Abstract
Objectives
Resistance to transportation and stressful veterinary visits are major causes for a decrease in feline veterinary care. Few options exist for oral sedatives to reduce cats’ anxiety prior to veterinary visits. The purpose of this study was to evaluate the safety and efficacy of oral trazodone for use as a single dose agent for sedation in cats.
Methods
Six laboratory cats were given single 50, 75 and 100 mg doses of trazodone and placebo. Trazodone 100 mg and placebo treatments were randomized. Pre- and post-study laboratory values and physical examinations were compared. During each 4 h period post-treatment, sedation was measured via accelerometers and video observations scored by an observer blinded to treatment. Examinations were performed on the cats 90 mins after treatment, and their behavioral responses scored by the same blinded observer.
Results
No adverse effects or changes in physical examinations or laboratory values were detected as a result of trazodone administration. Accelerometer data showed trazodone 50, 75 and 100 mg caused sedation as measured by activity reduction (83%, 46% and 66%, respectively). In contrast, there was a 14% activity increase after placebo. There was a significant reduction in video observation scores when cats were given trazodone 100 mg compared with placebo. Mean latency to peak sedation for trazodone 100 mg occurred at 2 h. Scores for behavioral response to examination, performed at 90 mins post-treatment, were not significantly different between cats receiving trazodone 100 mg and placebo.
Conclusions and relevance
Trazodone was well tolerated in this population of cats and caused appreciable sedation at all doses. Behavior during examination was not significantly different when cats received trazodone 100 mg compared with placebo. Further studies are recommended to investigate the use of oral trazodone in cats for the purpose of decreasing anxiety assocaited with transportation and examination.
Introduction
The latest census on pets in the USA shows that only half of all owned cats receive annual veterinary care, a decrease of 13.5% since 2006. 1 One of the major factors contributing to this decline is cats’ resistance to transportation and examination. 2 Many cats struggle against being placed in their carriers, and may even become aggressive to their owners. After the combined stressors of confinement and transportation, cats can be in a state of high arousal or anxiety, increasing the possibility of resistance and aggression during their examination. Handling difficult or fractious patients may preclude a thorough examination or lead to increased risk of injury to veterinary staff. 3 Physical parameters and laboratory values can be altered by anxiety and struggling, making interpretation of the data difficult for the veterinarian.4,5 These factors may result in decreased quality of patient care. Furthermore, many owners become distressed by their cat’s anxiety and may decide that the perceived traumatic event of the veterinary visit is more harmful to the cat’s health than the lack of veterinary care. 6
Recent efforts in veterinary practice have sought to decrease stress and increase the quality of care for feline patients.7,8 However, despite the best intentions of the veterinary care team, there still remain cats that are highly anxious prior to entering the veterinary hospital. The synthetic pheromone Feliway (Ceva) has shown variable success in reducing stress in the hospital or transportation setting.9–12 The few oral pharmacologic options that exist for cats to reduce the stress associated with veterinary visits have limitations in safety or anxiolytic effects.13–16 There is a critical need for a safe, oral sedative that can be administered by owners prior to confinement and transportation that will prevent or reduce the cascade of anxiety leading up to the veterinary visit and facilitate ease of examination.
The drug trazodone hydrochloride holds promise for this use in cats. Classified as a serotonin antagonist and reuptake inhibitor, trazodone has long been used in humans as an antidepressant, anxiolytic and hypnotic agent. 17 Trazodone has been documented as a safe and effective sedative and anxiolytic medication in canine patients.18–21 However, there are no published reports on oral trazodone’s sedative or anxiolytic effects in cats. The objectives of this study were to determine the safety and efficacy of oral trazodone for use as a sedative and anxiolytic agent in cats. We hypothesized that oral trazodone would be well tolerated and would produce mild sedation, as measured by reduced activity and decreased stress during examinations. The eventual outcome may be the use of trazodone to reduce distress from transportation and veterinary visits in feline patients and therefore increase owner presentation of cats to the veterinary hospital.
