Abstract
Background
Remdesivir has demonstrated benefit in some hospitalized patients with coronavirus disease 2019 (COVID-19) on supplemental oxygen and in nonhospitalized patients breathing room air. The durability of this benefit across time periods with different circulating severe acute respiratory syndrome coronavirus 2 variants of concern (VOC) is unknown. This comparative effectiveness study in patients hospitalized for COVID-19 and not receiving supplemental oxygen at admission compared those starting remdesivir treatment in the first 2 days of admission with those receiving no remdesivir during their hospitalization across different VOC periods.
Method
Using a large, multicenter US hospital database, in-hospital mortality rates were compared among patients hospitalized for COVID-19 but not requiring supplemental oxygen at admission between December 2020 and April 2022. Patients receiving remdesivir at hospital admission were matched 1:1 to those not receiving remdesivir during hospitalization, using propensity score matching. Cox proportional hazards models were used to assess 14- and 28-day in-hospital mortality rates or discharge to hospice.
Results
Among the 121 336 eligible patients, 58 188 remdesivir-treated patients were matched to 17 574 unique patients not receiving remdesivir. Overall, 5.4% of remdesivir-treated and 7.3% in the non-remdesivir group died within 14 days, and 8.0% and 9.8%, respectively, died within 28 days. Remdesivir treatment was associated with a statistically significant reduction in the in-hospital mortality rate compared with non-remdesivir treatment (14-day and 28-day adjusted hazard ratios [95% confidence interval], 0.75 [0.68–0.83] and 0.83 [0.76–0.90], respectively). This significant mortality benefit endured across the different VOC periods.
Conclusions
Remdesivir initiation in patients hospitalized for COVID-19 and not requiring supplemental oxygen at admission was associated with a significantly reduced in-hospital mortality rate. These findings highlight a potential survival benefit when clinicians initiated remdesivir on admission across the dominant variant eras of the evolving pandemic.
Keywords: comparative effectiveness research, COVID-19, hospitalization, mortality, remdesivir
Mortality rates from coronavirus disease 2019 (COVID-19) remain higher than those from influenza. Based on real-world data, remdesivir initiation was associated with significant mortality reduction among patients hospitalized for COVID-19 not requiring supplemental oxygen on admission across variants of concern periods.
With continued viral evolution contributing to episodic immunological escape, there is an ongoing need for effective therapeutics to treat coronavirus disease 2019 (COVID-19) that maintain antiviral activity against the prevailing variants of concern (VOC) for severe acute respiratory syndrome coronavirus 2 [1, 2]. In 2024, COVID-19 remains as consequential as influenza, with COVID-19 still accounting for more attributable deaths [3].
Remdesivir has been shown to be safe and effective at reducing mortality rates in hospitalized patients who require supplemental oxygen in the Adaptive COVID-19 Treatment Trial (ACTT-1) and SOLIDARITY trial, respectively [4, 5]. The PINETREE trial demonstrated that remdesivir also has efficacy in reducing the risk of hospitalization and death in non-hospitalized patients when viral replication is likely to be at its most active with high risk of progression to severe disease [6].
These 2 scenarios bookend hospitalized patients with moderate COVID-19, defined as those who do not require supplemental oxygen at admission. The SIMPLE Moderate clinical trial demonstrated the efficacy of remdesivir in improving clinical status among patients hospitalized for moderate COVID-19 [7]. Evidence from an individual patient data meta-analysis of 9 randomized clinical trials also indicated that remdesivir reduced 28-day mortality rates in patients requiring no or low-flow oxygen only [8].
Nonetheless, much of the evidence relating to remdesivir effectiveness, including findings from real-world studies, is based on data from the early phase of the COVID-19 pandemic [4, 5, 9, 10]. Since then, there has been an emergence of VOC, improvements in standards of care through the approval and authorization of different therapeutics, and widespread initial and follow-up vaccination.
The aim of the present study was to obtain up-to-date evidence relating to remdesivir effectiveness by comparing inpatient mortality rates according to remdesivir treatment among patients hospitalized for COVID-19 and not requiring supplemental oxygen on admission, across different VOC periods.
METHODS
Study Design and Data Source
This retrospective comparative effectiveness study used data extracted from PINC AI Healthcare Database (PHD, formerly Premier Healthcare Database; www.pinc-ai.com), a large, geographically diverse, all-payer hospital administrative billing database. The database captures patient, hospital and clinical characteristics, costs and charges, treatments, and diagnoses for approximately 25% of all hospitalizations occurring in the United States. Month, but not actual calendar date, is provided in the database due to privacy concerns. However, anchored to the day of admission, treatments and procedures during each subsequent day of the hospitalization from the day of admission until the day of discharge can be identified in the database. All data are captured for each day of the hospitalization relative to the hospital admission day.
Study Population
The study population comprised patients aged ≥18 years hospitalized for COVID-19 who had a documented primary diagnosis code of COVID-19 (International Classification of Diseases, 10th revision, Clinical Modification code U07.1) between 1 December 2020 and 30 April 2022 and who did not require supplemental oxygen during the first 2 days of the hospitalization. The use of the COVID-19 diagnosis code (U07.1) has been previously validated in the PINC AI Healthcare Database [11]. Furthermore, the diagnosis code for COVID-19 was also required to have been flagged as “present at admission.”
Furthermore, patients who did not require supplemental oxygen during the first 2 days of hospitalization, used as a proxy for disease severity, were identified by an absence of any charges related to oxygen supply and any charges for devices for low-flow oxygen, high-flow oxygen, noninvasive ventilation, invasive mechanical ventilation, and extracorporeal membrane oxygenation in the first 2 days of hospitalization. Some hospitals do not bill separately for supplemental oxygen supply or devices and instead include these charges in room charges. For these hospitals, it is not possible to identify supplemental oxygen use. Thus, only patients admitted to hospitals that reported separate charges for supplemental oxygen were included in the study.
