base. Conidiogenous cells enteroblastic, mono- and polyphialidic, lateral, subulate or subcylindrical, hyaline, thick-, smooth-walled, commonly with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci, commonly with a percurrent, terminal or subterminal proliferation; adelophialides occasionally present. Conidia aseptate, ovoid or broadly fusoid, both ends acutely pointed, straight, hyaline, thin-, smooth-walled, eguttulate, arranged in chains. Chlamydospores and sexual morph not observed.
Type: Polyphialocladium fusisporum L.W. Hou, L. Cai & Crous
Notes: Polyphialocladium is established here to accommodate two cultures that were previously incorrectly treated as Acremonium alternatum. Polyphialocladium is morphologically distinguished from other genera in Sarocladiaceae and Acremonium by its basitonously branched, and repeatedly sympodially proliferating conidiophores. Phylogenetic inference shows that this genus forms a fully supported clade basal to the genus Chlamydocillium, and is clearly separate from other genera of Sarocladiaceae (Figs 1, 3).
Polyphialocladium fusisporum L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845933. Fig. 94.
Fig. 94.

Polyphialocladium fusisporum (ex-type culture CBS 406.66). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Aggregated conidiophores with long conidial chains. E, F. Unbranched conidiophores. G–I. Branched conidiophores with mono- and polyphialides, and adelophialides. J. Conidiophores with proliferated phialides. K. Conidia. Scale bars = 10 μm.
Etymology: Referring to the fusoid conidia.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–1.7 μm wide. Conidiophores mostly aggregated, erect, straight or curved, arising directly from aerial or substratal mycelium, unbranched or basitonously branched, commonly repeatedly sympodially proliferating, with conidiogenous cells as short, lateral, cylindrical asymmetrical projections, bearing 1–5 levels and with 2–3 phialides per node, forming sporodochia-like structure, 18.5–169 μm long, up to 254 μm long, 1.5–3.6 μm wide at base, with 1–4(–5) septa, hyaline, smooth-walled, occasionally chondroid, thick and rough-walled at base, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate or subcylindrical, hyaline, thick-, smooth-walled, (7.5–)10.5–48 μm long, 1–2.7 μm wide at base, commonly with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci, commonly with percurrent or subterminal proliferations; polyphialides with up to two conidiogenous loci; adelophialides present, 8–10 × 0.5–1 μm. Conidia aseptate, broadly fusoid or ovoid, straight, with pointed/acute base at both ends, hyaline, thin-, smooth-walled, 3.2–6.3 × 1.6–3.1 μm, eguttulate, arranged in long chains, forming sporodochia-like structure in old cultures. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 27–29 mm diam, flat, white and felty at centre, dirty white and membranous at periphery, margin entire, reverse pale luteous at centre, buff at periphery; On MEA reaching 27 mm diam, raised, radially folded, rugose, membranous and dirty white at centre, dusty and creamy white at periphery, margin crenate, reverse pale orange, with buff radial lines; On PDA reaching 28–30 mm diam, flat, dusty, dirty white at centre, olivaceous buff at periphery, margin filiform, reverse pale olivaceous buff, with buff radial lines; On SNA reaching 7–8 mm diam, flat, membranous, dirty white, margin entire, reverse colourless. Without odour on all media.
Typus: Germany, Kiel-Kitzeberg, from wall in greenhouse, Feb. 1966, W. Gams, No. C 603 (holotype CBS H-8092, ex-type culture CBS 406.66).
Additional material examined: Germany, Kitzeberg area, Schüttbrehm, from carpophore of Gymnopilus sp. (Hymenogastraceae), unknown collection date and collector, dep. C. Decock, culture CBS 114602 = MUCL 8431.
Notes: Polyphialocladium fusisporum is represented by two cultures, one from the wall of a greenhouse and the other from a carpophore of Gymnopilus sp. in Germany. Both were formerly identified as Acremonium alternatum. However, they fall in a single lineage, phylogenetically distant from the ex-type culture of A. alternatum in Bionectriaceae, but closely related to Chlamydocillium in Sarocladiaceae (Fig. 1, 3). Morphologically, Po. fusisporum is characterised by producing basitonously branched and repeatedly sympodially proliferating conidiophores with multiple septa, while conidiophores of A. alternatum are unbranched or poorly branched.
Clade W
Plectosphaerellaceae W. Gams, Summerb. & Zare, Nova Hedwigia 85: 476. 2007.
Classification: Glomerellales, Sordariomycetes.
Type genus: Plectosphaerella Kleb.
Clade W13
Parafuscohypha L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845939.
Etymology: Morphologically resembling the genus Fuscohypha, but phylogenetically distinct.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores mostly aggregated, erect, straight or slightly bent, arising directly from aerial or substratal mycelium, or from the mycelial ropes and coils, unbranched or basitonously or verticillately branched, bearing 1–3(–5) levels with 2–3 phialides per node, commonly repeatedly sympodially proliferating, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, occasionally forming the sporodochia-like structure, chondroid at base, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Conidiogenous cells enteroblastic, monophialidic or polyphialidic, terminal, lateral, subulate or subcylindrical, hyaline, thick-, smooth-walled, with conspicuous periclinal thickening and cylindrical or slightly flared collarette at conidiogenous loci, commonly with a percurrent, terminal or subterminal proliferation. Conidia aseptate, broad ovoid, ellipsoidal or tear-shaped, with pointed/acute base and obtuse apices, straight, hyaline, smooth-walled, arranged in dry conidial heads. Chlamydospores and sexual morph absent.
Type: Parafuscohypha proliferata L.W. Hou, L. Cai & Crous
Notes: The monotypic genus Parafuscohypha is proposed here to accommodate Pf. proliferata. This species is represented by a single lineage in a poorly resolved clade in Plectosphaerellaceae, and is closely related to the genus Fuscohypha (Fig. 4). Morphologically it is distinct. Parafuscohypha differs from Fuscohypha species by forming repeatedly basitonously, verticillately branched, and repeatedly sympodially proliferating conidiophores on which conidiogenous cells are seen as short lateral, cylindrical asymmetrical projections. Only unbranched or verticillate branched conidiophores are seen in Fuscohypha (Giraldo & Crous 2019).
Parafuscohypha proliferata L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845940. Fig. 95.
Fig. 95.

Parafuscohypha proliferata (ex-type culture CBS 308.74). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Proliferating conidiophores and polyphialides with conidial heads. F–I. Branched conidiophores with mono-, polyphialides and percurrently proliferating phialides. J. Conidia. Scale bars = 10 μm.
Etymology: Referring to the proliferating conidiophores.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1–3.4 μm wide, mycelial coils and ropes commonly present. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous. Conidiophores mostly aggregated, rarely solitary, (sub-)erect, straight or slightly bent, arising directly from aerial or substratal mycelium, or from the mycelial ropes and coils, unbranched, basitonously or verticillately branched at lower part, commonly repeatedly sympodially proliferating, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, bearing 1–3(–5) levels with 2–3 phialides per node, occasionally forming the sporodochia-like structure, 39–162.5 μm long, up to 185.5 μm long, 1.8–4 μm wide at base, with multiple septa, hyaline, smooth-walled, occasionally chondroid and rough-walled at base, pale brown metabolic occasionally present on the basal or middle part, cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate or subcylindrical, hyaline, thick-, smooth-walled, 15–65 μm long, 1.2–3.5 μm wide at base, commonly with conspicuous periclinal thickening and cylindrical or slightly flared collarette at conidiogenous loci, commonly with repeatedly percurrent proliferation; polyphialides with up to three conidiogenous loci. Conidia aseptate, broad ovoid, ellipsoidal or tear-shaped, straight, with distinct apiculate base and rounded apices, hyaline, thin-, smooth-walled, 2.5–4 × 1.6–2.5 μm, eguttulate, arranged in dry conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 48–50 mm diam, flat, margin entire, with sparse aerial mycelium, dusty, slightly granulose, dirty white, colourless ar periphery, reverse concolourous; On MEA reaching 50–55 mm diam, radially folded at centre, flat at periphery, with moderate aerial mycelium, felty, buff at centre, with a white and grey ring, vinaceous buff at periphery, margin filiform, reverse luteous, with buff radial lines; On PDA reaching 42–45 mm diam, flat, margin filiform, with sparse aerial mycelium, dusty, creamy white, reverse pale olivaceous buff; On SNA reaching 50–53 mm diam, flat, membranous without aerial mycelium, colourless, margin fimbriate, reverse colourless. Without odour on all media.
Typus: Netherlands, Friesland Province, Ameland, near Ballum, from dead twig of Acer pseudoplatanus (Aceraceae), unknown collection date, isol. Oct. 1973, coll. and isol. W. Gams (holotype CBS H-24702, ex-type culture CBS 308.74).
Notes: The culture CBS 308.74 was received at the CBS culture collection as Acremonium domschii. Its asexual morph is acremonium-like, with basitonously, verticillately branched or sympodially proliferating conidiophores, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, based on which could be clearly differentiated from the closely related species Fuscohypha expansa (Fig. 95). Based on phylogenetic analysis, culture CBS 308.74 is not a member of Acremonium s. str as circumscribed in the present study, but is closely related to Fuscohypha, distant from Allomusicillium domschii (syn. Acremonium domschii; Fig. 4).
Clade W14
Brunneomyces A. Giraldo et al., Mycol. Progr. 16: 357. 2017.
Mycelium consisting of branched, septate, hyaline, smooth- and thin-walled hyphae, often becoming dark brown, verrucose and thick-walled with age. Conidiophores erect, unbranched or poorly branched, often proliferating sympodially, showing conidiogenous cells as short lateral and cylindrical projections, forming sporodochia-like structure in some species, occasionally chondroid at base, septate. Conidiogenous cells enteroblastic, mono- and polyphialidic, hyaline, terminal, lateral or intercalary (adelophialides), subulate, lageniform or cylindrical, usually with short cylindrical collarettes, often subhyaline or pale brown, and with a distinct periclinal thickening at the conidiogenous locus; commonly with repeatedly percurrent or subterminal proliferation; polyphialides with up to five conidiogenous loci present in some species. Conidia arranged in chains, collapsing as conidial heads in some species, aseptate, obovoid, fusoid, cylindrical to ellipsoidal, with elongated and truncate hilum or narrowly extruding at the base, obtuse apices, or with symmetrically thick and truncate bases and apices in some species, hyaline or brown. Chlamydospores present in some species, lateral or intercalary, mostly in chains, ellipsoid, subglobose or doliiform, hyaline, smooth-, thick-walled. Sexual morph unknown (emended from Giraldo et al. 2017).
Type: Brunneomyces brunnescens (W. Gams) A. Giraldo et al.
Other accepted species with available sequences: Brunneomyces europaeus A. Giraldo, Gené & Guarro, Brunneomyces hominis A. Giraldo, Deanna A. Sutton & Gené, B. polyphialidus L.W. Hou, L. Cai & Crous, B. pseudozeylanicus (W. Gams) L.W. Hou, L. Cai & Crous
Brunneomyces polyphialidus L.W. Hou, L. Cai & Crous, sp. nov. MycoBank MB 845941. Fig. 96.