Materials and methods
Animals
The subjects were six purpose-bred, neutered male domestic shorthair laboratory cats aged 6 months (one cat, weighing 3.0 kg) to 3–5 years (five cats, weighing 4.3–4.7 kg). The cats were individually housed in wire enclosures (188 cm high × 147 cm deep × 91 cm wide) in a single room within a laboratory animal facility. Each enclosure was furnished with an elevated shelf, a ‘hide’ box, litter pan, water bowl and toys. Cats were managed on a 12/12 h light/dark cycle, fed a measured feline dry diet (Hill’s Science Diet Adult Light) twice daily and maintained at an ideal body condition score (5/9), as referenced on a standard score chart (Purina Body Condition System, https://www.purinaveterinarydiets.com/media/1209/body_condition_chart.pdf). The five adult cats had previously been used as control subjects for a separate study prior to enrollment in the current investigation. These cats had been conditioned to entering a carrier voluntarily during their previous study. 22 The juvenile cat was recently acquired by the facility and had not received any prior conditioning. Housing and study protocols were approved by the North Carolina State University Institutional Animal Care and Use Committee.
Drug safety
Each cat received a pre-enrollment physical examination, chemistry panel, complete blood count and urinalysis. After the study, the physical examinations and laboratory tests were repeated in order to rule out any changes attributable to administration of trazodone.
Trazodone dosage and administration
Each cat served as its own control, receiving four treatments over a 4 week period: trazodone 50, 75 and 100 mg PO (10.6–16.7 mg/kg, 16.0–25 mg/kg and 21.3–33.3 mg/kg doses, respectively) and one placebo. These doses were chosen based on the commercial availability of trazodone, manufactured as 50 and 100 mg tablets. All cats were given the 50 mg dose at the time of the first treatment and the 75 mg dose at the second treatment. This was done as a precaution owing to the lack of previous trazodone safety data available and concerns about serotonin syndrome, which has been reported in cats. 23 The third and fourth treatments were randomized to be either the 100 mg dose or placebo.
Treatments were administered between 10:30 am and 1:00 pm, with doses staggered such that each cat received medication 90 mins prior to examination. There was a washout period of 4–7 days between treatment days. Trazodone tablets were cut into quarters and hidden in 1 tablespoon of canned food (Hill’s Prescription Diet a/d) or malleable treats (Pill Pockets). This was done rather than ‘pilling’ the cats so as to reduce any stress associated with forced tablet administration. For the trazodone 50 and 75 mg treatments, a 50 mg tablet was used (four quarters and six quarters, respectively). For the 100 mg treatment, a 100 mg tablet was used, also divided into quarters. For the placebo treatment, food or treats were given to the subjects with no tablet. On days when trazodone or placebo was administered, the cats were fed a reduced portion meal (1/8 cup) in the morning, approximately 3 h prior to the treatment. This was done to reduce the amount of food in the upper gastrointestinal tract that might delay absorption of trazodone. 24 It also ensured the cats’ hunger and motivation to eat food or treats containing medication voluntarily. The remainder of the cats’ daily ration was given after completing observations for the day.
Accelerometer activity monitors
In order to record locomotor activity as a measure of sedation in their enclosures, each cat was fitted with a pre-equilibrated accelerometer activity monitor (Actical; Mini Mitter) attached to its collar. The monitor was applied 1 week prior to initiation of the study to allow a period of acclimation to the device. The accelerometer was worn and data collected for the 4 week duration of the study. Monitors were set to record at intervals of 30 s. Each monitor’s time stamp was synchronized with the local time (Eastern Standard Time). At the end of the study, data were downloaded to a personal computer with a telemetric reader.
Activity observations
As a second measure of sedation, focal observational samples of the cats’ activity in their enclosures were recorded by a handheld digital video camera. The recordings were made prior to administering treatment (trazodone or placebo) and every 30 mins for 4 h after treatment. Each cat was given an Enclosure Activity Score from 1 (sedate) to 5 (very active) for each time interval. (See supplementary material Appendix 1 for score criteria.)
Examination
Examinations were performed 90 mins after administration of each treatment (trazodone or placebo). Treatment was staggered for each cat by 30 mins to allow adequate time for each cat’s examination. Prior to removing the cat from its enclosure for examination, a carrier was placed outside its door. The enclosure door was opened and the cat was given the opportunity to walk into the carrier freely. If the cat chose to not enter the carrier, a treat was tossed inside the carrier as an incentive. If the cat did not enter for a treat, it was gently picked up and placed at the opening of the carrier and guided inside. After being enclosed in the carrier, the cat was carried to the examination room, located 35 m down the hall from the room where the cats were housed.