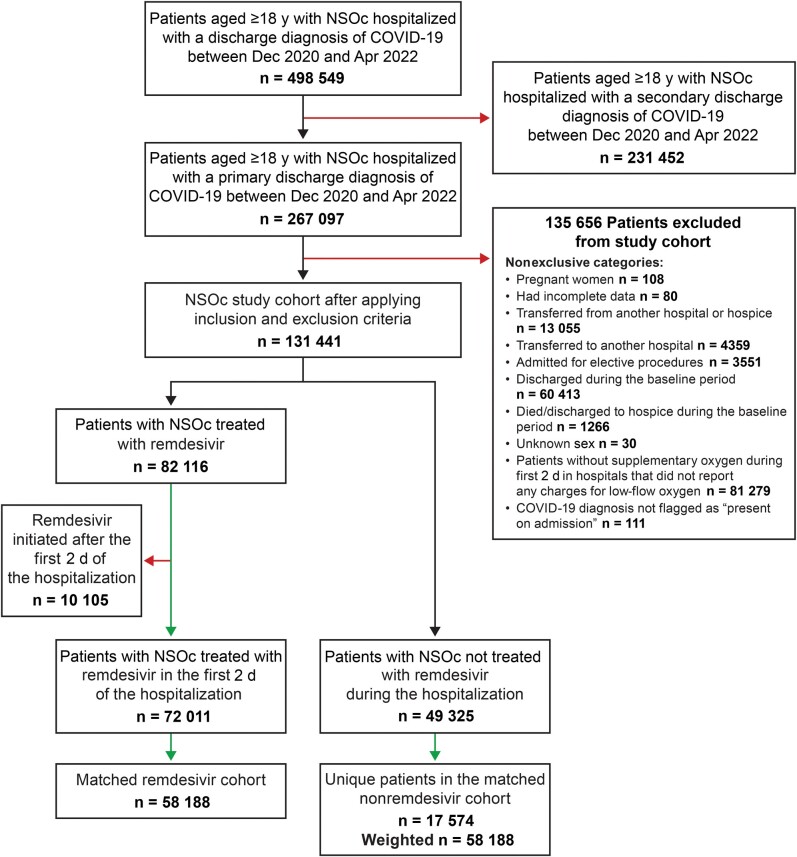
Patients were excluded from both exposure groups if they met any of the following criteria: pregnancy, incomplete data, death or discharge within 2 days of admission, transfer from hospice, transfer to or from another hospital, admission for elective procedure, or initiation of remdesivir after the first 2 days of hospitalization. Figure 1 presents the study flow.
Figure 1.
Study population. Abbreviations: COVID-19, coronavirus disease 2019; NSOc, no supplemental oxygen charges (in hospitals that were demonstrated to charge for supplemental oxygen).
Patients in the remdesivir group were those administered ≥1 dose of remdesivir within the first 2 days of hospital admission. The non-remdesivir comparator group was defined as patients not administered remdesivir at any time during their hospitalization. Patients crossing over to initiate remdesivir later in the admission were excluded given the specific research question to examine patients with the primary diagnosis of COVID-19 present on admission and to compare those receiving prompt antiviral therapy with those not receiving it. In addition, patients who initiated antiviral therapy at a later time during their admission were likely to have had confounding reasons, and thus identifying a corresponding clinical match was not feasible. For both exposure groups, index period was considered as the first 2 days of hospitalization and patient follow-up started on the day after the index period.
Ethical Approval and Patient Consent
Ethical approval and informed consent was not required for this study. This analysis of data from the US PINC AI Healthcare Database was conducted under an exemption from institutional review board oversight for US-based studies as the dataset utilized was derived from healthcare records that were de-identified and not re-identifiable.
Main Outcome and Covariates
Baseline was defined as the first 2 days following admission. This definition was chosen since actual time stamps are unavailable in the database, so that for a patient admitted to hospital at 23:59, that patient's day 2 would start at 00:00. The definition for baseline therefore provided all patients a window of a minimum of 24 hours in which clinical decisions were made and implemented.
All-cause in-hospital mortality rates were assessed at 14 and 28 days following the first 2 days of hospitalization during which treatment with remdesivir was ascertained. The timing of outcome assessment (14 and 28 days) was chosen to align with clinical trial definitions [4, 5]. In-hospital mortality was defined as a discharge status of either “expired” or “hospice.” Patients were followed up until death or the end of follow-up. Patients discharged alive and not into a hospice setting were censored at 14 and 28 days, respectively.
The following measures were captured at baseline: demographics (age group, sex, race, ethnicity, and primary payer), key comorbid conditions (obesity, chronic obstructive pulmonary disorder, cardiovascular disease, diabetes mellitus, renal disease, cancer, and immunosuppressive conditions), conditions recorded as part of admit diagnosis (sepsis, respiratory failure, hypoxemia, and pneumonia), hospital characteristics (hospital bed size, teaching, region, and urban/rural status), COVID-19 severity (hospital ward at admission and admission diagnoses), concomitant COVID-19 treatments (anticoagulants, corticosteroids, convalescent plasma, specific immunomodulatory agents considered individually [baricitinib, tocilizumab]), admission month, and admission source (skilled nursing or intermediate care facility or other).
Variable definitions are provided in the Supplementary Table 1. These covariates were considered to be confounders for COVID-19 related outcomes as they were indicators of differences in patient demographics, hospital-related variations in care, and disease severity. Similar variables have been assessed in other observational studies on COVID-19 using the same database [12–14].
Statistical Analysis
All analyses were conducted for the overall cohort and stratified by periods defined by prevailing VOC: pre-Delta (December 2020 to April 2021), Delta-predominant, (May–November 2021) and Omicron-predominant (pre-BA4/5, December 2021 to April 2022).