Fig. 96.

Brunneomyces polyphialidus (ex-type culture CBS 166.80). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D, E. Conidiophores with conidial chains and heads. F, G. Proliferating conidiophores. H, I. Polyphialides. J. Branched conidiophores. K. Conidiophores radiating out from coils formed by the mycelia. L. Conidia. Scale bars = 10 μm.
Etymology: Named after the polyphialides.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 0.9–2.2 μm wide. Conidiophores solitary or aggregated, erect, straight or curved, arising directly from aerial or substratal mycelium, radiating out from coils formed by the mycelia, unbranched or branched, bearing 1–5 levels and with 1–3 phialides per node, repeatedly proliferate sympodially, showing conidiogenous cells as short, lateral, cylindrical, asymmetrical projections, occasionally chondroid at base, (12–)15.5–76 μm long, up to 107.5 μm long, 1.7–3 μm wide at base, 1–2(–4)-septate, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate or subcylindrical, hyaline, thick-, smooth-walled, 9.3–51.5 μm long, 1.2–2.9 μm wide at base, commonly with conspicuous periclinal thickening and minute collarette at conidiogenous loci, commonly with repeatedly percurrent or subterminal proliferation; polyphialides with up to five conidiogenous loci present. Conidia aseptate, obovoid, cylindrical, with elongated and truncate hilum at basal ends and obtuse apices, straight, hyaline, thick-, smooth-walled, with two large guttules, 4.9–8 × 1.9–2.4 μm, arranged in long chains, collapse as conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 33–35 mm diam, flat, with sparse aerial mycelium, dusty, white, with dirty white zones, margin entire, reverse creamy white; On MEA reaching 20 mm diam, flat, radially folded, with moderate aerial mycelium, felty, white, margin fimbriate, reverse orange at centre, saffron at periphery, with buff radial lines; On PDA reaching 23–25 mm diam, flat, with sparse aerial mycelium, felty, rosy buff, with some olivaceous grey zones, margin crenate, reverse creamy white, with some pale olivaceous zones; On SNA reaching 22–24 mm diam, flat, with sparse aerial mycelium, dusty, white, margin lobate, reverse concolourous. Strong geosmin odour on OA, MEA, PDA media. Without any odour on SNA media.
Typus: Colombia, Chipaque, from leaf of Musa sp. (Musaceae), Dec. 1979, W. Gams, isol. H.A. van der Aa, No. 7280E (holotype CBS H-24703, ex-type culture CBS 166.80).
Notes: Although Brunneomyces polyphialidus is morphologically similar to the closely related B. hominis, it was isolated from a leaf of Musa sp., while B. hominis was isolated from a human host (Giraldo et al. 2017). Also, B. polyphialidus is distinguished from B. hominis by producing longer, more basitonously branched conidiophores (up to 107.5 μm vs up to 35 μm), and longer phialides [9.3–51.5 μm vs 12–20(–30) μm]. Conidia of B. polyphialidus are obovoid, also longer than those of B. hominis (4.9–8 × 1.9–2.4 μm vs 4–5(–6) × 2–2.5 μm), while that of B. hominis are ovoidal to ellipsoidal (Giraldo et al. 2017).
Brunneomyces pseudozeylanicus (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845942. Fig. 97.
Fig. 97.

Brunneomyces pseudozeylanicus (ex-type culture CBS 560.73). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D–G. Repeatedly branched conidiophores. H. Branched conidiophores with polyphialides. I, J. Percurrently and subterminally proliferated conidiophores. K. Conidia. Scale bars = 10 μm.
Basionym: Acremonium pseudozeylanicum W. Gams, Trans. Brit. Mycol. Soc. 64: 393. 1975.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.4–2.5 μm wide. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous, synnematogenous. Conidiophores mostly aggregated, (sub-)erect, arising directly from aerial or substratal mycelium, mostly repeatedly basitonously branched, bearing 1–5 levels and with 1–3(–4) phialides per node, commonly proliferating sympodially, showing conidiogenous cells as short, lateral, cylindrical asymmetrical projections, forming sporodochia-like structures, occasionally chondroid at base, 35.8–318 μm long, 1.6–4 μm wide at base, with 1–4 (–6) septa at basal, middle or apical part, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, subulate, cylindrical, hyaline, thick-, smooth-walled, (5.5–)11–33.5(–49.5) μm long, 1.5–2.6 μm wide at base, commonly with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci, commonly with repeatedly percurrent, subterminal proliferation; polyphialides present, with up to two conidiogenous loci. Conidia aseptate, fusoid, straight, with symmetrically thick and truncate bases and apices, hyaline, thick-, smooth-walled, 4.7–6.3 × 1.6–2 μm, eguttulate, arranged in long chains. Chlamydospores lateral or intercalary, mostly in chains, ellipsoid, subglobose or dolliform, hyaline, smooth-, thick-walled, 6–8 × 3.8–7.2 μm.Sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 17–19 mm diam, flat, with sparse aerial mycelium, thinly felty, moist, creamy white, buff at centre, margin entire, reverse buff; On MEA reaching 20–30 mm diam, flat, radially folded, with moderate aerial mycelium, felty, creamy white, margin entire, reverse pale orange, with buff radial lines; On PDA reaching 30 mm diam, flat, with sparse aerial mycelium, thinly felty, buff or pale salmon, margin filiform, reverse concolourous; On SNA reaching 15–18 mm diam, flat, with moderate aerial mycelium, felty, dirty white, margin entire, reverse creamy white.
Typus: Sri Lanka, Hikkaduwa, from decaying leaf of Cocos nucifera (Arecaceae), Jan. 1973, W. Gams (holotype of Acremonium pseudozeylanicum CBS 560.73 preserved as metabolically inactive culture, isotype IMI 185372, ex-type culture CBS 560.73 = ATCC 32184 = IMI 185372).
Notes: Brunneomyces pseudozeylanicus was originally described in the genus Acremonium, which is currently accommodated in Bionectriaceae (Fig. 1). It was transferred into Brunneomyces since it is not congeneric with Acremonium s. str. as defined by the type A. alternatum, but forms a well-supported clade basal to Brunneomyces in this study (Fig. 1 & 4). Morphologically, this species agrees with the characteristics of Brunneomyces by producing basitonously branched conidiophores and sympodially proliferating phialides. The morphological description of the ex-type culture (Fig. 97) differs slightly from the one in literature (Gams 1975) by having longer phialides [(5.5–)11–33.5(–49.5) μm vs 16–28 μm]. At the time the species was described (Gams 1975), phialides seen in culture were simple and unbranched, or formed part of once to twice basitonously branched conidiophores. Under similar conditions, we observed the conidiophores basitonously branched more than 5 times, and the sympodially proliferating phialides appearing as short lateral, cylindrical asymmetrical projections.
Clade W17
Allomusicillium L.W. Hou, L. Cai & Crous, gen. nov. MycoBank MB 845943.
Etymology: Allo = allos in Greek, different; name refers to its being morphologically similar to Musicillium, but phylogenetically different.
Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae. Conidiophores solitary or aggregated, erect or curved, arising directly from aerial or substratal mycelium, unbranched or basitonously branched, commonly repeatedly proliferating sympodially, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, at multiple levels and with one or more phialides per node, aseptate or septate, hyaline, smooth-walled, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate or subcylindrical, hyaline, thick-, smooth-walled, commonly with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci, commonly with repeatedly percurrent or subterminal proliferation; polyphialides with up to two conidiogenous loci. Conidia aseptate, ellipsoidal, short cylindrical, with slightly apiculate hilum at bases and obtuse apices, straight, hyaline, thin-, smooth-walled, eguttulate, arranged in long chains, collapsing as conidial heads. Chlamydospores and sexual morph absent.
Type: Allomusicillium domschii (W. Gams) L.W. Hou, L. Cai & Crous
Notes: Allomusicillium is phylogenetically basal to the clade containing Musicillium and Paramusicillium (Figs 1, 4), and has conidiophores that are morphologically distinct from those of the other genera. The conidiophores of Musicillium and Paramusicillium are generally unbranched or repeatedly verticillate towards the apex, bearing more than three phialides per node, and conidiogenous cells are monophialidic (Giraldo & Crous 2019). Allomusicillium is characterised by the basitonously branched conidiophores, commonly proliferating sympodially several times, and bearing fewer than three (1–2) phialides per node. Also, it commonly has mono- and polyphialides.
Allomusicillium domschii (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845944. Fig. 98.
Fig. 98.

Allomusicillium domschii (ex-type culture CBS 764.69). A–C. Colonies on MEA, PDA and SNA, respectively, after 14 d at 25 °C. D. Conidiophores on SNA. E–J, L. Conidiophores. K. Conidia. Scale bars = 10 μm.
Basionym: Acremonium domschii W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 124. 1971.
Description based on the ex-type culture CBS 764.69: Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.2–2.5 μm wide, mycelial ropes present. Sporulation abundant, phalacrogenous, nematogenous, plectonematogenous, occasionally synnematogenous. Conidiophores mostly aggregated, (sub-)erect, straight or slightly bent, arising directly from aerial or substratal mycelium, unbranched, mostly basitonously branched, commonly repeatedly proliferating sympodially, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, bearing 1–3 levels and with 1–2 phialides per node, forming sporodochia-like structures in old cultures, 37.5–150 μm long, up to 278 μm long, 1.2–3.5 μm wide at base, aseptate or with 1–2(–6) septa, hyaline, smooth-walled, in the lower parts mostly blackish and rough-walled in the older culture, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral or terminal, subulate or subcylindrical, hyaline, thick-, smooth-walled, (9.3–)12.5–57.5(–70) μm long, 1.1–2.5 μm wide at base, commonly with conspicuous periclinal thickening and cylindrical collarette at conidiogenous loci, commonly with repeatedly percurrent, terminal or subterminal proliferation; polyphialides with up to two conidiogenous loci. Conidia aseptate, ellipsoidal, straight, with slightly apiculate hilum at bases and obtuse apices, hyaline, thin-, smooth-walled, 3–4.5(–6.3) × 1.7–2.4 μm, eguttulate, arranged in long chains, collapsing as conidial heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 45 mm diam, flat, with sparse aerial mycelium, dusty, white, margin entire, reverse concolourous; On MEA reaching 32 mm diam, flat, radially fold, with abundant aerial mycelium, felty, dirty white, margin crenate, sparse crystal present, reverse orange, with few buff radial lines; On PDA reaching 48–50 mm diam, flat, with moderate aerial mycelium, thinly felty, creamy white, with buff and filiform margin, reverse creamy white with buff zones; On SNA reaching 42–43 mm diam, flat, with sparse aerial mycelium, dusty, colourless, margin dendritic, reverse colourless.
