Abstract
Introduction
Most patients on peritoneal dialysis (PD) in the United States are on automated PD (APD) utilizing several liters of PD solution daily for their treatment. The ordering, delivery, and storage of PD solutions can be challenging and is an important factor that can dissuade patients from doing PD. The generation of PD solutions at home is a strategy that could potentially be used to overcome this problem. The APD Solution Generation System (SGS) allowed for PD solution generation using tap water in patients’ homes.
Methods
In this study, we set out to evaluate the performance of the SGS in prevalent, adult patients with end-stage kidney disease, who are on maintenance PD. We evaluated the primary safety (microbiological testing) and efficacy (chemical composition) of the product water generated by the SGS device.
Results
Twenty-two patients from 12 different United States centers were enrolled, of which 14 patients completed the study. The results of the primary safety and efficacy end point analyses of the product water showed that all 64 samples met the International Organization for Standardization (ISO) specifications. Secondary safety analysis found a total of 34 adverse events (AEs) in 12 patients. Of these AEs, 3, namely, culture negative peritonitis, bacterial peritonitis, and atrial fibrillation were deemed serious treatment-emergent AEs.
Conclusion
This study demonstrated that the SGS can successfully generate PD solution in patients’ homes, while meeting chemical composition and ISO microbiological standards. Lessons learned from this clinical trial will be useful in optimizing product development and future clinical trials.
Keywords: automated peritoneal dialysis, peritoneal dialysis, Solution Generation System
Graphical abstract
Approximately 12% of all patients on dialysis in the United States are currently being treated with PD.1 Most of these patients are on APD, utilizing a cycler to deliver their treatments. PD solutions come prepackaged in bags, and patients use several liters of PD solutions during each daily dialysis therapy. These solutions contain variable amounts of glucose, all of which are hyperosmolar compared to plasma, thereby creating an osmotic gradient, which facilitates the transfer of fluid and solutes between the plasma and peritoneal cavity. Boxes containing bags of solution are often delivered to patients’ homes on a monthly basis. The ordering, delivery, and storage of these solutions can therefore be a challenge, impacting patients’ living spaces and lifestyle. Lacking enough storage space for supplies, particularly in urban settings, is an important factor that can dissuade patients from choosing PD.2
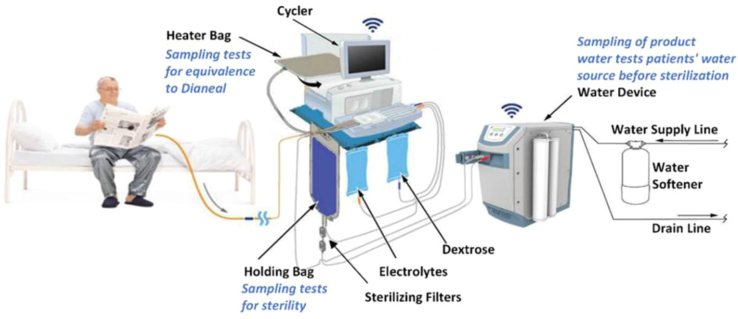
Generation of PD dialysis solutions at home is a strategy that could potentially be used to overcome this problem. The APD SGS developed by Baxter International Inc. for this proof-of-concept study allowed for PD solution generation using tap water in patients’ homes. The system consisted of the Amia APD Cycler, PD fluid concentrates (dextrose concentrate and electrolyte concentrate), a disposable set (containing a cassette, water line with holding bag and 2 sterilizing grade filters, heater bag, patient line, dextrose concentrate line, last fill solution line, electrolyte concentrate line, and a drain line), a bag tray, a water softener (also referred to as ion exchanger), and a commercial dialysis water treatment device (WD) (Figure 1). The WD consisted of a pretreatment filter pack, reverse osmosis membrane, heater, conductivity and temperature sensors, and ports for patient connection. The water softener was an accessory to the WD.
Figure 1.
APD Solution Generation System (SGS) setup.
The SGS used a study-specific version of the Amia APD Cycler with Baxter’s Sharesource platform, an online platform that reports patient treatments and events, with the additional capability of managing the SGS used in the study. The system was designed to produce dialysis solution as prescribed by the clinician and formulated to have a nominal solution chemistry equivalent to the composition of the factory manufactured Dianeal Low Calcium (2.5 mEq/l) bags, which come in 3 standard dextrose concentrations as follows: 1.5%, 2.5%, and 4.25%.