Propensity score (PS) methods were used to match patients in the treatment and comparator groups [15, 16]. The PS was estimated separately for each VOC period using logistic regression models that included covariates such as demographics, key comorbid conditions, hospital characteristics, admission diagnoses, hospital ward on admission, and concomitant medications. All covariates were retained in the model irrespective of their P value.
To account for differences in hospital COVID-19 management practices that may have evolved with each VOC time frame, a 1:1 preferential within-hospital matching approach with replacement with a caliper distance of 0.2 times the standard deviation of the logit of the PS was implemented, as follows. First, patients receiving remdesivir were matched to patients in the nonremdesivir group within the caliper distance, using a greedy nearest-neighbor approach and exact matching on the age group (18–49, 50–64, or ≥65 years) in 2–3-month blocks of admission month within the VOC period and within the same hospital. The unmatched patients in the remdesivir group were then matched to patients in the non-remdesivir group within the caliper distance, using a greedy nearest-neighbor approach and exact matching on the age group (18–49, 50–64, ≥65 years) in 2–3-month blocks of admission month within the VOC period in another remdesivir-using hospital of the same bed size (<200, 200–499, or ≥500).
A 1:1 matching with replacement approach was undertaken to allow for most of the remdesivir-treated patients to be matched and included in the analysis. There was no limit to the number of times a patient in the non-remdesivir group was available for matching to a remdesivir-treated patient [15]. In addition, a time constraint was imposed such that the matched pair of patients were in the hospital for ≥3 days. This emulates previous study design approaches [4, 10].
In the matched cohort, time to death was assessed using Kaplan-Meier curves and compared using log-rank tests. Furthermore, Cox proportional hazards models were used to derive adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs), adjusted for hospital-level cluster effects, and key covariates of age, admission month, hospital admission ward (documented bed charges for intensive care unit [ICU]/step-down unit vs general ward), and baseline COVID-19 treatments irrespective of their absolute standardized difference. Assumptions of using the Cox proportional hazards models were met. A robust (sandwich) variance estimator was used to account for potential replications of patients induced by a matching with replacement approach, which resulted in conservative (wider) 95% CIs.
Additional sensitivity analyses were conducted, as follows: (1) excluding patients that were admitted to the ICU/step-down unit in the first 2 days of hospitalization; (2) 1:1 PS matching without replacement; (3) considering only discharge status of “expired” to define the outcome of interest; (4) excluding patients who were discharged to hospice to remove their impact on the decision whether or not to treat with remdesivir; and (5) including only patients with available baseline laboratory data (approximately 25% of the study cohort) and estimated glomerular filtration rate (eGFR) ≥30 mL/min/1.73 m2 in the initial study cohort of patients and redoing the PS development and matching.
RESULTS
Of the 121 336 eligible patients with COVID-19 not requiring supplemental oxygen on admission, 72 011 (59.3%) were treated with remdesivir in the first 2 days of the hospitalization, and 49 325 (40.7%) were not treated with remdesivir (Figure 1).
Before matching, a lower proportion of remdesivir-treated patients had risk factors associated with progression to severe disease. Specifically, remdesivir-treated patients were younger than those in the non-remdesivir group (47.9% vs 60.4%, respectively, aged ≥65 years) with a lower proportion of patients with immunocompromised conditions (23.6% vs 37.8%), cancer (4.0% vs 4.9%), or diabetes mellitus (36.2% vs 39.9%). Remdesivir-treated patients were more likely to be obese than those in the non-remdesivir group (34.6% vs 25.6%, respectively) before matching (Table 1).
Table 1.
Demographic and Hospital Characteristics of Patients Hospitalized for Coronavirus Disease 2019, December 2020 to April 2022
| Characteristic | Before Matching | After Matchinga | |||||
|---|---|---|---|---|---|---|---|
| Patients, No. (%) | ASD | Patients, No. (%) | ASD | ||||
| No RDV (n = 49 325) | RDV (n = 72 011) | No RDV (n = 58 188b) | RDV (n = 58 188 ) | ||||
| Age group, y | 18–49 | 7768 (15.7) | 15 664 (21.8) | 0.24 | 11 904 (20.5) | 11 904 (20.5) | 0.00 |
| 50–64 | 11 755 (23.8) | 21 886 (30.4) | 17 478 (30.0) | 17 478 (30.0) | |||
| ≥65 | 29 802 (60.4) | 34 461 (47.9) | 28 806 (49.5) | 28 806 (49.5) | |||
| Female sex | 25 509 (51.7) | 35 655 (49.5) | 0.04 | 28 748 (49.4) | 28 991 (49.8) | 0.01 | |