Typus: Germany, Kr. Eckernförde, Kaltenhofer Moor, from Inonotus obliquus (Hymenochaetaceae), collection date unknown, isol. Dec. 1965, coll. and isol. W. Gams, No. 1130a (holotype of Acremonium domschii CBS H-6607, isotype CBS H-6608, ex-type culture CBS 764.69).
Notes: The monotypic genus Allomusicillium is highly supported in the phylogenetic analysis (Fig. 4) and is represented by a mycophilic species, A. domschii, isolated from Inonotus obliquus (Hymenochaetaceae, Agaricomycetes). This species was originally names Acremonium domschii. Morphologically, Al. domschii can be distinguished from its most closely related species, Paramusicillium asperulatum, in its production of hyaline, basitonously branched conidiophores, which are commonly repeatedly proliferating sympodially, while those of Para. asperulatum are brown almost up to the first whorl, then are simple or repeatedly verticillate towards the apex (Giraldo & Crous 2019).
Clade X
Cephalothecaceae Höhn., Ann. Mycol. 15: 362. 1917.
Classification: Cephalothecales, Sordariomycetes.
Type genus: Cephalotheca Fuckel
Phialemonium W. Gams & McGinnis, Mycologia 75: 978. 1983.
Colonies spreading, flat, velvety, plane, or slightly floccose, white, yellow grey, ochraceous or dark olivaceous grey. Mycelium consisting of hyaline, smooth, branched, septate hyphae. Conidiophores absent or poorly differentiated, erect, unbranched, basitonously or verticillately branched, sympodially proliferating, with multiple septa. Phialides lateral and terminal, subulate, subcylindrical, ampulliform, awl-shaped, or flask-shaped, often inflated at base, with or without barely visible collarettes, with a percurrently sympodial proliferation in some species. Adelophialides often present, short cylindrical. Polyphialides with up to four conidiogenous loci present in species. Short stipe bearing 1–3 phialides in an irregular arrangement could be present in some species. Conidia hyaline or pigmented, aseptate, ellipsoidal, oblong, cylindrical, obovate, globose, citriform or limoniform, mostly smooth-walled or inconspicuously rugose, arranged in slimy heads or in long chains, with or without connectives or scar at both ends. Chlamydospores present or absent. Sexual morphs albertiniella-like or cephalotheca-like (emended from Perdomo et al. 2013, Crous et al. 2015b).
Type: Phialemonium obovatum W. Gams & McGinnis
Other accepted species with available sequences: Phialemonium atrogriseum (Panas.) Dania García, Perdomo, Gené, Cano & Guarro, Ph. thermophilum (W. Gams & J. Lacey) L.W. Hou, L. Cai & Crous
Phialemonium thermophilum (W. Gams & J. Lacey) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 845945. Fig. 99.
Fig. 99.

Phialemonium thermophilum (ex-type culture CBS 734.71). A–C. Colonies on OA, MEA and PDA, respectively, after 14 d at 25 °C. D. Conidiophores with conidial heads. E. Branched conidiophores. F, G. Unbranched conidiophores. H. Polyphialides. I, J. Conidiophores with percurrent, terminal proliferating phialides and polyphialides. K. Conidia. Scale bars = 10 μm.
Basionym: Acremonium thermophilum W. Gams & J. Lacey, Trans. Brit. Mycol. Soc. 59: 520. 1972.
Description based on the ex-type culture CBS 734.71: Mycelium consisting of branched, septate, hyaline, smooth-, thin-walled hyphae, 1.5–2.5 μm wide. Sporulation abundant, phalacrogenous. Conidiophores solitary or aggregated, erect, straight to flexuous, arising directly from aerial or substratal mycelium, poorly unbranched, mostly repeatedly basitonously, verticillately branched or repeatedly, sympodially proliferating, showing conidiogenous cells as short lateral, cylindrical asymmetrical projections, bearing 1–3 levels and with 1–2 phialides per node, up to 200 μm long, 1–3.3 μm wide at base, with multiple septa, hyaline, smooth-walled, basal cell rather thick-walled, irregularly encrusted and warty, with cell walls usually thicker than those of vegetative hyphae. Phialides lateral, awl-shaped, subulate or subcylindrical, hyaline, thick-, smooth-walled, 17–70(–86) μm long, 1–2.2 μm wide at base, commonly with inconspicuous periclinal thickening and conspicuous minute collarette at conidiogenous loci, commonly with repeatedly percurrent, terminal proliferation; polyphialides with up to four conidiogenous loci. Conidia aseptate, ellipsoidal, oblong or short cylindrical, with symmetrically rounded ends, straight, hyaline, thin-, smooth-walled, eguttulate, 2.4–4.9 × 1.2–2 μm, arranged in slimy heads. Chlamydospores and sexual morph not observed.
Culture characteristics after 14 d at 25 °C: Colonies on OA reaching 22–25 mm diam, flat, with sparse aerial mycelium, dusty, white, with creamy white and entire margin, reverse umber at centre, creamy white at periphery, slight aromatic odour; On MEA reaching 26–27 mm diam, flat, with moderate aerial mycelium, felty, vinaceous buff, with white and entire margin, reverse saffron, slight aromatic odour; On PDA reaching 29 mm diam, flat, with sparse aerial mycelium, dusty, pale brown vinaceous at centre, pale rosy buff to creamy white at periphery, margin entire, reverse pale rosy buff, strong aromatic odour; On SNA reaching 22 mm diam, flat, with sparse aerial mycelium, dusty, white, margin filiform, reverse white, odour yeast-like (revised from Gams & Lacey 1972).
Typus: UK, England, Rothamsted, from heated hay, unknown collection date and collector, isol. J. Lacey, CBS H-24631 (holotype CBS 734.71 preserved as metabolically inactive culture, ex-type culture CBS 734.71 = ATCC 24622).
Additional material examined: UK, from heated hay, unknown collection date and collector, isol. J. Lacey, No. C 918, culture CBS 733.71.
Notes: When Acremonium thermophilum was originally described (Gams & Lacey 1972), it was classified in Acremonium sect. Nectriodea based on its thick-walled conidiophores and basitonous ramification. The SSU and LSU sequences supported A. thermophilum as a distinct species from the ex-epitype culture (CBS 407.66) of A. alternatum in Bionectriaceae, but clustered in the Cephalothecaceae (Summerbell et al. 2011). The ex-type culture (CBS 734.71) examined in this study forms a single clade and is positioned on a very long branch in Phialemonium, which supports this species as unique (Fig. 1). It is hereby combined in the genus Phialemonium. Most morphological characters of the ex-type strain CBS 734.71 agree with the original description of A. thermophilum from literature. However, colonies observed on MEA media described in literature are pale ochraceous, fawn to light umber towards the centre, while colonies observed on MEA media are vinaceous buff, with white and entire margin. In addition, phialides observed in the present study are longer than those described in literature [17–70(–86) μm vs 18–38 μm], possibly resulting from the variation among different media used for morphological observations (Gams & Lacey 1972).
Clade Y
Coniochaetaceae Malloch & Cain, Canad. J. Bot. 49: 878. 1971.
Classification: Coniochaetales, Sordariomycetes.
Type genus: Coniochaeta (Sacc.) Cooke
Coniochaeta (Sacc.) Cooke, Grevillea 16: 16. 1887.
Type: Coniochaeta lignicola (Nannf.) Z.U. Khan, Gené & Guarro
Coniochaeta psammospora (W. Gams) L.W. Hou, L. Cai & Crous, comb. nov. MycoBank MB 848121.
Basionym: Acremonium psammosporum W. Gams, Cephalosporium-artige Schimmelpilze (Stuttgart): 61. 1971.
Description and illustration: Gams (1971).
Typus: France, Forêt d’Argonne, from decaying wood, unknown collector and collection date (holotype CBS H-9730, ex-type culture CBS 590.63 = LCP 879).
DISCUSSION
This manuscript provides a molecular phylogenetic overview and morphological data of species in Acremonium known from culture, aiming to facilitate their circumscription and to clarify the phylogenetic positions of the species currently accepted in Acremonium. This study represents the largest sampling of acremonium-like fungi that has ever been subjected to multi-locus DNA sequencing analyses, including most strains examined in Gams (1971) and Summerbell et al. (2011). Phylogenetic analyses based on combined multi-locus data of rpb2 and tef-1α with LSU and ITS confirmed the conclusions of Glenn et al. (1996) and Summerbell et al. (2011) that taxa with acremonium-like asexual morphs are highly polyphyletic, occurring in at least three orders of Sordariomycetes. The present study further reveals that the most acremonium-like fungi belong in Hypocreales, and that Acremonium s. str. is restricted to the Bionectriaceae (Zare et al. 2007, Perdomo et al. 2011, Summerbell et al. 2011, Giraldo et al. 2012). Beyond that, species with acremonium-like asexual morphs are shown to belong in Cephalothecaceae, Clavicipitaceae, Cordycipitaceae, Myrotheciomycetaceae, Nectriaceae, Niessliaceae, Plectosphaerellaceae and Sarocladiaceae, as well as five new families of Hypocreales, namely Chrysonectriaceae, Neoacremoniaceae, Nothoacremoniaceae, Pseudoniessliaceae and Valsonectriaceae.
Bionectriacaeae
Thirty-nine well-supported monophyletic genera were resolved in Bionectriaceae (Fig. 2), laying a foundation for additional genera and species to be recognised and described. Previously recognised genera were also well resolved, agreeing with Rossman et al. (2001) and Summerbell et al. (2011). Genera Bulbithecium, Emericellopsis, Fusariella, and Synnemellisia classified as incertae sedis in Hypocreales (Lumbsch & Huhndorf 2007, Hyde et al. 2020b), are shown to belong in the Bionectriaceae.
Bionectriaceae has diverse asexual morphologies of acremonium-like, clonostachys-like, verticillium-like and sesquicillium-like (Rossman et al. 1999, Summerbell et al. 2011). According to the phylogenetic analyses in the present study, more than half of the Acremonium species recognised by Gams et al. (1971) could be accommodated in Bionectriaceae. The phylogenetic relationship of Acremonium representing sections Simplex, Gliomastix and Nectrioidea was assessed and the results reveals that, apart from the core species belonging to Acremonium s. str. and other existing genera e.g. Emericellopsis, Gliomastix or Lasionectria, 10 additional genera could be delineated in Bionectriaceae. Among them, three monophyletic genera, Gossypinidium, Monohydropisphaera, and Musanaesporium, are represented by a single lineage. This is consistent with the results of previous studies, showing “Acremonium” to be a relatively plesiomorphic assemblage of asexual morphs (Summerbell et al. 2011).