In this study, we set out to evaluate the performance of the SGS in prevalent, adult patients end-stage kidney disease, who are on maintenance PD.
Methods
This is a multicenter open label, single arm, prospective, descriptive study, undertaken to evaluate the performance of the APD SGS under a United States Food and Drug Administration Investigational New Drug Application (IND# 141130). Institutional review board approval was received before study initiation, and the study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki, the International Conference of Harmonization Good Clinical Practice, applicable United States Code of Federal Regulations, and all applicable regulatory requirements and laws, including for the archival of essential documents. This study’s reporting was designed to be consistent with the principles established by the CONSORT 2010 statement extension to randomized pilot and feasibility trials, as adapted for the reporting of nonrandomized intervention development studies.3,4
The primary efficacy objective of this study was to evaluate the chemical composition of the final dialysis solution produced by patients using the SGS during simulated treatment, compared to the specifications of Baxter’s approved Dianeal Low Calcium PD Solution.
The primary safety objective of this study was to evaluate whether the product water from the WD meets ISO standard for microbiological (including endotoxin) and chemical contamination,5 and whether water in the holding bag meets system microbiological requirements, when produced in patients’ homes using the SGS, for a simulated treatment.
The secondary objective of this study was to evaluate the safety of the Amia APD SGS while treating patients with end-stage kidney disease, by collecting data on reported incidents of AEs, serious AEs, adverse device events, serious adverse device events, incidence of device alarms, and vital signs, and assessing the PD adequacy by calculating total Kt/Vurea.
Detailed inclusion and exclusion criteria, study plan, and statistical methods can be found in the Supplementary Material.
Results
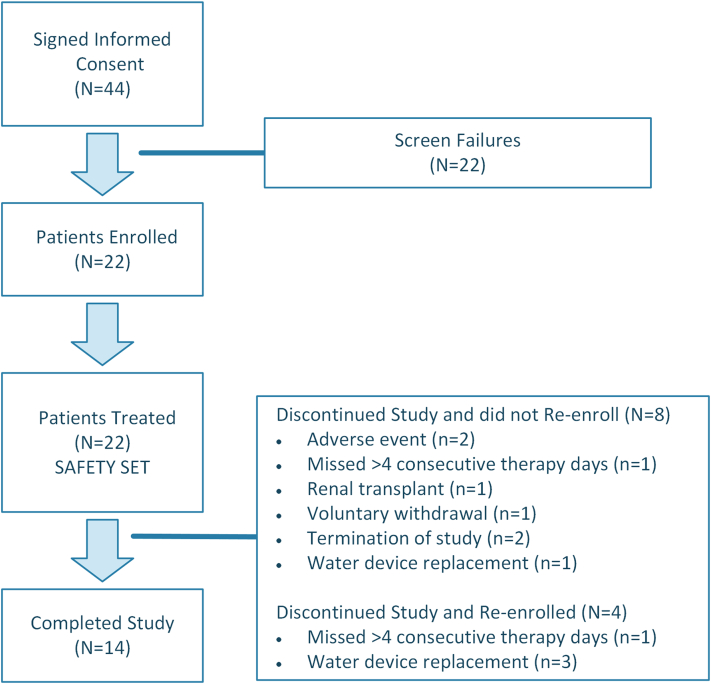
Twenty-two patients from 12 different United States centers were enrolled, of which 14 patients completed the study. Patients were followed-up with for 12 weeks; disposition of the enrolled patients is shown in Figure 2. The study was terminated early (14 months), before reaching the planned patient enrollment. Safety concerns were not a reason for early termination of the study, but rather ongoing operational and recruiting challenges, which were in part caused by the COVID-19 pandemic. The demographics and baseline characteristics of the patients are available in Supplementary Table S1.
Figure 2.
Enrollment and disposition of patients.