| Race | White | 32 828 (66.6) | 50 910 (70.7) | 0.13 | 40 847 (70.2) | 41 195 (70.8) | 0.03 |
| Black | 11 069 (22.4) | 12 054 (16.7) | 9901 (17.0) | 9712 (16.7) | |||
| Asian | 940 (1.9) | 1666 (2.3) | 1350 (2.3) | 1343 (2.3) | |||
| Other | 4488 (9.1) | 7381 (10.2) | 6090 (10.5) | 5938 (10.2) | |||
| Ethnicity | Hispanic | 6037 (12.2) | 13 633 (18.9) | 0.22 | 11 295 (19.4) | 10 873 (18.7) | 0.01 |
| Non-Hispanic | 37 794 (76.6) | 49 316 (68.5) | 39 322 (67.6) | 39 790 (68.4) | |||
| Unknown | 5494 (11.1) | 9062 (12.6) | 7571 (13.0) | 7525 (12.9) | |||
| Primary payer | Commercial | 9119 (18.5) | 22 054 (30.6) | 0.33 | 16 737 (28.8) | 17 222 (29.6) | 0.05 |
| Medicare | 31 116 (63.1) | 35 364 (49.1) | 30 441 (52.3) | 29 473 (50.7) | |||
| Medicaid | 5590 (11.3) | 8653 (12.0) | 6664 (11.5) | 6786 (11.7) | |||
| Other | 3500 (7.1) | 5940 (8.2) | 4346 (7.5) | 4707 (8.1) | |||
| Admission month | Dec 2020 | 6533 (13.2) | 8915 (12.4) | 0.41 | 7458 (12.8) | 7386 (12.7) | 0.15 |
| Jan 2021 | 5948 (12.1) | 9318 (12.9) | 7566 (13.0) | 7638 (13.1) | |||
| Feb 2021 | 2501 (5.1) | 3770 (5.2) | 3134 (5.4) | 3037 (5.2) | |||
| Mar 2021 | 1743 (3.5) | 2943 (4.1) | 2286 (3.9) | 2383 (4.1) | |||
| Apr 2021 | 1966 (4.0) | 3668 (5.1) | 2868 (4.9) | 2868 (4.9) | |||
| May 2021 | 1050 (2.1) | 2061 (2.9) | 1588 (2.7) | 1599 (2.7) | |||
| Jun 2021 | 462 (0.9) | 900 (1.2) | 704 (1.2) | 693 (1.2) | |||
| Jul 2021 | 1501 (3.0) | 3183 (4.4) | 2506 (4.3) | 2638 (4.5) | |||
| Aug 2021 | 4030 (8.2) | 9525 (13.2) | 7878 (13.5) | 7746 (13.3) | |||
| Sep 2021 | 3492 (7.1) | 5922 (8.2) | 4692 (8.1) | 4857 (8.3) | |||
| Oct 2021 | 2261 (4.6) | 3070 (4.3) | 2665 (4.6) | 2500 (4.3) | |||
| Nov 2021 | 2386 (4.8) | 3348 (4.6) | 2766 (4.8) | 2766 (4.8) | |||
| Dec 2021 | 4222 (8.6) | 5265 (7.3) | 4071 (7.0) | 4266 (7.3) | |||
| Jan 2022 | 8388 (17.0) | 7870 (10.9) | 6311 (10.8) | 6116 (10.5) | |||
| Feb 2022 | 2025 (4.1) | 1591 (2.2) | 1289 (2.2) | 1224 (2.1) | |||
| Mar 2022 | 371 (0.8) | 274 (0.4) | 176 (0.3) | 202 (0.3) | |||
| Apr 2022 | 446 (0.9) | 388 (0.5) | 230 (0.4) | 269 (0.5) | |||
| Admission source: transfer from SNF or ICF | 1077 (2.2) | 877 (1.2) | 0.07 | 949 (1.6) | 791 (1.4) | 0.02 | |
| Hospital size, no. of beds | <100 | 2157 (4.4) | 3864 (5.4) | 0.1 | 2512 (4.3) | 2928 (5.0) | 0.06 |
| 100–199 | 6216 (12.6) | 10 219 (14.2) | 8361 (14.4) | 7945 (13.7) | |||
| 200–299 | 9896 (20.1) | 13 047 (18.1) | 10 468 (18.0) | 10 544 (18.1) | |||
| 300–399 | 10 435 (21.2) | 15 306 (21.3) | 12 370 (21.3) | 12 811 (22.0) | |||
| 400–499 | 4846 (9.8) | 5515 (7.7) | 4985 (8.6) | 4468 (7.7) | |||
| ≥500 | 15 775 (32.0) | 24 060 (33.4) | 19 492 (33.5) | 19 492 (33.5) | |||
| Rural/urban status | Urban | 44 181 (89.6) | 65 660 (91.2) | 0.05 | 53 831 (92.5) | 53 269 (91.5) | 0.04 |
| Rural | 5144 (10.4) | 6351 (8.8) | 4357 (7.5) | 4919 (8.5) | |||
| Teaching hospital | 22 748 (46.1) | 29 401 (40.8) | 0.11 | 24 017 (41.3) | 24 147 (41.5) | 0.00 | |
| Region | Midwest | 10 565 (21.4) | 12 114 (16.8) | 0.17 | 8702 (15.0) | 9637 (16.6) | 0.08 |
| Northeast | 7294 (14.8) | 11 447 (15.9) | 10 418 (17.9) | 9458 (16.3) | |||
| South | 25 658 (52.0) | 35 862 (49.8) | 28 393 (48.8) | 28 828 (49.5) | |||
| West | 5808 (11.8) | 12 588 (17.5) | 10 675 (18.3) | 10 265 (17.6) | |||
| Comorbid conditions | Obesity | 12 649 (25.6) | 24 908 (34.6) | 0.2 | 19 634 (33.7) | 20 264 (34.8) | 0.02 |
| COPD | 10 868 (22.0) | 16 595 (23.0) | 0.02 | 13 621 (23.4) | 13 525 (23.2) | 0.00 | |
| Cardiovascular disease | 40 855 (82.8) | 51 979 (72.2) | 0.26 | 43 325 (74.5) | 42 896 (73.7) | 0.02 | |
| Diabetes mellitus | 19 686 (39.9) | 26 069 (36.2) | 0.08 | 21 764 (37.4) | 21 662 (37.2) | 0.00 | |
| Renal disease | 15 078 (30.6) | 11 314 (15.7) | 0.36 | 10 734 (18.4) | 9541 (16.4) | 0.05 | |
| Cancer | 2404 (4.9) | 2849 (4.0) | 0.04 | 2508 (4.3) | 2354 (4.0) | 0.01 | |
| Immunocompromised condition | 18 654 (37.8) | 17 022 (23.6) | 0.31 | 15 827 (27.2) | 14 213 (24.4) | 0.06 | |
| General ward on admission | 43 574 (88.3) | 63 386 (88.0) | 0.01 | 51 182 (88.0) | 51 350 (88.2) | 0.01 | |
| Admission diagnosis | Sepsis | 182 (0.4) | 211 (0.3) | 0.01 | 179 (0.3) | 179 (0.3) | 0.00 |
| Pneumonia | 1926 (3.9) | 4066 (5.6) | 0.08 | 3442 (5.9) | 3355 (5.8) | 0.01 | |
| Other treatments at baseline | Anticoagulants | 40 943 (83.0) | 65 236 (90.6) | 0.23 | 49 439 (85.0) | 52 756 (90.7) | 0.15 |
| Corticosteroids | 29 286 (59.4) | 67 165 (93.3) | 0.87 | 54 457 (93.6) | 54 407 (93.5) | 0.00 | |
| Convalescent plasma | 793 (1.6) | 5163 (7.2) | 0.27 | 3536 (6.1) | 3740 (6.4) | 0.01 | |
| Tocilizumab | 487 (1.0) | 1460 (2.0) | 0.09 | 1367 (2.3) | 1288 (2.2) | 0.01 | |
| Baricitinib | 898 (1.8) | 1924 (2.7) | 0.06 | 1348 (2.3) | 1678 (2.9) | 0.04 | |
Abbreviations: ASD, absolute standardized difference; COPD, chronic obstructive pulmonary disease; ICF, intermediate care facility; ICU, intensive care unit; RDV, remdesivir; SNF, skilled nursing facility.