Among the 39 genera accepted in the present study, sexual morphs are known for 14 of them. Most genera have nectria-like sexual morphs with perithecial ascomata, and acremonium- or cylindrocarpon-like asexual morphs, including Geonectria, Lasionectria, Lasionectriella, Lasionectriopsis, Nectriopsis, Ochronectria, Paracylindrocarpon, Protocreopsis, Stilbocrea and Verrucostoma, many of which were previously treated as members of Nectria (Doi 1977, Samuels 1978, Rossman et al. 1999, Hirooka et al. 2010, Lechat & Fournier 2016b, Crous et al. 2016b, Tibpromma et al. 2018, Lechat et al. 2018b, 2019). Other genera were found to have cleistothecial ascomata with globose ascospores, such as Bulbithecium, Emericellopsis, Hapsidospora and Roumegueriella (van Beyma 1940, Malloch & Cain 1970, Udagawa & Muroi 1990, Udagawa et al. 1994, Rossman et al. 2001). The one exception is Hydropisphaera s. str. circumscribed in the present study. Hydropisphaera comprises four nectria-like species, H. cyatheae, H. fungicola, H. peziza and H. suffulta, plus one cleistothecial genus Heleococcum typified by He. aurantiacum. Heleococcum aurantiacum is characterised by producing cleistothecial ascomata lacking a nectrioid centrum, with deliquescent asci and cylindrical, 1-septate ascospores (Tubaki 1967, Rossman et al. 1999). It is allied with the type of Hydropisphaera, H. peziza, based on molecular data (Fig. 2), which also agrees with the results of Rossman et al. (2001) and Summerbell et al. (2011). Although species in the genera of Bionectriaceae are mostly known from their sexual morphs, asexual taxa can also be accommodated in these genera: Acremonium s. str., Fusariella, Gliomastix, Ovicillium, Pseudoacremonium, Septofusidium and Stanjemonium, as well as the ten newly described genera only known from their asexual morphs. Their whose sexual morphs, if any, remain to be discovered.
The genus Emericellopsis includes acremonium-like fungi in Hypocreales (Gonçalves et al. 2020). In the present study, Emericellopsis typified by E. terricola comprised 21 species, including the type of Stilbella, S. fimetaria, and five species that were initially described as Acremonium, namely A. exuviarum, A. fuci, A. gamsianum, A. potronii and A. tubakii (Fig. 2). This finding agrees with the conclusions of previous studies (Grum-Grzhimaylo et al. 2013, Gonçalves et al. 2020). Species of Stilbella and similar asexual genera have been known to be associated with four distinct orders, namely Claviciptales, Eurotiales, Hypocreales and Helotiales (Seifert 1985). Most Stilbella species have nectria-like sexual morphs, which are KOH negative. Only one species, Stilbella emericellopsis, produces an emericellopsis-like sexual morph with cleistothecial ascomata, which was introduced as asexual morph of E. synnematicola (Seifert 1985), and their connection had been proven by single ascospore and conidium isolations (Mathur & Thirumalachar 1960, Seifert 1985). In the present study, the ex-neotype culture of the lectotype of Stilbella, S. erythrocephalum (now regarded as S. fimetaria; CBS 558.84) was found to fall within the Emericellopsis clade (Fig. 2), closely related to ex-type culture of E. synnematicola (CBS 176.60). The two genera are clearly synonyms, but the choice of generic name has been the topic of debate. Although Stilbella (1900) represents an earlier name than Emericellopsis (1940), and is much better known to field biologists and amateurs, most species cluster elsewhere and will have to be renamed, and only two taxa belong to Emericellopsis including the type of Stilbella (S. emericellopsis and S. fimetaria). To avoid numerous name changes, we thus recommend using Emericellopsis as the generic name for this clade, with the genus Stilbella reduced to synonymy with Emericellopsis.
The monotypic genera Bullanockia (Crous et al. 2016a), Stromatonectria (Jaklitsch & Voglmayr 2011) and Xanthonectria (Lechat et al. 2016a) were originally erected based on phylogenies established with single LSU sequences or combined ITS and LSU sequences, which placed the three genera in the Bionectriaceae (Hypocreales). According to our phylogenetic analysis based on additional loci and cultures, these three genera clustered within two different clades representing the two recently established families in the Hypocreales: Bullanockia and Xanthonectria fall within the Xanthonectriaceae, and Stromatonectriaceae was established based on Stromatonectria (Perera et al. 2023) (Fig. 1).
The taxonomic placement of the type of Xanthonectria, X. pseudopeziza (basionym: Sphaeria pseudopeziza) has long been uncertain due to the lack of clear affinities with other genera. Morphologically, X. pseudopeziza resembles some species of Hydropisphaera, Ijuhya, Pronectria and Protocreopsis, within the Bionectriaceae, in having multi-septate ascospores. Xanthonectria pseudopeziza produces (3–)5–7(–9)-septate ascospores, clearly unlike the members of Bionectriaceae, which commonly produce ascospores that are aseptate or 1–3(–4)-septate (Rossman et al. 1999). Recently, the family, Xanthonectriaceae, was established to accommodate this genus (Perera et al. 2023). Although Bullanockia was described based on the acremonium-like asexual morph, it is phylogenetically distant from the clade of Bionectriaceae (Fig. 1). Morphological characters of sexual morph of Bullanockia await to be discovered. With regard to Stromatonectria, this genus was originally placed in the Bionectriaceae based on the characteristic KOH negative ascomata and stromata as well as molecular data (Jaklitsch & Voglmayr 2011). It differs in the production of pycnidial asexual morphs e.g. closed, compound conidiomata in vivo, which have never been reported in Bionectriaceae. The family, Stromatonectriaceae, was established to accommodate this genus (Perera et al. 2023). In summary, both the phylogenetic analyses and morphological characters suggest Bullanockia, Stromatonectria and Xanthonectria should be excluded from Bionectriaceae, which confirmed the result of Perera et al. (2023). The genus Peethambara has been transferred to Stachybotryaceae (Crous et al. 2014), while the genus Paranectria was moved to Nectriaceae in this study based on its only available ITS sequence (Paranectria affinis K(M):253675, MZ159749.1) which is close to Paracremonium spp. (Supplementary Fig. S1).
Several genera that had little or no DNA sequence data available prior to this study, including Fusariella, Geosmithia, Mycocitrus, Nectriella, Nectriopsis, Septofusidium, Selinia, Trichonectria and Valsonectria. Trichonectria and Septofusidium remain polyphyletic according to our phylogenetic analyses. Other genera with more DNA sequence data, such as Bionectria, Clonostachys and Ijuhya, remain para- and polyphyletic. In the present study, Hydropisphaera is restricted to species in the Bionectriaceae consisting of six species for which molecular data were available.
Hydropisphaera cirsii, H. pseudoarenula and H. saulensis were recently described by Lechat & Fournier (2020) based on morphological characters and LSU sequence data. However, phylogenetic analysis in our study revealed these species to be distant from Hydropisphaera s. str. Hydropisphaera cirsii and H. pseudoarenula formed a separate clade close to Fusariella, and H. saulensis clustered with Synnemellisia (Supplementary Fig. S2). This result further suggested Hydropisphaera s. lat. is polyphyletic, and species previously identified as Hydropisphaera await to be re-disposed.
Although the generic placement could not be resolved for all bionectriaceous genera in this study, the newly introduced acremonium-like genera and their related sexual morphs represent an important step in resolving the taxonomy and phylogeny of the family.
Niessliaceae
Niessliaceae was originally introduced by Kirschstein (1939) for a group of ascomycetes with minute, superficial, reticulate ascomata. A total of 12 genera were previously included in the family (Gams et al. 2019). Species of its type genus, Niesslia, based on N. chaetomium, were linked to the hyphomycetous asexual genus Monocillium, which shares characters with Acremonium, such as aculeate phialides and hyaline conidia arranged in heads. Gams (1971) distinguished Monocillium from Acremonium based on morphological characters. He particularly stressed that Monocillium had thick-walled phialides and lacked branched conidiophores. In the present study, ex-type cultures of four Acremonium s. lat. species (A. cavaraeanum, A. guillematii, A. incrustatum and A. nigrosclerotium), one Cephalosporium species (C. ballagii) clustered within Niessliaceae (Fig. 1). However, because of the unresolved phylogeny of Niessliaceae and lack of molecular sequence data for N. chaetomium, the taxonomy of these taxa has not been resolved.
Chrysonectriaceae, Neoacremoniaceae, Nothoacremoniaceae, Pseudoniessliaceae and Valsonectriaceae
Although most species of Acremonium s. lat. are accommodated in Bionectriaceae, several species previously identified as “Acremonium” have proved to be phylogenetically scattered in Cephalothecales, Coniochaetales, Glomerellales and Hypocreales (Fig. 1). Five new families, Chrysonectriaceae, Neoacremoniaceae (including Neoacremonium gen. nov.), Nothoacremoniaceae (including Nothoacremonium gen. nov.), Pseudoniessliaceae (including Pseudoniesslia gen. nov.) and Valsonectriaceae are introduced here in the order Hypocreales for species previously treated as members of the genus Acremonium.
Chrysonectria was originally introduced in the family Nectriaceae (Lechat et al. 2018a). In this study, Chrysonectria crystallifera formed a separate lineage together with C. finisterensis in Hypocreales, representing the new family Chrysonectriaceae (Fig. 1). It is unique based on molecular phylogeny as well as morphology.
Nothoacremoniaceae is based on Nothoacremonium, comprising the tropical mycoparasite species “Acremonium” exiguum (Fig. 1; Gams 1975), and five other cultures, which are known to be saprobic or parasitic on human or plants, were described as No. subcylindricum and No. vesiculophorum in this present study (Fig. 2).
Neoacremoniaceae includes five species that occur on diverse plant hosts, including Musaceae and Poaceae. Two species (N. flavum and N. minutisporum) produce a strong geosmin odour or yellow pigment in culture, which suggests why these taxa are interesting for their secondary metabolites. An interesting result generated is that CBS 538.93, the ex-type culture of Parapyrenis maritima, originally described as belonging to Xylariales (Aptroot 1995), actually clustered close to Neoacremonium minutisporum (synonym: Acremonium minutisporum) (Fig. 1). However, Pa. maritima was only described based on a sexual morph, making it impossible to reconcile morphologically with an acremonium-like species. Therefore, whether Pa. maritima truly produces an acremonium-like asexual morph or whether the culture was contaminated by an acremonium-like species at some point before or after it was deposited, remains to be determined.
The single species in the family Pseudoniessliaceae, Pseudoniesslia minutispora, was originally described as a member of Niesslia. Although the preliminary phylogenetic analysis of this species was not shown in the protologue, the phylogenetic analysis in the present study agrees with the original comments in the protologue of Niesslia minutispora (Gams et al. 2019), placing this species in a distinct lineage with hypocrealean affinities (Fig. 1). It can be differentiated from Niesslia by the production of sporodochial conidiomata and by its conidiophores with terminal whorls of relatively long phialides (Gams et al. 2019).
Valsonectriaceae contains two subclades and includes 16 cultures that were previously identified as two Acremonium species, A. roseolum and A. inflatum. They share key morphological characters: sympodially proliferating conidiophores and phialides with a bulbous lower region. Most species in the monophyletic genus Valsonectria produce fusoid, broadly fusoid or ovoid conidia with hila at both ends, and conidia arranged in long chains. The only exception is V. inflata, conidia of which are (sub-)globose, green to grey-green and arranged in slimy heads, suggesting the two subclades possibly represents two different genera. Currently, a total of eight species with available sequences are included in the family Valsonectriaceae. They are known to be saprobic on dead plant stems or to be isolated from soil in diverse environments from different continents, including Europe, North America, and Asia.