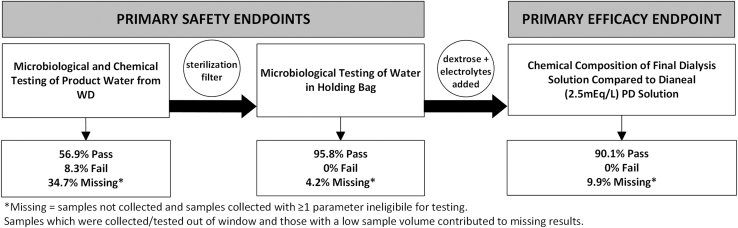
The results of the primary safety (microbiological testing) and efficacy (chemical composition) end point analysis of the product water showed that out of the 71 samples collected, all 64 tested samples that had results available, met ISO standard specifications (Figure 3). The expected composition of the Amia APD generated solution is shown in Supplementary Table S2. For the chemical composition of the dialysis solution, all 64 samples that were tested met specifications (Table 1).
Figure 3.
Results of primary safety and efficacy endpoints. PD, peritoneal dialysis.
Table 1.
Primary efficacy end point: results of overall testing of the chemical composition of the dialysis solution
| Visit | Total tests | Met specifications, n (%) | Did not meet specifications, n (%) | Missing resultsa, n (%) |
|---|---|---|---|---|
| All visits | 71 | 64 (90.1) | 0 (0) | 7 (9.9) |
| Visit 1 (1.5% dextrose) | 23 | 21 (91.3) | 0 (0) | 2 (8.7) |
| Visit 2 (2.5% dextrose) | 20 | 19 (95.0) | 0 (0) | 1 (5.0) |
| Visit 3 (4.25% dextrose) | 14 | 12 (85.7) | 0 (0) | 2 (14.3) |
| Visit 4 (1.5% dextrose) | 14 | 12 (85.7) | 0 (0) | 2 (14.3) |
Missing results comprised: (i) samples that were never collected and (ii) samples that were collected, but had at least 1 parameter that was not able to be analyzed.
For individual parameter testing of the chemical composition of the dialysis solution (primary efficacy end point), all parameters for which a sample was able to be analyzed met specifications for all tests and visits (Supplementary Table S3). There were 2 missing results for each of the following parameters: 5-hydroxymethylfurfual, color, and sodium. There were 3 missing results for dextrose hydrous assay.
With the exception of 1 patient, all patients maintained dialysis treatment adequacy with total Kt/Vurea values ≥ 1.7 as recommended by the International Society for Peritoneal Dialysis6 (Supplementary Table S4).
The results of the primary safety and sensitivity analysis of microbiological and chemical testing of the product water from the WD (presterilizing filter) showed that out of a total of 72 tests which were available, 41 (56.9%) met specifications, 6 (8.3%) did not meet specifications, and 25 (34.7%) results were missing (Table 2 and Supplementary Table S5).
Table 2.
Primary safety end point: microbiological and chemical testing of the product water from the water device
| Visit | Total tests | Met specifications, n (%) | Did not meet specifications, n (%) | Missing resultsa, n (%) |
|---|---|---|---|---|
| All visits | 72 | 41(56.9) | 6 (8.3) | 25 (34.7) |
| Visit 1 | 24 | 9 (37.5) | 0 (0) | 15 (62.5) |
| Visit 2 | 20 | 12 (60.0) | 3 (15.0) | 5 (25.0) |
| Visit 3 | 14 | 11 (78.6) | 1 (7.1) | 2 (14.3) |
| Visit 4 | 14 | 9 (64.3) | 2 (14.3) | 3 (21.4) |
Missing results comprised: (i) samples that were never collected and (ii) samples that were collected, but had at least 1 parameter that was not able to be analyzed.
The primary safety analysis of microbiological testing of the product water from the holding bag showed that out of a total of 71 tests that were available for most recent results, 68 (95.8%) met specifications, and 3 (4.2%) results were missing (Table 3). There were no tests that did not meet specifications.
Table 3.
Primary safety end point: microbiological testing of the water from the holding bag
| Visit | Total tests | Met specifications, n (%) | Did not meet specifications, n (%) | Missing resultsa, n (%) |
|---|---|---|---|---|
| All visits | 71 | 68 (95.8) | 0 (0) | 3 (4.2) |
| Visit 1 (1.5% dextrose) | 23 | 23 (100.0) | 0 (0) | 0 (0) |
| Visit 2 (2.5% dextrose) | 20 | 20 (100.0) | 0 (0) | 0 (0) |
| Visit 3 (4.25% dextrose) | 14 | 13 (92.9) | 0 (0) | 1 (7.1) |
| Visit 4 (1.5% dextrose) | 14 | 12 (85.7) | 0 (0) | 2 (14.3) |
Missing results comprised: (i) samples that were never collected and (ii) samples that were collected, but had at least 1 parameter that was not able to be analyzed.