aMatching with replacement approach.
bWeighted.
After matching, the study population comprised 58 188 remdesivir-treated patients and 17 574 unique patients in the non-remdesivir group (weighted to 58 188 patients, as control patients were available for matching more than once). After matching, admission month and baseline anticoagulant treatment covariate had an absolute standardized difference value of >0.10; these were included as covariates in the Cox proportional hazards model (Table 1). Of the matched study population, 49.5% were aged ≥65 years and up to 27.2% were immunocompromised, up to 37.4% had diabetes mellitus, up to 4% had cancer, and up to 34.8% were obese (Table 1).
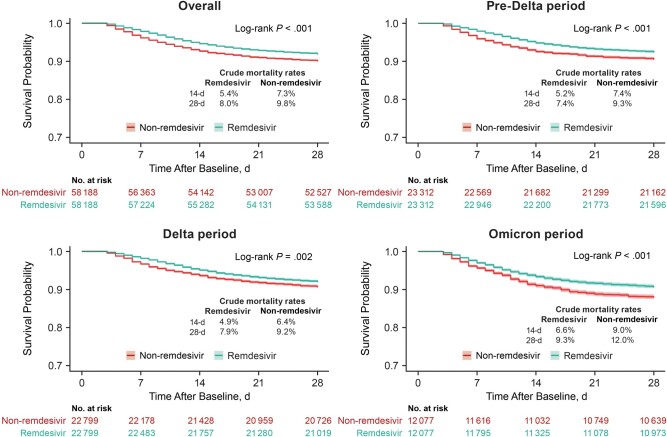
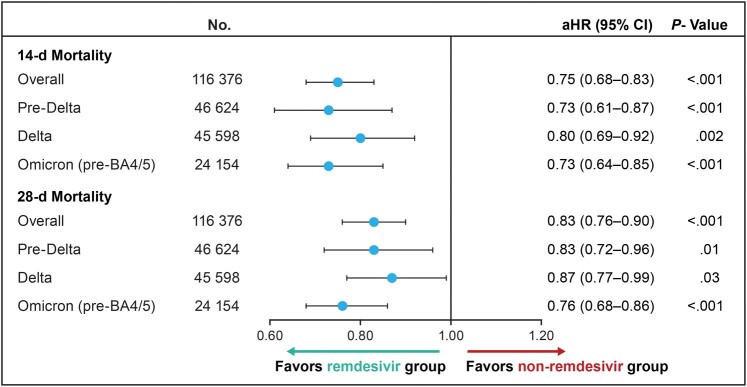
Across VOC periods, 3125 (5.4%) of the remdesivir-treated patients and 4267 (7.3%) in the nonremdesivir group died within 14 days, and 4649 (8.0%) of remdesivir-treated and 5716 (9.8%) in the non-remdesivir group died within 28 days. In the unadjusted analysis, the mortality risk was significantly lower in remdesivir-treated patients than in the non-remdesivir group (log-rank P < .001) (Figure 2). After adjustment, remdesivir use was associated with a significant reduction in inpatient mortality rate compared with the nonremdesivir group (14-day aHR, 0.75 [95% CI, .68–.83]; 28-day aHR, 0.83 [.76–.90]) (Figure 3).
Figure 2.
Kaplan-Meier curves and 95% confidence bands for time to in-hospital death or transfer to hospice among patients hospitalized for coronavirus disease 2019 (COVID-19), not requiring supplemental oxygen at admission, across the COVID-19 variant time periods. Sample sizes for the nonremdesivir group are weighted because matching with replacement approach was used. Time after baseline refers to the time during which outcomes were assessed after the 2-day period in which remdesivir treatment administration was identified (baseline).
Figure 3.
In-hospital mortality rates at 14 and 28 days among patients hospitalized for coronavirus disease 2019 (COVID-19) and not requiring supplemental oxygen at admission, across the COVID-19 variant time periods (adjusted Cox proportional hazards model). Estimates were adjusted for age, admission month, hospital ward on admission (intensive care unit/step-down vs general ward), and baseline treatments (anticoagulants, convalescent plasma, corticosteroids, baricitinib, or tocilizumab). Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval.