DNA barcodes
Morphological characteristics of Acremonium s. lat. specifically are reduced and relatively conserved. Features used for species delimitation in previous studies, such as shape and dimensions of conidiogenous cells and conidia, do not provide robust distinctions. The complication is further confirmed because acremonium-like species clustering in different phylogenetic clades share many overlapping features. This complicated situation is similar to what has been observed in other asexual morphs e.g. Fusarium, Phoma and Septoria, suggesting that because of the little-differentiated morphology, the clear-cut distinction of this group of fungi is impossible without the support from DNA barcodes and phylogenetic analyses. Researchers and diagnosticians therefore must rely on DNA sequence data to support morphological and ecological conclusions (Verkley et al. 2013, Chen et al. 2015, Crous et al. 2021b).
In recent years, the single ITS and LSU loci, or combined ITS-LSU, and SSU-LSU have been used in studies of acremonium-like species and related sexual genera. Although the ITS and LSU sequences have proven useful in species delimitation and the use of two DNA barcode loci has been applied in several taxonomic groups (Vu et al. 2019), use of a single locus or even combinations of loci have failed to provide sufficiently clear genera and species boundaries for acremonium-like species, especially species in Acremonium s. lat. These species were found to be highly polyphyletic among diverse families of Hypocreales, and were not well-supported statistically (Summerbell et al. 2011). In this study we carried out a more comprehensive analysis of the acremonium-like fungi by employing additional loci. Results of this study show that sequences of rpb2 and tef-1α could delineate the majority of acremonium-like species, suggesting these two loci could serve as reliable DNA barcodes for species delimitation in acremonium-like species in Hypocreales. The single tef-1α locus can be used to recognise more than 90 % of the species in Bionectriaceae; while the rpb2 can be used to recognise more than 94 % of the species. This indicates that the rpb2 region provides the best signal for species recognition in these fungi, which agrees with the result of Lombard et al. (2015) who proposed rpb2 as barcode for Nectriaceae.
Acknowledgments
LH acknowledges funding from the China Scholarship Council (CSC; 201904910749) for supporting her visit to the Westerdijk Institute (WI). This study was partially supported by Chinese Academy of Sciences (CAS: KFJ-BRP-009 and CAS-NSTDA: NO. 153211KYSB20200039). We are thankful to M. Starink-Willemse, M. Hernández-Restrepo, M. Verweij and M. Sandoval-Denis for providing technical assistance to LH during her visit to WI, and to A. van Iperen, Y. Vlug and T. Merkx for depositing the isolates and specimens in the culture collection and fungarium. LH extends thanks to F. Liu, Q. Chen, P. Zhao, J. Liang and all other members in Cai’s laboratory for providing care and help during her Ph.D. study. TR acknowledges funding from the Tromsø Research Foundation (ID: 18_CANS_AS). PWC is grateful to the European Union’s Horizon 2020 research and innovation program (RISE) under the Marie Skłodowska-Curie grant agreement No. 101008129, project acronym ‘Mycobiomics’, and the Dutch NWO Roadmap grant agreement No. 2020/ENW/00901156, project ‘Netherlands Infrastructure for Ecosystem and Biodiversity Analysis – Authoritative and Rapid Identification System for Essential biodiversity information’ (acronym NIEBA-ARISE) for funding. We are very grateful to the reviewers whose suggestions helped improve this manuscript.
DECLARATION ON CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
Supplementary Material: https://studiesinmycology.org/
Phylogenetic tree inferred from a Maximum Likelihood analysis based on a concatenated alignment of ITS and LSU sequences of 401 strains representing Hypocreales and related orders (Cephalothecales, Coniochaetales, Glomerellales). The RAxML bootstrap support values (MLBS) above 50 % are given at the nodes. The scale bar represents the expected number of changes per site. Strains with special status are indicated with a letter after the accession number (T: ex-type). The tree is rooted to Saccharata proteae CBS 115206.
Phylogenetic tree inferred from a Maximum Likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tef-1α sequences of 468 strains representing Bionectriaceae and outgroups. The RAxML bootstrap support values (MLBS) above 50 % are given at the nodes. The scale bar represents the expected number of changes per site. Strains with special status are indicated with a letter after the accession number (T: ex-type). The tree is rooted to Paracremonium inflatum CBS 485.77, Sarocladium oryzae CBS 180.74, S. zeae CBS 800.69, and Xenoacremonium recifei CBS 137.35.
Strains used in this study with details of their host, location, and GenBank accessions numbers
REFERENCES
- Abdel-Hafez SI, el-Kady IA, Mazen MB, et al. (1987). Mycoflora and trichothecene toxins of paddy grains from Egypt. Mycopathologia 100: 103–112. [DOI] [PubMed] [Google Scholar]
- Alfaro-García A, Armengol J, Bruton BD, et al. (1996). The taxonomic position of the causal agent of Acremonium collapse of muskmelon. Mycologia 88: 804–808. [Google Scholar]
- Aptroot A. (1995). Redisposition of some species excluded from Didymosphaeria (Ascomycotina). Nova Hedwigia 60: 325–379. [Google Scholar]
- Ashrafi S, Helaly S, Schroers HJ, et al. (2017). Ijuhya vitellina sp. nov., a novel source for chaetoglobosin A, is a destructive parasite of the cereal cyst nematode Heterodera filipjevi. PLoS ONE 12: e0180032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer S, Ludwig-Müller J. (2014). Effects of the endophyte Acremonium alternatum on oilseed rape (Brassica napus) development and clubroot progression. Albanian Journal of Agricultural Sciences 13: 15–20. [Google Scholar]
- Backus MP, Orpurt PA. (1961). A new Emericellopsis from Wisconsin, with notes on other species. Mycologia 53: 64–83. [Google Scholar]
- Beljakova LA. (1974). Genus Emericellopsis van Beyma (Eurotiaceae). Mikologiya i Fitopatologiya 8: 385–396. [Google Scholar]
- Berkeley MJ. (1860). Outlines of British Fungology. Lovell Reeve, London. [Google Scholar]
- Berkeley MJ, Broome CE. (1850). Notices of British fungi (438–501). Annals and Magazine of Natural History 5: 455–466. [Google Scholar]
- Berkeley MJ, Broome CE. (1852). Notices of British Fungi (615–639). Annals and Magazine of Natural History 9: 317–329. [Google Scholar]
- Bobeck DR, Pearce CJ. (2017). Agricultural microbial inoculant compositions and uses thereof. United States patent application US 15/702, 417. Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- Booth C. (1959). Studies of Pyrenomycetes: IV Nectria (Part I). Mycological Papers 73: 1–115. [Google Scholar]
- Burton HS, Abraham EP. (1951). Isolation of antibiotics from a species of Cephalosporium. Cephalosporins P1, P2, P3, P4 and P5. Biochemical Journal 50: 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda RF. (1987). Fungi Cubenses II. Instituto de Investigaciones Fundamentales en Agricultura Tropical, Alejandro de Humboldt, Ciudad de La Habana, Cuba, ACC Publishing. [Google Scholar]
- Chabelska-Frydman C. (1964). A new species of Fusariella from Israel. Canadian Journal of Botany 42: 1485–1488. [Google Scholar]
- Chen Q, Jiang JR, Zhang GZ, et al. (2015). Resolving the Phoma enigma. Studies in Mycology 82: 137–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements FE, Shear CL. (1931). The genera of fungi, 2nd edition. H. W. Wilson Company Publishing, New York, USA. [Google Scholar]
- Cooke MC. (1884). Synopsis Pyrenomycetum. Grevillea 12: 102–113. [Google Scholar]
- Crous PW, Braun U, Hunter GC, et al. (2013). Phylogenetic lineages in Pseudocercospora. Studies in Mycology 75: 37–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Cowan DA, Maggs-Kölling G, et al. (2020). Fungal Planet description sheets: 1112–1181. Persoonia 45: 251–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Hernández-Restrepo M, Schumacher RK, et al. (2021a). New and Interesting Fungi. 4. Fungal Systematics and Evolution 7: 255–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Lombard L, Sandoval-Denis M, et al. (2021b). Fusarium: more than a node or a foot-shaped basal cell. Studies in Mycology 98: 1–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Luangsa-ard JJ, Wingfield MJ, et al. (2018a). Fungal Planet description sheets: 785–867. Persoonia 41: 238–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Schumacher RK, Wingfield MJ, et al. (2015a). Fungal Systematics and Evolution: FUSE 1. Sydowia 67: 81–118. [Google Scholar]
- Crous PW, Shivas RG, Quaedvlieg W, et al. (2014). Fungal Planet description sheets: 214–280. Persoonia 32: 184–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. (2016a). Fungal Planet description sheets: 469–557. Persoonia 37: 218–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Guarro J. (2015b). Fungal Planet description sheets: 320–370. Persoonia 34: 167–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Richardson DM, et al. (2016b). Fungal Planet description sheets: 400–468. Persoonia 36: 316–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Wingfield MJ, Burgess TI, et al. (2018b). Fungal Planet description sheets: 716–784. Persoonia 40: 240–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous PW, Verkley GJM, Groenewald JZ, et al. (eds) (2019). Fungal Biodiversity, 2nd ed. Westerdijk Fungal Biodiversity Institute, Utrecht, Netherlands: [Westerdijk Laboratory Manual Series 1]. [Google Scholar]
- Darriba D, Taboada GL, Doallo R, et al. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Saha R, Dar SA, et al. (2010). Acremonium species: a review of the etiological agents of emerging hyalohyphomycosis. Mycopathologia 170: 361–375. [DOI] [PubMed] [Google Scholar]
- Davidson DE, Christensen M. (1971). Emericellopsis stolkiae sp. nov. from saline soils in Wyoming. Transactions of the British Mycological Society 57: 385–391. [Google Scholar]