Once in the examination room, the carrier was placed on an examination table, and the cats were given the option to exit the carrier willingly or for a treat lure. If the cat did not exit voluntarily, the top of the carrier was removed. If the cat resisted removal from the bottom half of the carrier, it was left in place. Each step of the physical examination was performed in a predetermined order, starting with less invasive components and finishing with procedures that required more restraint.
Auscultation of the heart and lungs were performed first, followed by peripheral lymph node palpation, abdominal palpation, otoscopic examination, ophthalmoscopic examination, oral examination, mock jugular venepuncture in a sternal position and finishing with mock cystocentesis performed in lateral recumbency. For the mock venepuncture and cystocentesis, the needle of the syringe was placed intradermally or subcutaneously. All cats were handled with minimal restraint. If needed, a towel and/or technician assistance were employed. Treats were also given to all cats to encourage compliance. If a cat became too fractious, the examination was discontinued and the cat returned to its enclosure. At the end of the examination, each cat was given the opportunity to enter its carrier voluntarily for return to its respective enclosure. All examinations and behavioral ratings were performed by an investigator masked to treatment (JMO).
Behavioral response to examination
For each step of the examination (removal from carrier, auscultation, etc), cats were given a behavioral response score (modified from Rand et al). 5 The cats were scored for six different behaviors (vocalization, struggling, aggression, hypersalivation, immobility response and open-mouth breathing) on a scale of 0–4 (0 = none, 1 = mild, 2 = moderate, 3 = marked, 4 = too severe to complete examination). Scores for each behavior for each step of the examination were combined for a total behavioral response score. This was performed for each cat for each treatment.
Stress measurement
Cats were assessed for stress at three time points: while in their carriers prior to the examination (pre-exam), out of the carrier during the examination (exam) and in their carrier after examination (post-exam). Stress was measured using McCune’s cat stress assessment scale ranging from 1 (relaxed) to 7 (terrorized). 25 (See supplementary material Appendix 2 for scale description.) Stress scores for pre-exam, exam and post-exam time points were combined for an overall stress score. This was performed for each cat for each treatment.
Statistical analysis
Data were tested for statistical significance using SAS version 9.3 (SAS Institute). Because the 50 mg and 75 mg doses of trazodone were not randomized, the data obtained during these treatment days were not used for statistical analysis of dose effects. Data from trazodone 100 mg and placebo treatments were compared. Non-parametric statistics were used for ordinal data; after testing for normality, parametric statistics were used for continuous data. Physiological parameters (heart rate, respiratory rate), total behavioral response scores during examination and overall stress scores were analyzed using non-parametric Wilcoxon signed rank tests. Accelerometer data were blocked into half-hour increments from the time of treatment administration to 4 h post-treatment. Using non-parametric one-way ANOVA in order to find the largest decrease in activity, each half-hour time block was compared with the previous half-hour block. The time at which the largest decrease in activity occurred was referred to as time of peak sedation. In addition, the first 2 h of activity were compared with the second 2 h of activity in order to determine the percentage change in activity. Enclosure activity scores were analyzed for a 2-point reduction. A Fisher’s exact test was used to compare the incidence of 2-point score reduction between trazodone 100 mg and placebo. The time of the lowest enclosure activity score was referred to as time of peak sedation. Enclosure activity scores were compared with accelerometer data using one-way ANOVA. Because of the small sample size, a P value <0.1 was considered significant. 26
Results
Drug safety
All cats were medicated successfully, except one cat, which failed to ingest all food and medication at the 100 mg dose. No adverse effects such as anorexia, vomiting, diarrhea, ataxia, tremor, paradoxical excitation or behavioral disinhibition were observed in any cat throughout the course of the study. Physical examination of the cats at the conclusion of the study revealed no abnormalities. Laboratory values obtained at the end of the study were not appreciably different from values obtained prior to the onset of the study. Heart rates (Wilcoxon signed rank test, n = 5; P = 0.5) and respiratory rates were not significantly affected by trazodone 100 mg compared with placebo (Wilcoxon signed rank test, n = 4; P = 0.375). In some instances, owing to the cat’s aggression, heart and respiratory rates could not be obtained.
