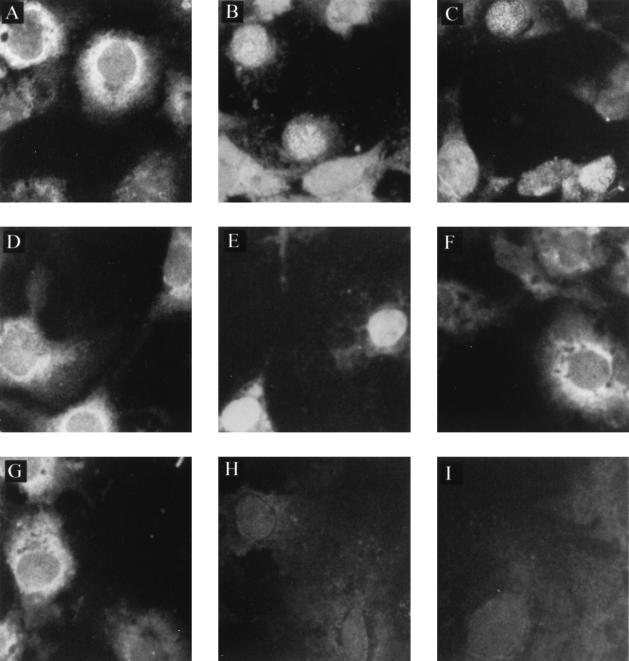
FIG. 3.
Subcellular localization of p10 and p10 deletion mutants in Cos-7 cells cotransfected with p24P. Cos-7 cells (American Tissue Culture Collection, Rockville, Md.) were cultured at a concentration of 2.5 × 105 cells per 35-mm-diameter tissue culture, in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin (Life Technologies, Gaithersburg, Md.) per ml. After overnight culture at 37°C to achieve a 50 to 60% confluency, the cells were transfected with plasmid DNA by the use of Lipofectamine (Life Technologies) according to the manufacturer's protocol. At 48 h posttransfection, aliquots of cells were spotted onto slides and left at 37°C for 16 h, such that the same transfected culture could be assayed for more than one protein by immunofluorescence. Cells were then fixed and permeabilized on the slides with 3.7% formaldehyde for 20 min followed by 0.5% Triton for 10 min. The Cos-7 cells were transfected with the pcORFx1-FLAG (10 μg) alone (A), the pcDL-p24 plasmid (20 μg) alone (B and I), or with both constructs (C). The Cos-7 cells were also transfected with pc1-51-FLAG alone (D) or pc52-261-FLAG alone (F) or cotransfected with pc1-51-FLAG plus pcDL-p24 (E) and pc52-261-FLAG plus pcDL-p24 (G). Mock-transfected cells (H) served as control. The cells were stained with the anti-FLAG monoclonal antibody (A and C to I) or the p24-specific antiserum (B). A fluorescein isothiocyanate-conjugated protein G (Sigma) was used to detect the staining under an epifluorescence microscope. Magnification, ×400.