Within each VOC period, remdesivir treatment was associated with a significant reduction in the inpatient mortality rate compared with the non-remdesivir group at both 14 days (pre-Delta, Delta-predominant, and Omicron-predominant aHR [95% CI], 0.73 [.61–.87], 0.80 [.69–.92], and 0.73 [.64–.85], respectively) and 28 days (0.83 [.72–.96], 0.87 [.77–.99], and 0.76 [.68–.86], respectively) (Figure 3).
Similar results were obtained using 1:1 matching without replacement (Supplementary Tables 2 and 3). Sensitivity analyses to assess the inclusion of hospice in the mortality outcome yielded similar findings as the primary analysis (Supplementary Table 3). Further, a sensitivity analysis conducted by excluding patients that were admitted to the ICU/step-down unit in the first 2 days of hospitalization revealed consistent results (14-day aHR, 0.76 [95% CI, .68–.85]; 28-day, 0.83 [.76–.91]) (Supplementary Table 3). In addition, sensitivity analysis restricted to a subset of hospitals that reported laboratory data, using a study cohort with an available eGFR value ≥30 mL/min/1.73 m2 and using 1:1 matching without replacement also led to consistent findings (Supplementary Table 4).
DISCUSSION
In this large, multicenter, healthcare database study, the data suggest that prompt remdesivir treatment on admission was associated with a statistically significant reduction in in-hospital mortality rates compared with not initiating remdesivir during hospitalization among patients admitted for COVID-19 that did not require supplemental oxygen on admission. These findings were consistent across VOC periods, indicating that remdesivir was effective regardless of VOC, with no evidence of meaningful variant escape.
There were 49 325 patients not requiring supplemental oxygen at admission and not administered remdesivir during their hospitalization for COVID-19. This database encompasses information from approximately 25% of US hospitalizations, and the results showed that remdesivir treatment was associated with a 17% reduction in the 28-day mortality risk and improved outcomes. Although the absolute number-needed-to-treat of 55 in the overall study period and 37 in the Omicron period is higher than for severe COVID-19, the relative mortality reduction remains comparable.
These findings should be considered in the context of existing evidence. The major remdesivir clinical trials (ACTT-1 and SOLIDARITY trials) were not designed or powered to detect significant differences in mortality rates between subgroups based on baseline supplemental oxygen requirement. However, the non-statistically significant point estimates for mortality in an individual metanalysis of 3 randomized controlled trials (ACTT-1, SOLIDARITY, and SIMPLE Moderate) indicate the potential effectiveness of remdesivir in reducing the all-cause mortality rate in patients not receiving supplemental oxygen initially (relative risk, 0.78 [95% CI, .41–1.50]) [17]. Evidence from trials has also established the clinical benefits of remdesivir in outpatient settings, in hospitalized patients not receiving supplemental oxygen, and in hospitalized patients requiring low-flow or high-flow oxygen, thereby supporting the effectiveness of remdesivir across a broad spectrum of COVID-19 disease intensity [4, 5, 7, 18].
The current results extend and corroborate findings from a previous US PINC AI Healthcare Database study by expanding the study time period and increasing the study sample size while complementing the findings from a study using the HealthVerity healthcare data ecosystem, which applied different statistical methods using a distinct data source [12, 19]. In the previous PINC AI Healthcare Database study conducted among patients with COVID-19 hospitalized between August and November 2020, remdesivir use was associated with a reduction in mortality rate compared with the non-remdesivir group in patients with supplemental oxygen charges at baseline (14-day aHR, 0.69 [95% CI, .57–.83]; 28-day aHR, 0.80 [.68–.94]) [12]. Similarly, the study using HealthVerity data revealed a lower mortality rate in remdesivir-treated patients across the spectrum of disease severity, including those not receiving supplemental oxygen [19].
These findings, along with the confirmed safety profile of remdesivir, indicate that early administration of remdesivir when viral replication is most active was associated with improved outcomes in this study population. The findings also provide further evidence of a lack of meaningful escape of VOC from remdesivir at the population level, aligning with findings of in vitro studies [20–22].
Despite this accumulating evidence, clinical guidelines currently have disparate recommendations relating to the treatment of patients not requiring supplemental oxygen at the time of admission [23–26]. Many of the trials informing these guidelines were conducted during the early phase of the pandemic before widespread vaccination, when much was unknown regarding the treatment of COVID-19, when there were fewer VOC, and when a smaller proportion of the population had been infected and recovered from previous COVID-19 infections. Given these changes and since further placebo-controlled trials in this specific population are unlikely, real-world evidence can provide further information to clinicians.
This study has important strengths. First, it used a large administrative database with adequate patient numbers to enable assessment of the effectiveness of remdesivir across multiple waves of the pandemic. Second, the study sample for the analysis was restricted to patients with a primary diagnosis of COVID-19 to ensure that the sample comprised patients who were hospitalized due to COVID-19 rather than with COVID-19. COVID-19 infection may be identified during admission for unrelated indications with an incidentally positive test, and such patients are likely very distinct from patients admitted because of COVID-19 infection [27].
A key limitation of the present study is the potential for residual confounding due to imbalances in unmeasured variables between the treatment groups even after PS matching. To minimize the potential for residual confounding, patients were matched according to the PS, age group, admission month, and hospital. The study cohort likely comprised patients with heterogeneous time-since-symptom onset since this information is not available in this database; patients were therefore matched on presenting characteristics only.
Although chronic kidney disease, a potential historical contraindication for remdesivir administration before its subsequent expanded label across all levels of renal function [28], was accounted for in the derivation of PS, laboratory values of renal function such as creatinine were not accounted for, since these data were only available for a small subset of patients (n = 29 829/116 376; ie, 26% of the matched cohort). However, among this subset of patients, the median baseline creatinine levels [interquartile range] were similar across treatment groups after matching (remdesivir group, 1.0 [0.8–1.3] mg/dL; non-remdesivir group, 1.0 [0.8–1.4] mg/dL). Moreover, a sensitivity analysis was performed using a full PS development and matching using only patients admitted to a hospital with laboratory data available in the database and with a baseline eGFR level ≥30 mL/min/1.73 m2 (the eGFR from the historical label for remdesivir before its expansion to include patients across any spectrum of renal function) (Supplementary Table 4) [28].