Secondary safety analysis revealed a total of 34 AEs in 12 patients (Table 4). Of these, the following 3 events in 3 patients were deemed serious treatment-emergent AEs: culture negative peritonitis, bacterial peritonitis, and atrial fibrillation. None of the treatment-emergent AEs led to death; however, they led to the withdrawal of 2 patients from the study, both due to peritonitis which were considered as possibly associated with the study device.
Table 4.
Summary of AEs
| Parameter | Number of patients (N = 22), n (%) | Number of events | Number of events per patient year |
|---|---|---|---|
| Any AE (serious or nonserious) | 12 (54.5) | 34 | 4.33 |
| Any TEAEs | 8 (36.4) | 25 | 5.32 |
| Any serious TEAEs | 3 (13.6) | 3 | 0.64 |
| Required or prolonged hospitalization | 2 (9.1) | 2 | 0.43 |
| Required intervention to prevent permanent impairment/damage (devices) | 1 (4.5) | 1 | 0.21 |
| Other important medical event | 1 (4.5) | 1 | 0.21 |
| Any study device-related TEAEsa | 2 (9.1) | 2 | 0.43 |
| Any typical PD therapy-related TEAEsa | 4 (18.2) | 11 | 2.34 |
| Any other Baxter product-related TEAEsa | 1 (4.5) | 1 | 0.21 |
| Any study device-related treatment-emergent SAEsa | 2 (9.1) | 2 | 0.43 |
| Any typical PD therapy-related treatment-emergent SAEsa | 2 (9.1) | 2 | 0.43 |
| Any other Baxter product-related treatment-emergent SAEsa | 1 (4.5) | 1 | 0.21 |
| Any TEAEs of special interest | 2 (9.1) | 2 | 0.43 |
| Any TEAEs leading to withdrawal | 2 (9.1) | 7 | 1.49 |
| Any TEAEs leading to death | 0 | 0 | 0 |
AE, adverse event; PD, peritoneal dialysis; SAE, serious adverse event; TEAE, treatment-emergent adverse event.
An AE is considered “related” if the AE relation is assessed by the investigator as “probably associated” or “possibly associated.”
Device deficiencies (DDs), which included product or device malfunction or failure, were noted in many patients. There were 96 DDs in 20 patients and 14 device alarms in 6 patients (Table 5 and Supplementary Table S6). However, the number of DDs per day on the device was low at 0.05/d and none of the DDs led to any AE. The most common DDs were related to the WD and the Amia APD Cycler.
Table 5.
Summary of device deficiencies and alarms
| Parameter | Number of patients (N = 22), n (%) | Number of events | Number of events per total device days |
|---|---|---|---|
| Any device deficiency | 20 (90.9) | 96 | 0.05 |
| Any device alarm | 6 (27.3) | 14 | 0.01 |
Discussion
This study demonstrated that the SGS can successfully generate PD solution in patients’ homes, while meeting chemical composition and ISO microbiological standards. Similar systems have been tested and are currently in use for home hemodialysis; however, this is the first device of its kind to be used in PD. This approach has the potential to increase PD utilization, while reducing storage space requirements and ordering burden for patients, supply activities and expenditures for providers, used consumables, and potentially overall cost of treatment. SGS could also help with supply chain issues like many faced during the pandemic. Beyond saving storage space and bypassing potential supply problems, this device offers the possibility of creating dextrose solutions with variable concentrations, allowing a more individualized therapy in contrast to the current system that limits the patient to use only the following 3 prefixed dextrose concentration solutions: 1.5%, 2.5%, and 4.25%.
There were several limitations in the design and conduct of this study. With regard to study design, this study involved patients to be limited in terms of any travel during the study and required significant time commitment. It is important to remember that PD patients tend to be more independent, enjoy travel, and employed. Therefore, even though an attempt was made to design the trial in a patient-centric manner, these requirements made it difficult to recruit, retain, and follow the protocol procedures. Because the clinical trial was practically carried out in the patients’ homes, this added to the operational complexity with reliance on coordination of schedules between the study sponsor, clinical trial vendors, the investigational site, and the patients while respecting and protecting patient privacy. Attempts to maximize patientricity contributed to protocol deviations and missing samples.