- De Hoog GS, Guarro J, Gené J, et al. (2020). Atlas of Clinical Fungi, 3rd e-edition. Utrecht/Reus. [Google Scholar]
- De Hoog GS, Van den Ende AHGG. (1998). Molecular diagnostics of clinical strains of filamentous Basidiomycetes. Mycoses 41: 183–189. [DOI] [PubMed] [Google Scholar]
- Desmazières JBHJ. (1846). Treizième notice sur quelques plantes cryptogames récemment découvertes en France, et qui vont paraître en nature dans la collection publiée par l’auteur. Annales des Sciences Naturelles, ser. III. Botanique 6: 62–84. [Google Scholar]
- Dickinson CH. (1968). Gliomastix Guéguen. Mycological Papers 115: 1–24. [Google Scholar]
- Döbbeler P, Davison PG. (2017). Frullania as a hotspot for hypocrealean ascomycetes: ten new species from Southeastern North America. Nova Hedwigia 106: 209–256. [Google Scholar]
- Doi Y. (1967). A revision of the Hypocreales with cultural observations. II. On Mycocitrus phyllostachydis (Syd.) Doi, a perfect state of Cephalosporium. Bulletin of the National Science Museum Tokyo 10: 31–36. [Google Scholar]
- Doi Y. (1968). Revision of the Hypocreales with cultural observations. I. The genus Pelonectriella, with a note on bambusicolous Hypocreales with large persistent stroma. Bulletin of the National Science Museum Tokyo 11: 179–184. [Google Scholar]
- Doi Y. (1977). Protocreopsis, a new genus of the Hypocreales. Kew Bulletin 31: 511–555. [Google Scholar]
- Domsch KH, Gams W, Anderson TH. (2007). Compendium of soil fungi, 2nd edition. IHW Verlag Publishing, Eching, Germany. [Google Scholar]
- Dumortier BCJ. (1822). Commentationes Botanicae. C. Casterman-Dieu, Tournay, Belgium. [Google Scholar]
- Ellis MB. (1971). Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew Publishing, Surrey, England. [Google Scholar]
- Ellis MB. (1976). More Dematiaceous Hyphomycetes. Commonwealth Mycological Institute, Kew Publishing, Surrey, England. [Google Scholar]
- Engler A, Prantl K. (1900). Die natürlichen Pflanzenfamilien nebst ihren Gattungen und wichtigeren Arten insbesondere den Nutzpflanzen: I. Tl., 1. Abt.: Fungi (Eumycetes): 1–570. [Google Scholar]
- Fletcher KIG, Sim J, Williams N, et al. (2017). Novel lineage of a green alga and Acremonium stroudii (Ascomycota) sp. nov. reported from Ascension Island. Journal of the Marine Biological Association of the United Kingdom 97: 669–679. [Google Scholar]
- Fujikawa H, Wauke T, Kusunoki J, et al. (1997). Contamination of microbial foreign bodies in bottled mineral water in Tokyo, Japan. Journal of Applied Microbiology 82: 287–291. [DOI] [PubMed] [Google Scholar]
- Fernández-Trujillo JP, Martínez JA, Salmerón MC, et al. (1997). Isolation of Acremonium species causing postharvest decay of peaches in Spain. Plant Disease 81: 958–958. [DOI] [PubMed] [Google Scholar]
- Fries EM. (1822). Systema Mycologicum 2: 1–274. Sweden, Lund. [Google Scholar]
- Fries EM. (1823). Systema Mycologicum, sistens fungorum ordines, genera et species hucusque cognitas, II, pars II: 276–620. [Google Scholar]
- Fuckel L. (1866). Fungi Rhenani Exsiccati, Supplementi. Fasc. 2: 1601–1700. [Google Scholar]
- Gams W. (1968). Typisierung der Gattung Acremonium. Nova Hedwigia 16: 141–145. [Google Scholar]
- Gams W. (1971). Cephalosporium-artige Schimmelpilze (Hyphomycetes). G. Fischer Publishing, Stuttgart, Germany. [Google Scholar]
- Gams W. (1975). Cephalosporium-like Hyphomycetes: some tropical species. Transactions of the British Mycological Society 64: 389–404. [Google Scholar]
- Gams W. (1978). Connected and disconnected chains of phialoconidia and Sagenomella gen. nov. segregated from Acremonium. Persoonia 10: 97–112. [Google Scholar]
- Gams W, Lacey J. (1972). Cephalosporium-like hyphomycetes: two species of Acremonium from heated substrates. Transactions of the British Mycological Society 59: 519–522. [Google Scholar]
- Gams W, O’Donnell K, Schroers HJ, et al. (1998). Generic classification of some more hyphomycetes with solitary conidia borne on phialides. Canadian Journal of Botany 76: 1570–1583. [Google Scholar]
- Gams W, Stielow B, Gräfenhan T, et al. (2019). The ascomycete genus Niesslia and associated monocillium-like anamorphs. Mycological Progress 18: 5–76. [Google Scholar]
- Gams W, van Zaayen A. (1982). Contribution to the taxonomy and pathogenicity of fungicolous Verticillium species. I. Taxonomy. Netherlands Journal of Plant Pathology 88: 57–78. [Google Scholar]
- Giraldo A, Crous PW. (2019). Inside Plectosphaerellaceae. Studies in Mycology 92: 227–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo A, Gené J, Cano J, et al. (2012). Two new species of Acremonium from Spanish soils. Mycologia 104: 1456–1465. [DOI] [PubMed] [Google Scholar]
- Giraldo A, Gené J, Cano J, et al. (2014). Acremonium with catenate elongate conidia: phylogeny of Acremonium fusidioides and related species. Mycologia 106: 328–338. [DOI] [PubMed] [Google Scholar]
- Giraldo A, Gené J, Sutton DA, et al. (2015). Phylogeny of Sarocladium (Hypocreales). Persoonia 34: 10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo A, Gene J, Sutton DA, et al. (2017). New acremonium-like species in the Bionectriaceae and Plectosphaerellaceae. Mycological Progress 16: 349–368. [Google Scholar]
- Glenn AE, Bacon CW, Price R, et al. (1996). Molecular phylogeny of Acremonium and its taxonomic implications. Mycologia 88: 369–383. [Google Scholar]
- Gonçalves MFM, Vicente TFL, Esteves AC, et al. (2020). Novel halotolerant species of Emericellopsis and Parasarocladium associated with macroalgae in an estuarine environment. Mycologia 112: 154–171. [DOI] [PubMed] [Google Scholar]
- Gonçalves VN, Oliveira FS, Carvalho CR, et al. (2017). Antarctic rocks from continental Antarctica as source of potential human opportunistic fungi. Extremophiles 21: 851–860. [DOI] [PubMed] [Google Scholar]
- Gomes RR, Glienke C, Videira SIR, et al. (2013). Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31: 1–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosklags JH, Swift ME. (1957). The perfect stage of an antibiotic-producing Cephalosporium. Mycologia 49: 305–317. [Google Scholar]
- Grum-Grzhimaylo AA, Georgieva ML, Debets AJM, et al. (2013). Are alkalitolerant fungi of the Emericellopsis lineage (Bionectriaceae) of marine origin? IMA Fungus 4: 213–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarro J, Gams W, Pujol I, et al. (1997). Acremonium species: new emerging opportunists — in vitro antifungal susceptibilities and review. Clinical Infectious Diseases 25: 1222–1229. [DOI] [PubMed] [Google Scholar]
- Guarro J, Palacio A, Gené J, et al. (2009). A case of colonization of a prosthetic mitral valve by Acremonium strictum. Revista Iberoamericana de Micologia 26: 146–148. [DOI] [PubMed] [Google Scholar]
- Guéguen F. (1905). Gliomastix (Torula) chartarum n. gen. n. sp.; contribution a l’étude de la formation endogène des conidies. Bulletin de la Société Mycologique de France 21: 230–241. [Google Scholar]
- Gupta A, Jain H, Lynde C, et al. (2000). Prevalence and epidemiology of onychomycosis in patients visiting physicians’ offices: a multicenter Canadian survey of 15,000 patients. Journal of the American Academy of Dermatology 43: 244–248. [DOI] [PubMed] [Google Scholar]
- Hagestad OC, Hou L, Andersen JH, et al. (2021). Genomic characterization of three marine fungi, including Emericellopsis atlantica sp. nov. with signatures of a generalist lifestyle and marine biomass degradation. IMA Fungus 12: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton-Miller JMT. (2000). Sir Edward Abraham’s contribution to the development of the cephalosporins: a reassessment. International Journal of Antimicrobial Agents 15: 179–184. [DOI] [PubMed] [Google Scholar]
- Hirooka Y, Kobayashi T, Ono T, et al. (2010). Verrucostoma, a new genus in the Bionectriaceae from the Bonin Islands, Japan. Mycologia 102: 418–429. [DOI] [PubMed] [Google Scholar]
- Hou LW, Groenewald JZ, Pfenning LH, et al. (2020). The phoma-like dilemma. Studies in Mycology 96: 309–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes SJ. (1949). Studies on micro-fungi, 1. The genus Fusariella Saccardo. Mycological Papers 28: 1–11. [Google Scholar]
- Hughes SJ. (1958). Revisiones Hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany 36: 727–836. [Google Scholar]
- Hyde KD, Dong Y, Phookamsak R, et al. (2020a). Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity 100: 5–277. [Google Scholar]
- Hyde KD, Norphanphoun C, Maharachchikumbura SSN, et al. (2020b). Refined families of Sordariomycetes. Mycosphere 11: 305–1059. [Google Scholar]
- Ito T, Okane I, Nakagiri A, et al. (2000). Two species of Acremonium section Acremonium: A. borodinense sp. nov. and A. cavaraeanum rediscovered. Mycological Research 104: 77–80. [Google Scholar]
- Jaklitsch WM, Voglmayr H. (2011). Stromatonectria gen. nov. and notes on Myrmaeciella. Mycologia 103: 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RT, Frederick L. (1971). Cephalosporium salviniae sp. nov. a pathogen of Salvinia rotundifolia. Mycopathologia et Mycologia Applicata 43:195–200. [Google Scholar]
- Jørgensen CA. (1921). Heleococcum aurantiacuvi n. gen. et n. spec. Botanisk Tidsskrift 37: 417–420. [Google Scholar]
- Karsten PA. (1887). Symbolae ad mycologiam Fennicam. XXI. Meddelanden af Societas pro Fauna et Flora Fennica 14: 103–110. [Google Scholar]
- Kidd MN, Beaumont A. (1924). Apple rot fungi in storage. Transactions of the British Mycological Society 10: 98–118. [Google Scholar]
- Kim HJ, Li XJ, Kim DC, et al. (2021). PTP1B inhibitory secondary metabolites from an antarctic fungal strain Acremonium sp. SF-7394. Molecules 26: 5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschstein W. (1939). Über neue, seltene und kritische Ascomyceten und Fungi imperfecti. II. Annales Mycologici 37: 88–140. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2015). MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntze O. (1891). Revisio Generum Plantarum Vol. 2: 375–1011. [Google Scholar]
- Lechat C, Courtecuisse R. (2010). A new species of Ijuhya, I. antillana, from the French West Indies. Mycotaxon 113: 443–447. [Google Scholar]