Accelerometer activity monitors
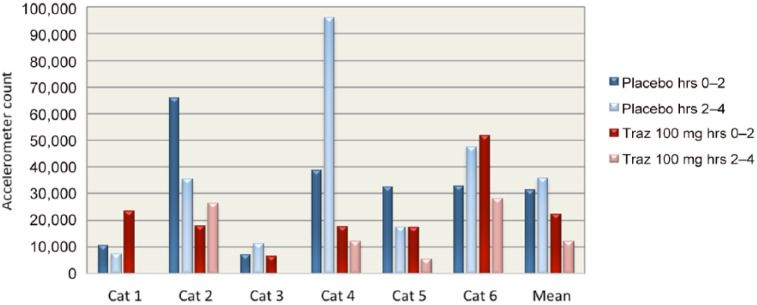
Accelerometer data showed substantial decreases in activity in cats after receiving trazodone. Cats were less active after receiving trazodone 100 mg compared with placebo for every half-hour increment after treatment was administered during the 4 h study window, with the exception of the first half-hour block when treatments were administered (see Figure 1). Peak sedation occurred at 2.5 h post-treatment with trazodone 100 mg. When accelerometer data were divided into the first 2 h post-treatment and the second 2 h post-treatment, cats had a decrease in mean activity on all doses of trazodone (83% with 50 mg, 30% with 75 mg and 46% with 100 mg) during the second 2 h. On placebo, cats had an increase in mean activity of 14% in the second 2 h of observation (see Figure 2). Cats were 66% less active during the second 2 h after trazodone 100 mg compared with the second 2 h after placebo (see Figure 3). Individual subject response to trazodone 100 mg and placebo are displayed in Figure 4.
Figure 1.
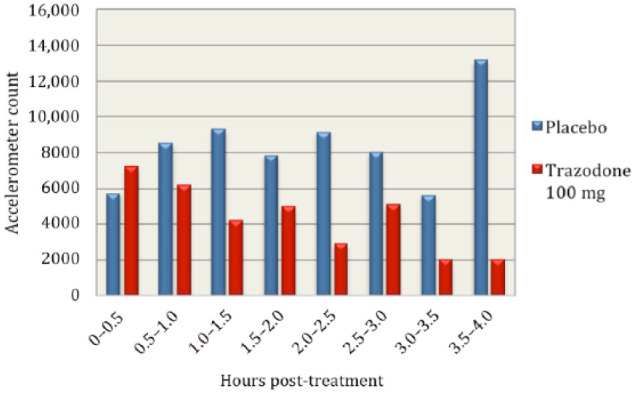
Mean accelerometer activity counts for all cats divided into half-hour increments for 4 h post-treatment with trazodone 100 mg and placebo
Figure 2.
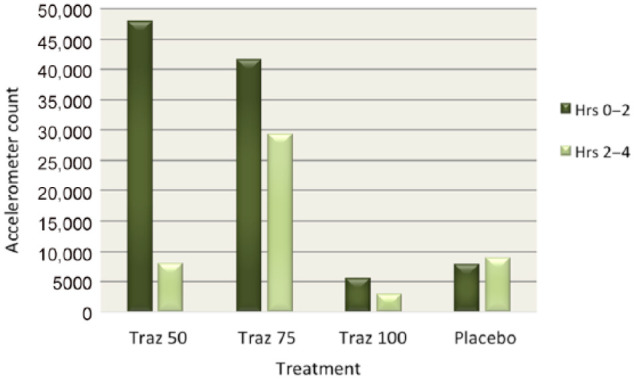
Mean accelerometer activity counts for all cats divided into the first 2 h post-treatment and the second 2 h post-treatment for all 50, 75 and 100 mg doses of trazodone (Traz) and placebo
Figure 3.
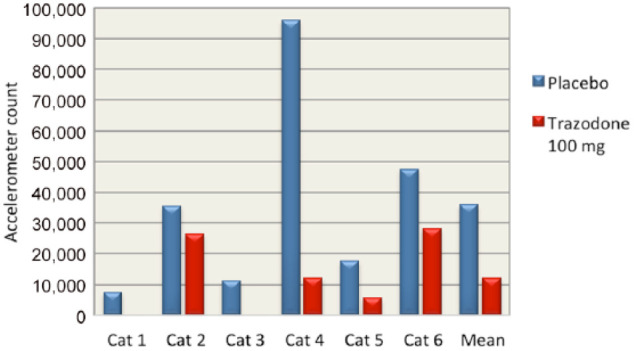
Total accelerometer activity count for each cat during the second 2 h post-treatment with trazodone 100 mg and placebo. The mean for all cats is also provided
Figure 4.