The main limitation originated from the complexities surrounding valid sample collection. Beyond the necessary coordination of availability of all parties involved, the window to obtain, ship, and test samples for the end points was very narrow and resulted in either missing samples or invalidation of results due to unforeseen circumstances (weather, flight delay, courier delay, out-of-range temperature excursions, etc.). Lastly, although central laboratories were used to analyze the end points, there were instances when, even though study samples were received, broken equipment or incorrect procedures caused some samples to be excluded, leading to missing results.
Although sufficient for an early feasibility study to establish proof-of-concept, this study also demonstrated the need for a better alignment between system design and usability with practical realities of home care in order to enable improvements toward effective clinical trial execution and eventual real-world experiences of potentially transformative devices such as the SGS. Challenges with product water sampling and testing were other major factors which contributed to suboptimal results in the intent-to-treat analysis. Lessons learned from this clinical trial will be instrumental in informing optimal product development and successful clinical trials in the future.
Disclosure
OES is a consultant for Outset Medical and Taylor Advisory. AW, BK, HT, and PR are employed by Baxter International Inc. NA has grants or contracts from NIH, Novartis, Otsuka, and Aztrazeneca. All the other authors declared no competing interests.
Acknowledgments
We thank the principal investigators at all the study sites, Dr. Divya Monga, Dr. Vesh Srivatana, Dr. Andres Serrano, Dr. Clinton Edwards, Dr. Mohanram Narayanan, Dr. Wael Hussein, Dr. Balazs Szamosfalvi, and Dr. Richard Fuquay for recruiting patients and conducting all aspects of the study at their respective sites. This study was funded by Baxter Healthcare Corporation.
Footnotes
Supplementary Methods.
Table S1. Demographics and baseline characteristics of enrolled patients.
Table S2. Composition of Amia APD generated solutions.
Table S3. Individual parameter testing of the chemical composition of the dialysis solution.
Tabe S4. Individual parameter summary of microbiological and chemical testing of the product water from the water device.
Table S5. Secondary efficacy end point: total Kt/V urea summary.
Table S6. Summary of device deficiency characteristics.
CONSORT Checklist.
Supplementary Material
Supplementary Methods. Table S1. Demographics and baseline characteristics of enrolled patients. Table S2. Composition of Amia APD generated solutions. Table S3. Individual parameter testing of the chemical composition of the dialysis solution. Table S4. Secondary efficacy end point: total Kt/V urea summary. Table S5. Individual parameter summary of microbiological and chemical testing of the product water from the water device. Table S6. Summary of device deficiency characteristics. CONSORT Checklist.
References
- 1.Johansen K.L., Chertow G.M., Gilbertson D.T., et al. US Renal Data System 2021 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2022;79(Suppl 1):A8–A12. doi: 10.1053/j.ajkd.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Shamy O., Muller T., Tokita J., Cummings Y., Sharma S., Uribarri J. Home Dialysis: A majority Chooses It, a Minority Gets It. blood Purif. Blood Purif. 2021;50:818–822. doi: 10.1159/000512539. [DOI] [PubMed] [Google Scholar]
- 3.Eldridge S.M., Chan C.L., Campbell M.J., et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355 doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lancaster G.A., Thabane L. Guidelines for reporting non-randomised pilot and feasibility studies. Pilot Feasibility Stud. 2019;5:1–6. doi: 10.1186/s40814-019-0499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Organization for Standardization . International Organization for Standardization; 2014. Water for Haemodialysis and Related Therapies.https://www.iso.org/standard/61862.html 2014. Accessed March 28, 2024. [Google Scholar]
- 6.Peritoneal Dialysis Adequacy Work Group Clinical practice guidelines for peritoneal adequacy, update 2006. Am J Kidney Dis. 2006;48(suppl 1):S91–S97. doi: 10.1053/j.ajkd.2006.05.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. Table S1. Demographics and baseline characteristics of enrolled patients. Table S2. Composition of Amia APD generated solutions. Table S3. Individual parameter testing of the chemical composition of the dialysis solution. Table S4. Secondary efficacy end point: total Kt/V urea summary. Table S5. Individual parameter summary of microbiological and chemical testing of the product water from the water device. Table S6. Summary of device deficiency characteristics. CONSORT Checklist.