- Lechat C, Farr DF, Hirooka Y, et al. (2010). A new species of Hydropisphaera, H. bambusicola, is the sexual state of Gliomastix fusigera. Mycotaxon 111: 95–102. [Google Scholar]
- Lechat C, Fournier J. (2008). Nectriella atrorubra C. Lechat & J. Fournier sp. nov. Fungal Planet 30: 1–2. [Google Scholar]
- Lechat C, Fournier J. (2013). Two new species of Lasionectria (Bionectriaceae, Hypocreales) from Guadeloupe and Martinique (French West Indies). Mycotaxon 121: 275–280. [Google Scholar]
- Lechat C, Fournier J. (2016a). Hydropisphaera znieffensis, a new species from Martinique. Ascomycete.org 8: 55–58. [Google Scholar]
- Lechat C, Fournier J. (2016b). Lasionectriella, a new genus in the Bionectriaceae, with two new species from France and Spain, L. herbicola and L. rubioi. Ascomycete.org 8: 59–65. [Google Scholar]
- Lechat C, Fournier J. (2017). Hydropisphaera foliicola, a new species from Martinique. Ascomycete.org 9: 6–8. [Google Scholar]
- Lechat C, Fournier J. (2019). Three new species of Ijuhya (Bionectriaceae, Hypocreales) from metropolitan France, French Guiana and Spain, with notes on morphological characterization of Ijuhya and allied genera. Ascomycete.org 11: 55–64. [Google Scholar]
- Lechat C, Fournier J. (2020). Three new species of Hydropisphaera (Bionectriaceae) from Europe and French Guiana. Ascomycete.org 12: 39–46. [Google Scholar]
- Lechat C, Fournier J, Moreau PA. (2016a). Xanthonectria, a new genus for the nectrioid fungus Nectria pseudopeziza. Ascomycete.org 8: 172–178. [Google Scholar]
- Lechat C, Fournier J, Richter T. (2016b). Protocreopsis caricicola (Hypocreales, Bionectriaceae), the first species of Protocreopsis reported from a north temperate area. Ascomycete.org 8: 30–32. [Google Scholar]
- Lechat C, Fournier J, Priou JP. (2018a). Chrysonectria, a new genus in the Nectriaceae with the new species C. finisterrensis from France. Ascomycete.org 10: 121–125. [Google Scholar]
- Lechat C, Fournier J, Vega M, et al (2018b). Geonectria, a new genus in the Bionectriaceae from France. Ascomycete.org 10: 81–85. [Google Scholar]
- Lechat C, Gardiennet A. (2009). Hydropisphaera castaneicola sp. nov. Bulletin Mycologique et Botanique Dauphiné-Savoie 192: 5–8. [Google Scholar]
- Lechat C, Moreau PA, Bender H. (2019). Lasionectriopsis, a new genus in the Bionectriaceae, based on the new species L. germanica. Ascomycete.org 11: 1–4. [Google Scholar]
- Leite CL, Pereira LT, Gerlach A, et al. (2018). Additional information on Mycocitrus aurantium (Bionectriaceae, Hypocreales), an unusual bamboo-inhabiting fungus found in South America. Biotemas 31: 1–9. [Google Scholar]
- Lin CG, Chen Y, Mckenzie EHC, et al. (2016). The genus Fusariella. Mycological Progress 15: 1313–1326. [Google Scholar]
- Lin HJ, Chien CY, Huang JW. (2004). Pathogenicity and host range of Acremonium lactucae sp. nov., the causal agent of leaf brown spot of lettuce. Plant Pathology Bulletin 13: 91–96. [Google Scholar]
- Lindau G. (1904). Rabenhorst’s Kryptogamen-Flora. Pilze-Fungi Imperfecti 1: 1–176. [Google Scholar]
- Lindau G. (1905). Beobachtungen über Hyphomyceten. 1. Verhandelingen van het Bataviaasch Genootschap der Kunsten en Wetenschappen 47: 63–76. [Google Scholar]
- Link HF. (1809). Observationes in ordines plantarum naturales. Dissertatio I. Magazin der Gesellschaft Naturforschenden Freunde Berlin 3: 3–42. [Google Scholar]
- Liu YJ, Whelen S, Hall BD. (1999). Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution 16: 1799–1808. [DOI] [PubMed] [Google Scholar]
- Locquin M. (1984). Mycologie générale et structurale. Masson, Paris, France: 1–551. [Google Scholar]
- Lombard L, Houbraken J, Decock C, et al. (2016). Generic hyper-diversity in Stachybotriaceae. Persoonia 36: 156–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L, van der Merwe NA, Groenewald JZ, et al. (2015). Generic concepts in Nectriaceae. Studies in Mycology 80: 189–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowen R. (1995). Acremonium section Lichenoidea section nov. and Pronectria oligospora species nov. Mycotaxon 53: 81–95. [Google Scholar]
- Lumbsch HT, Huhndorf SM. (2007). Outline of ascomycota–2007. Myconet 13: 1–58. [Google Scholar]
- Maire R. (1911). Remarques sur quelques Hypocréacées. Annales Mycologici 9: 315–325. [Google Scholar]
- Malloch D. (1989). An undescribed species of Leucosphaerina. Studies in Mycology 31: 107–111. [Google Scholar]
- Malloch D, Cain RF. (1970). Five new genera in the new family Pseudeurotiaceae. Canadian Journal of Botany 48: 1815–1825. [Google Scholar]
- Mathur PN, Thirumalachar MJ. (1960). A new Emericellopsis species with Stilbella type of conidia. Mycologia 52: 694–697. [Google Scholar]
- Matruchot L. (1911). Un nouveau champignon pathogène pour l’homme. Comptes Rendues des Séances Hebdomadaires de l’Académie des Sciences Paris 152: 325–327. [Google Scholar]
- Mirtalebi M, Banihashemi Z, Sabahi F, et al. (2017). Dieback of rose caused by Acremonium sclerotigenum as a new causal agent of rose dieback in Iran. Spanish Journal of Agricultural Research 14: 10–103. [Google Scholar]
- Möller A. (1901). Phycomyceten und Ascomyceten. Untersuchungen aus Brasilien. Botanische Mittheilungen aus den Tropen 9: 1–319. [Google Scholar]
- Möller C, Gams W. (1993). Two new hyphomycetes isolated from Antarctic lichens. Mycotaxon 48: 441–450. [Google Scholar]
- Morgan-Jones G, Gams W. (1982). Notes on Hyphomycetes. XLI. An endophyte of Festuca arundinacea and the anamorph of Epichloe typhina, new taxa in one of two new sections of Acremonium. Mycotaxon 15: 311–318. [Google Scholar]
- Nicot J. (1958). Quelques micromycètes des sables littoraux. Bulletin de la Société Mycologique de France 74: 221–235. [Google Scholar]
- Nong Y, Zhuang WY. (2005). Preliminary survey of Bionectriaceae and Nectriaceae (Hypocreales, Ascomycetes) from Jigongshan, China. Fungal Diversity 19: 95–107. [Google Scholar]
- Novicki TJ, Geise R, Limaye AP, et al. (2003). Genetic diversity among clinical isolates of Acremonium strictum determined during an investigation of a fatal mycosis. Journal of Clinical Microbiology 41: 2623–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell K. (1993). Fusarium and its near relatives. In: The fungal holomorph: mitotic, meiotic and pleomorphic speciation in fungal systematics (Reynolds DR, Taylor JW, eds.). CABI Pubilishing, Wallingford, UK: 225–233. [Google Scholar]
- Pang KL, Alias SA, Chiang MW, et al. (2010). Sedecimiella taiwanensis gen. et sp. nov., a marine mangrove fungus in the Hypocreales (Hypocreomycetidae, Ascomycota). Botanica Marina 53: 493–498. [Google Scholar]
- Perdomo H, García D, Gené J, et al. (2013). Phialemoniopsis, a new genus of Sordariomycetes, and new species of Phialemonium and Lecythophora. Mycologia 105: 398–421. [DOI] [PubMed] [Google Scholar]
- Perdomo H, Sutton DA, García D, et al. (2011). Spectrum of clinically relevant Acremonium species in the United States. Journal of Clinical Microbiology 49: 243–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera RH, Hyde KD, Jones EBG, et al. (2023). Profile of Bionectriaceae, Calcarisporiaceae, Hypocreaceae, Nectriaceae, Tilachlidiaceae, Ijuhyaceae fam. nov., Stromatonectriaceae fam. nov. and Xanthonectriaceae fam. nov. Fungal Diversity 118: 95–271. [Google Scholar]
- Pérez-Cantero A, Guarro J. (2020). Sarocladium and Acremonium infections: New faces of an old opportunistic fungus. Mycoses 63: 1203–1214. [DOI] [PubMed] [Google Scholar]
- Persoon CH. (1800). Observationes mycologicae Vol. 2: 1–106. [Google Scholar]
- Persoon CH. (1801). Synopsis methodica fungorum. Henrich Dieterich Publishing, Göttingen, Germany. [Google Scholar]
- Petch T. (1931). Notes on entomogenous fungi. Transactions of the British Mycological Society 16: 55–75. [Google Scholar]
- Petch T. (1932). Notes on entomogenous fungi. Transactions of the British Mycological Society 16: 209–245. [Google Scholar]
- Petrak F, Sydow H. (1936). Kritisch-systemische Originaluntersuchungen über Pyrenomyzeten, Sphaeropsideen und Melanconieen. VII. Annales Mycologici 34: 11–52. [Google Scholar]
- Plishka MJ, Tsuneda A, Currah RS. (2009). Morphology and development of Nigrosabulum globosum, a cleistothecial coprophile in the Bionectriaceae (Hypocreales). Mycological Research 113: 815–821. [DOI] [PubMed] [Google Scholar]
- Plowright CB. (1882). A monograph of British Hypomyces. Grevillea 11: 41–51. [Google Scholar]
- Rayner RW. (1970). A mycological colour chart. Commonwealth Mycological Institute and British Mycological Society, Kew, Surrey, UK. [Google Scholar]
- Rehner SA, Buckley E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97: 84–98. [DOI] [PubMed] [Google Scholar]
- Rehner SA, Samuels GJ. (1995). Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Canadian Journal of Botany 73: 816–823. [Google Scholar]
- Rogerson CT. (1970). The hypocrealean fungi (Ascomycetes, Hypocreales). Mycologia 62: 865–910. [PubMed] [Google Scholar]
- Ronquist F, Teslenko M, Van der Mark P, et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossman AY. (1983). The phragmosporous species of Nectria and related genera (Calonectria, Ophionectria, Paranectria, Scoleconectria and Trichonectria). Mycological Papers 150: 1–164. [Google Scholar]
- Rossman AY, Farr DF, Platas G, et al. (2008). Hydropisphaera fungicola Rossman, Farr & Newcombe, sp. nov. Fungal Planet 24: 1–2. [Google Scholar]
- Rossman AY, McKemy JM, Pardo-Schultheiss RA, et al. (2001). Molecular studies of the Bionectriaceae using large subunit rDNA sequences. Mycologia 93: 100–110. [Google Scholar]
- Rossman AY, Samuels GJ, Rogerson CT, et al. (1999). Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Studies in Mycology 42: 1–248. [Google Scholar]
- Rossman AY, Seifert KA, Samuels GJ, et al. (2013). Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales) proposed for acceptance and rejection. IMA Fungus 4: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy RY, Rai B. (1968). Fusariella indica sp. nov. Transactions of the British Mycological Society 51: 333–335. [Google Scholar]
- Rämä T, Nordén J, Davey ML. et al. (2014). Fungi ahoy! Diversity on marine wooden substrata in the high North. Fungal Ecology 8: 46–58. [Google Scholar]