Total accelerometer activity count for each cat when given trazodone (Traz) 100 mg and placebo. Activity is divided into the first 2 h post-treatment and the second 2 h post-treatment. The mean for all cats is also provided
Activity observations
Enclosure activity scores of 1 (‘sedate’) and 2 (‘calm’) were only observed in cats when they received trazodone (at any of the doses). No cats received scores of 1 or 2 when they received placebo. Four of the six cats had a ⩾2-point reduction in their observed enclosure activity when given trazodone 100 mg. The times to the 2-point reduction occurred at 1.0, 1.5, 2.5 and 3.0 h post-treatment. Mean enclosure activity scores for cats receiving trazodone 100 mg and placebo are displayed in Figure 5. There was a statistically significant difference between the enclosure activity scores of cats when they received trazodone 100 mg compared with placebo (Fisher’s exact test, n = 12; P = 0.06). There was a highly significant positive correlation between the enclosure activity scores and the accelerometer counts (one-way ANOVA, df = 4, f = 3.51; P = 0.0095).
Figure 5.
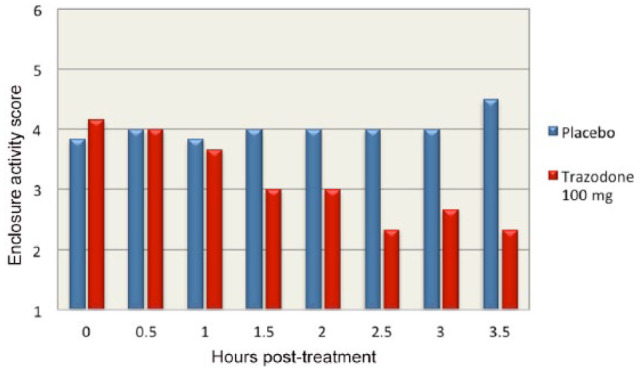
Mean enclosure activity score for all cats divided into half-hour increments for 4 h post-treatment with trazodone 100 mg and placebo
Behavioral response to examination
Total behavioral response scores given for each cat reflected only struggling, aggression and vocalization. Hypersalivation, open-mouth breathing and immobility were not observed during examination in any of the cats with any trazodone or placebo treatments. The scores revealed no significant difference between cats that received trazodone 100 mg and placebo (Wilcoxon signed rank test, n = 6; P = 0.594). A separate analysis of the behavioral response score for aggression and struggling alone were analyzed. Even with vocalization excluded, there was no difference between the trazodone 100 mg and placebo treatments (Wilcoxon signed rank test, n = 6; P = 0.844).
Stress measurement
The mean overall stress score for before, during and after examination was 2.4 out of 7.0 for cats that received trazodone 100 mg and for cats that received placebo. Therefore, there was no difference between the trazodone 100 mg and placebo groups for the overall stress scores recorded before, during and after the examinations (Wilcoxon signed rank test, n = 6; P = 1.000).
Discussion
The results of this study demonstrate that oral trazodone at single doses of 50, 75 and 100 mg/cat was well tolerated. None of the cats in this study showed any laboratory abnormalities, adverse effects or signs of serotonin syndrome. With regard to cat stress and behavior during examination, trazodone did not produce an effect, either positive or negative, based on the behavioral response and overall stress scores. This could be explained by the timing of the examinations. In our study, examinations occurred 90 mins after dosing. The enclosure activity score data showed that the mean largest reduction in activity occurred at 2 h after treatment with trazodone 100 mg. The accelerometer data showed that the median lowest activity level occurred at 2.5 h post-trazodone 100 mg. Thus, these findings suggest that the examinations were performed before the time of peak sedation. A recent pharmacokinetics study of single-dose trazodone in dogs showed that trazodone achieved mean peak plasma concentrations at 445 ± 271 mins after oral dosing, illustrating a wide range of individual response times. 21 Although trazodone plasma concentrations were not measured in our study, the accelerometer data suggest that peak plasma concentrations occur later than 90 mins post dosing, with a wide range of peak sedation times (1.0, 1.5, 2.5 and 3.0 h after dosing).