A limitation of the PS matching approach is the exclusion of patients who are not matched leading to exclusion of the corresponding information from these patients. No data were available regarding clinical history before hospital admission including antivirals or other therapeutics administered before hospitalization, so this could not be accounted for in the analyses. Since outpatient remdesivir use was not approved by the Food and Drug Administration until January 2022 and the effect of remdesivir was consistent in VOC time periods before and after this approval, the impact of this limitation on the study's findings is negligible.
This study also relied on separate billing charges relating to the use of supplemental oxygen to identify the study population that did not receive supplemental oxygen. Given that some hospitals include charges for supplemental oxygen within the room charge, it was necessary to exclude patients who were admitted to hospitals that did not report any low-flow oxygen charges. This minimized the risk of including patients who did receive supplemental oxygen in the study cohort, but it is still possible that some patients who were on supplemental oxygen at baseline may have been misclassified and included in this study cohort.
Previous research has demonstrated that patients without supplemental oxygen at admission, as ascertained using this approach, were associated appropriately with lower mortality, as compared to patients using supplemental oxygen at admission [12]. Furthermore, missing or incomplete data remains an issue in any observational study. However, in our study cohort, we had only 80 patients (of 121 336 in total) with missing or incomplete information. Hence, we did not attempt to impute any missing data in our analysis. Finally, the infecting lineage of individual COVID-19 cases included in this study is unknown; therefore, VOC periods in which specific variants were predominant were used as a proxy for actual VOC. The study covers the pre-BA4/5 period and as such does not provide information for VOC after this time frame.
During the study period, of the 121 336 patients who did not require supplemental oxygen at admission to US hospitals for COVID-19, 49 325 (40.7%) did not receive remdesivir. In this real-world database, treatment with remdesivir was associated with a 17% lower relative mortality risk by day 28 across VOC periods.
There remains a lower but meaningful absolute mortality risk in patients with COVID-19 admitted without hypoxemia compared to those admitted with hypoxemia [12, 14]. Conducting a randomized controlled trial appropriately powered for mortality is impractical in this patient population. Evidence of the association between remdesivir use and lower mortality rate from this large observational study may inform clinicians in the management of patients with COVID-19 admitted without hypoxemia.
Supplementary Material
Acknowledgments
The authors acknowledge the medical writing and editing support provided by Stephanie H. Read and Amy Porter.
Author contributions. All listed authors contributed substantially to the manuscript and agreed to the final submitted version.
Availability of data. The data that support the findings of this study are available from PINC AI (https://www.pinc-ai.com). Restrictions apply to the availability of these data, which were used under license for this study.
Financial support. This work was supported by Gilead Sciences, Inc.
Potential conflicts of interest. E. M., E. L., C. D. T., M. T., M. B., and R. H. report employement and being stockholders of Gilead Sciences during the conduct of the study. A. C., and H. J. report funding for study and medical writing provided to their institution (Certara), from Gilead Sciences, during the conduct of the study. C. C. M. reports payment or honoraria for lectures/speaking from AstraZeneca and participation on an advisory board for Gilead Sciences. A. C. K reports grants from the National Institutes of Health Adaptive COVID-19 Treatment Trial. R. L. G. reports grants or contracts to his institution from AstraZeneca, Eli Lilly, Gilead Sciences, Johnson & Johnson, Pfizer, Regeneron, and Roivant Sciences (Kinevant Sciences); participation on advisory boards and/or consulting fees from AbbVie, AstraZeneca, Eli Lilly, Gilead Sciences, GSK Pharmaceuticals, and Roche; payment or honoraria for lectures/speaking from Gilead Sciences and Pfizer (the latter unrelated to infectious diseases); travel support from Gilead Sciences; de minimis investment in AbCellera; and a gift in kind to his institution from Gilead Sciences to facilitate an unrelated academic-sponsored clinical trial (NCT03383419).
Contributor Information
Essy Mozaffari, Medical Affairs, Gilead Sciences, Foster City, California, USA.
Aastha Chandak, Evidence & Access, Certara, New York, New York, USA.
Chidinma Chima-Melton, Division of Pulmonary & Critical Care Medicine, Department of Medicine, University of California–Los Angeles Health, Torrance, California, USA.
Andre C Kalil, Division of Infectious Diseases, Department of Internal Medicine, University of Nebraska Medical Center, Omaha, Nebraska, USA.
Heng Jiang, Evidence & Access, Certara, Paris, France.
EunYoung Lee, Medical Affairs, Gilead Sciences, Foster City, California, USA.
Celine Der-Torossian, Medical Affairs, Gilead Sciences, Foster City, California, USA.
Mark Thrun, Medical Affairs, Gilead Sciences, Foster City, California, USA.
Mark Berry, Medical Affairs, Gilead Sciences, Foster City, California, USA.
Richard Haubrich, Medical Affairs, Gilead Sciences, Foster City, California, USA.
Robert L Gottlieb, Center for Advanced Heart and Lung Disease, Baylor University Medical Center, Dallas, Texas, USA; Baylor Scott & White Research Institute, Dallas, Texas, USA; Department of Internal Medicine, Burnett School of Medicine at TCU, Fort Worth, Texas, USA; Department of Internal Medicine, Texas A&M Health Science Center, Dallas, Texas, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. World Health Organization . World Health Organization Health emergency dashboard. Available at: https://extranet.who.int/publicemergency. Accessed 7 February 2024.