- Saccardo PA. (1882). Fungi boreali-americani. Michelia 2: 564–582. [Google Scholar]
- Saccardo PA. (1883). Sylloge Fungorum 2: 1–815. (Padova). [Google Scholar]
- Saccardo PA. (1884). Sylloge fungorum omnium hucusque cognitorum, Vol. 4. Sumptibus Auctoris, Patavii. [Google Scholar]
- Samson RA. (1974). Paecilomyces and some allied Hyphomycetes. Studies in Mycology 6: 1–119. [Google Scholar]
- Samuels GJ. (1973). The myxomyceticolous species of Nectria. Mycologia 65: 401–420. [Google Scholar]
- Samuels GJ. (1976a). A revision of the fungi formerly classified as Nectria subgenus Hyphonectria. Memoirs of the New York Botanical Garden 26: 1–126. [Google Scholar]
- Samuels GJ. (1976b). Perfect states of Acremonium: The genera Nectria, Actiniopsis, Ijuhya, Neohenningsia, Ophiodictyon, and Peristomialis. New Zealand Journal of Botany 14: 231–260. [Google Scholar]
- Samuels GJ. (1978). Some species of Nectria having Cylindrocarpon imperfect states. New Zealand Journal of Botany 16: 73–82. [Google Scholar]
- Samuels GJ. (1988). Fungicolous, lichenicolous, and myxomyceticolous species of Hypocreopsis, Nectriopsis, Nectria, Peristomialis, and Trichonectria. Memoirs of the New York Botanical Garden 48: 1–78. [Google Scholar]
- Seaver FJ, Waterston JM. (1940). Contributions to the mycoflora of Bermuda—I. Mycologia 32: 388–407. [Google Scholar]
- Seifert KA. (1985). A monograph of Stilbella and some allied Hyphomycetes. Studies in Mycology 27: 1–235. [Google Scholar]
- Seifert KA, Morgan-Jones G, Gams W, et al. (2011). The Genera of Hyphomycetes. CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands. [Google Scholar]
- Sigler L, Zuccaro A, Summerbell RC, et al. (2004). Acremonium exuviarum sp. nov., a lizard-associated fungus with affinity to Emericellopsis. Studies in Mycology 50: 409–414. [Google Scholar]
- Smith G. (1962). Some new and interesting species of micro-fungi. III. Transactions of the British Mycological Society 45: 387–394. [Google Scholar]
- Spatafora JW, Blackwell M. (1993). Molecular systematics of unitunicate perithecial ascomycetes: The Clavicipitales-Hypocreales connection. Mycologia 85: 912–922. [Google Scholar]
- Spatafora JW, Blackwell M. (1994). The polyphyletic origins of ophiostomatoid fungi. Mycological Research 98: 1–9. [Google Scholar]
- Spegazzini C. (1881). Fungi argentini additis nonnullis brasiliensibus montevideensibusque. Pugillus quartus (Continuacion). Anales de la Sociedad Científica Argentina 12: 193–227. [Google Scholar]
- Spegazzini C. (1911). Mycetes Argentinenses (Series V). Anales del Museo Nacional de Historia Natural Buenos Aires. ser. 3 13: 329–467. [Google Scholar]
- Stamatakis A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolk AC. (1955). Emericellopsis minima sp. nov. and Westerdykella ornata gen. nov., sp. nov. Transactions of the British Mycological Society 38: 419–424. [Google Scholar]
- Subramanian CV. (1972). Conidial chains, their nature and significance in the taxonomy of Hyphomycetes. Current Science 41: 43–49. [Google Scholar]
- Sukapure RS, Thirumalachar MJ. (1963). Studies on Cephalosporium species from India-I. Mycologia 55: 563–569. [Google Scholar]
- Sukapure RS, Thirumalachar MJ. (1965). Studies on Cephalosporium species from India-III. Sydowia 19: 171–175. [Google Scholar]
- Sukapure RS, Thirumalachar MJ. (1966). Studies of Cephalosporium species from India-II. Bulletin of the Torrey Botanical Club 93: 305–311. [Google Scholar]
- Summerbell RC, Gueidan C, Guarro J, et al. (2018). The Protean Acremonium. A. sclerotigenum/egyptiacum: Revision, Food Contaminant, and Human Disease. Microorganisms 6: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell RC, Gueidan C, Schroers HJ, et al. (2011). Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Studies in Mycology 68: 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell RC, Scott JA. (2015). Acremonium. In: Molecular Biology of Food and Water Borne Mycotoxigenic and Mycotic Fungi (Paterson RRM, Lima N, eds). CRC Press, Boca Raton, USA: 115–128. [Google Scholar]
- Sun JZ, Liu XZ, Hyde KD, et al. (2017). Calcarisporium xylariicola sp. nov. and introduction of Calcarisporiaceae fam. nov. in Hypocreales. Mycological Progress 16: 433–445. [Google Scholar]
- Sung GH, Hywel-Jones NL, Sung JM, et al. (2007a). Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Studies in Mycology 57: 5–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung GH, Sung JM, Hywel-Jones NL, et al. (2007b). A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localized incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution 44: 1204–1223. [DOI] [PubMed] [Google Scholar]
- Sutton J, Mason TG. (2017). Isolated strain of Clonostachys rosea for use as a biological control agent. United States Patent Application US Patent No. 15/667, 861, Washington, DC: U.S. Patent and Trademark Office. [Google Scholar]
- Tian J, Lai D, Zhou L. (2017). Secondary metabolites from Acremonium fungi: Diverse structures and bioactivities. Mini Reviews in Medicinal Chemistry 17: 603–632. [DOI] [PubMed] [Google Scholar]
- Tibpromma S, Hyde KD, McKenzie EHC, et al. (2018). Fungal diversity notes 840–928: micro-fungi associated with Pandanaceae. Fungal Diversity 93: 1–160. [Google Scholar]
- Tichelaar GM. (1972). Acremonium gamsii nov. sp. (Hyphomycetes). Acta Botanica Neerlandica 21: 197–199. [Google Scholar]
- Tubaki K. (1967). An undescribed species of Heleococcum from Japan. Transactions of the Mycological Society of Japan 8: 5–10. [Google Scholar]
- Tubaki K. (1973). Aquatic sediment as a habitat of Emericellopsis, with a description of an undescribed species of Cephalosporium. Mycologia 65: 938–941. [Google Scholar]
- Udagawa S, Muroi T. (1990). Bulbithecium, a new genus of cleistocarpic coprophilous ascomycetes. Bulletin of the National Science Museum Tokyo 16: 13–19. [Google Scholar]
- Udagawa SI, Uchiyama S, Kamiya S. (1994). A new species of Roumegueriella. Mycoscience 35: 409–412. [Google Scholar]
- van Beyma FH. (1940). Beschreibung einiger neuer Pilzarten aus dem Centraalbureau voor Schimmelcultures, Baarn (Nederland), VI. Mitteilung. Antonie van Leeuwenhoek 6: 263–290. [PubMed] [Google Scholar]
- van Beyma FH. (1942). Beschreibung einiger neuer Pilzarten aus dem Centraalbureau voor Schimmelcultures, Baarn (Nederland). VII. Mitteilung. Antonie van Leeuwenhoek 8: 105–122. [PubMed] [Google Scholar]
- Verkley GJM, Quaedvlieg W, Shin HD, et al. (2013). A new approach to species delimitation in Septoria. Studies in Mycology 75: 213–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Höhnel F. (1916). Mykologisches. Österreichische Botanische Zeitschrift 66: 94–112. [Google Scholar]
- Vu D, Groenewald M, De Vries M, et al (2019). Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Hester M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R, Sun BL. (1994). Ancient and recent patterns of geographic speciation in the oyster mushroom Pleurotus revealed by phylogenetic analysis of ribosomal DNA sequences. Proceedings of the National Academy of Sciences of the United States of America 91: 4599–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese J. (1916). Beiträge zur Kenntnis der Hypocreaceen (I. Mitteilung) – Sitzungsberichte der Akademie der Wissenschaften mathematisch-naturwissenschaftliche Klasse 125: 465–575. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, et al., eds). Academic Press, San Diego, California, USA: 315–322. [Google Scholar]
- Wijayawardene NN, Hyde KD, Lumbsch HT, et al. (2018). Outline of ascomycota: 2017. Fungal diversity 88: 167–263. [Google Scholar]
- Yeh YH, Kirschner R. (2014). Sarocladium spinificis, a new endophytic species from the coastal grass Spinifex littoreus in Taiwan. Botanical Studies 55: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zare R, Gams W. (2001). A revision of Verticillium section Prostrata. IV. The genera Lecanicillium and Simplicillium gen. nov. Nova Hedwigia 73: 1–50. [Google Scholar]
- Zare R, Gams W. (2016). More white verticillium-like anamorphs with erect conidiophores. Mycological Progress 15: 993–1030. [Google Scholar]
- Zare R, Gams W, Starink-Willemse M, et al. (2007). Gibellulopsis, a suitable genus for Verticillium nigrescens, and Musicillium, a new genus for V. theobromae. Nova Hedwigia 85: 463–490. [Google Scholar]
- Zeng ZQ, Zhuang WY. (2016). A new fungicolous species of Hydropisphaera (Bionectriaceae, Hypocreales) from central China. Phytotaxa 288: 279–284. [Google Scholar]
- Zhuang WY, Zeng ZQ. (2017). Cocoonihabitus sinensis gen. et sp. nov. on remaining leaf veins of cocoons in a new family (Cocoonihabitaceae fam. nov.) of Hypocreales. Mycosystema 36: 1591–1598. [Google Scholar]
- Zuccaro A, Summerbell RC, Gams W, et al. (2004). A new Acremonium species associated with Fucus spp., and its affinity with a phylogenetically distinct marine Emericellopsis clade. Studies in Mycology 50: 283–298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic tree inferred from a Maximum Likelihood analysis based on a concatenated alignment of ITS and LSU sequences of 401 strains representing Hypocreales and related orders (Cephalothecales, Coniochaetales, Glomerellales). The RAxML bootstrap support values (MLBS) above 50 % are given at the nodes. The scale bar represents the expected number of changes per site. Strains with special status are indicated with a letter after the accession number (T: ex-type). The tree is rooted to Saccharata proteae CBS 115206.
Phylogenetic tree inferred from a Maximum Likelihood analysis based on a concatenated alignment of LSU, ITS, rpb2 and tef-1α sequences of 468 strains representing Bionectriaceae and outgroups. The RAxML bootstrap support values (MLBS) above 50 % are given at the nodes. The scale bar represents the expected number of changes per site. Strains with special status are indicated with a letter after the accession number (T: ex-type). The tree is rooted to Paracremonium inflatum CBS 485.77, Sarocladium oryzae CBS 180.74, S. zeae CBS 800.69, and Xenoacremonium recifei CBS 137.35.
Strains used in this study with details of their host, location, and GenBank accessions numbers