- 2. Ferdinands JM, Rao S, Dixon BE, et al. . Waning of vaccine effectiveness against moderate and severe COVID-19 among adults in the US from the VISION network: test negative, case-control study. BMJ 2022; 379:e072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention . Weekly U.S. influenza surveillance report. Available at: https://www.cdc.gov/flu/weekly/index.htm. Accessed 9 January 2023.
- 4. Beigel JH, Tomashek KM, Dodd LE, et al. . Remdesivir for the treatment of COVID-19-final report. N Engl J Med 2020; 383:1813–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. WHO Solidarity Trial Consortium . Remdesivir and three other drugs for hospitalised patients with COVID-19: final results of the WHO solidarity randomised trial and updated meta-analyses. Lancet 2022; 399:1941–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brown SM, Katz MJ, Ginde AA, et al. . Consistent effects of early remdesivir on symptoms and disease progression across at-risk outpatient subgroups: treatment effect heterogeneity in PINETREE study. Infect Dis Ther 2023; 12:1189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spinner CD, Gottlieb RL, Criner GJ, et al. . Effect of remdesivir vs standard care on clinical status at 11 in patients with COVID-19: a randomized clinical trial. JAMA 2020; 324:1048–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Amstutz A, Speich B, Mentré F, et al. . Effects of remdesivir in patients hospitalised with COVID-19: a systematic review and individual patient data meta-analysis of randomised controlled trials. Lancet Respir Med 2023; 11:453–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garibaldi BT, Wang K, Robinson ML, et al. . Real-world effectiveness of remdesivir in adults hospitalized with coronavirus disease 2019 (COVID-19): a retrospective, multicenter comparative effectiveness study. Clin Infect Dis 2021; 75:e516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garibaldi BT, Wang K, Robinson ML, et al. . Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open 2021; 4:e213071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kadri SS, Gundrum J, Warner S, et al. . Uptake and accuracy of the diagnosis code for COVID-19 among US hospitalizations. JAMA 2020; 324:2553–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mozaffari E, Chandak A, Zhang Z, et al. . Remdesivir treatment in hospitalized patients with COVID-19: a comparative analysis of in-hospital all-cause mortality in a large multi-center observational cohort. Clin Infect Dis 2021; 75:e450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mozaffari E, Chandak A, Gottlieb RL, et al. . Remdesivir reduced mortality in immunocompromised patients hospitalized for COVID-19 across variant waves: findings from routine clinical practice. Clin Infect Dis 2023; 77:1626–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mozaffari E, Chandak A, Gottlieb RL, et al. . Remdesivir is associated with reduced mortality in COVID-19 patients requiring supplemental oxygen including invasive mechanical ventilation across SARS-CoV-2 variants. Open Forum Infect Dis 2023; 10:ofad482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci 2010; 25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Read SH, Khachatryan A, Chandak A, et al. . Comparative effectiveness research in COVID-19 using real-world data: methodological considerations. J Comp Eff Res 2021; 10:1259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaka AS, MacDonald R, Greer N, et al. . Major update: remdesivir for adults with COVID-19. Ann Intern Med 2021; 174:663–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gottlieb RL, Vaca CE, Paredes R, et al. . Early remdesivir to prevent progression to severe COVID-19 in outpatients. N Engl J Med 2021; 386:305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chokkalingam AP, Hayden J, Goldman JD, et al. . Association of remdesivir treatment with mortality among hospitalized adults with COVID-19 in the United States. JAMA Netw Open 2022; 5:e2244505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pitts J, Lu X, Du Pont V, et al. . Remdesivir retains potent antiviral activity against the SARS-CoV-2 delta variant and other variants of concern. Presented at: International Society for Influenza and Other Respiratory Viruses (ISIRV), ISIRV-WHO Conference on COVID-19, Influenza and RSV: Surveillance-Informed Prevention and Treatment Virtual Conference 2021, 19–21 October 2021.
- 21. Takashita E, Yamayoshi S, Simon V, et al. . Efficacy of antibodies and antiviral drugs against omicron BA.2.12.1, BA.4, and BA.5 subvariants. N Engl J Med 2022; 387:468–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Food and Drug Administration . FDA announces bebtelovimab is not currently authorized in any US region. 2022. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-announces-bebtelovimab-not-currently-authorized-any-us-region. Accessed 7 February 2023.
- 23. National Institutes of Health . Coronavirus Disease 2019 (COVID-19) treatment guidelines. 2023. Available at: https://files.covid19treatmentguidelines.nih.gov/guidelines/archive/covid19treatmentguidelines-12-20-2023.pdf. Accessed 9 January 2023. [PubMed]
- 24. World Health Organization . Therapeutics and COVID-19: living guideline. Available at: https://iris.who.int/bitstream/handle/10665/362843/WHO-2019-nCoV-therapeutics-2022.5-eng.pdf. Accessed 14 January 2023. Therapeutics and COVID-19: living guideline. Available at: https://iris.who.int/bitstream/handle/10665/362843/WHO-2019-nCoV-therapeutics-2022.5-eng.pdf. Accessed 14 January 2023.
- 25. The National Institute for Health and Care Excellence (NICE) . COVID-19 rapid guideline: managing COVID-19. 2022. Available at: https://www.nice.org.uk/guidance/ng191. Accessed 9 January 2023. [PubMed]
- 26. Chalmers JD, Crichton ML, Goeminne PC, et al. . Management of hospitalised adults with coronavirus disease 2019 (COVID-19): a European respiratory society living guideline. Eur Respir J 2021; 57:2100048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Klann JG, Strasser ZH, Hutch MR, et al. . Distinguishing admissions specifically for COVID-19 from incidental SARS-CoV-2 admissions: national retrospective electronic health record study. J Med Internet Res 2022; 24:e37931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gilead Sciences . Veklury (remdesivir): prescribing information. 2023. Available at: https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. Accessed 27 March 2024.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.