It is also possible that the scoring system used for behavioral response to examination was not adequate. Three of the six behaviors (hypersalivation, immobility response and open-mouth breathing) were not observed at any point in the study. Vocalization did not appear to coincide with distress and was, in fact, more frequently observed to occur with affiliative or playful interactions with the investigators. Thus, only changes in aggression and struggling behaviors, scored on a 0–4 Likert scale, were measured. This was likely insufficient for detecting any effects of trazodone on behavior.
There was a high degree of variability in individual cats’ activity level, as well as their responses to trazodone, as depicted in Figure 4. In this figure it is important to note that cat 2 showed an increase in activity after the administration of trazodone 100 mg. This cat had been difficult to medicate over the course of the study and was recorded to have not ingested all of its food or the full 100 mg trazodone dose. The increased activity was suspected to be due to the incomplete dose or could reflect this cat’s individual response to treatment.
Cats used in this study had already been conditioned to entering their carriers for a previous study. 22 Therefore, they did not exhibit any signs of stress in response to seeing or hearing their carriers, with placement in the carrier or with transportation while inside the carrier. Carrier conditioning eliminated our ability to determine the effects of trazodone on transportation distress, one of the major causes for feline anxiety related to veterinary visits.
The most notable effect of trazodone in this study was on activity. Locomotor activity has been used as a measure of sedation in humans and animals.27–30 Trazodone 100 mg produced a significant reduction in activity in the cats, as measured by both the accelerometers and enclosure activity scores. All doses of trazodone produced substantial decreases in activity based on the accelerometers, with the 50 mg dose (the first dose given) causing the largest reduction. Figure 2 illustrates interesting discrepancies in the activity between different treatment days. The cats were much more active on treatment days 1 and 2, when trazodone 50 mg and 75 mg were administered compared with treatment days 3 and 4, when the randomized trazodone 100 mg and placebo doses were given. This could be a reflection of the time point in the course of the study. The 50 mg and 75 mg doses were given at the beginning of the study, when cats would have been more stimulated by the presence of the investigators. The 100 mg dose and placebo days were later in the study, and the cats may have habituated to the investigators’ entrances and departures.
There were limitations in this study. The small sample size reduces the power of statistical analysis, despite the crossover design, and limits our ability to generalize the findings. The study would have also been strengthened if all doses of trazodone could have been randomized. Subjects were limited to neutered male cats. Additionally, cats were housed in a single room with visual access to the other cats. It is possible that cats were influenced by the stimuli of the other cats’ activities. One cat did not receive a full dose on one treatment day (100 mg). While administration of the treatments remained non-force-based (ie, voluntarily ingested), not all cats received the medication in similar types or amounts of food. This may have slightly altered absorption rates of the trazodone but it is unlikely to have had a sizeable effect on observations and measurements recorded.
There are few oral sedatives that owners can administer to cats prior to stressful events. Benzodiazepines have the potential to cause paradoxical excitation and behavioral disinhibition. 13 The benzodiazepine diazepam has been used as a sedative for cats. However, there have been documented cases of idiosyncratic hepatotoxicity from oral administration of diazepam.14,15 Thus, oral diazepam for cats has fallen out of favor with most veterinarians. 16 The phenothiazine acepromazine may be used for tranquilization but is not a true anxiolytic agent. 13 Acepromazine also has the potential for undesirable side effects, such as ataxia, paradoxical excitation, motor restlessness and hypotension. 13 Oral trazodone has been shown in previous studies to be well tolerated by dogs.18,20,21 It is prescribed for both situational and daily use and produces behavioral calming and mild sedation,18–20 consistent with the findings of this investigation.
Conclusions
Trazodone was well tolerated in this population of laboratory cats at single oral doses of 50, 75 and 100 mg. No adverse behavioral or physiological effects were observed. Compared with placebo, trazodone (100 mg) produced significant sedation as measured by decreased activity. Peak sedation was noted between 2 and 2.5 h after oral trazodone administration in food. Cats exhibited the greatest sedative effect when given the 50 mg dose, but as this was the first dose given, an order effect could not be ruled out. The experimental design did not allow for a statistical comparison of the effects of the three doses. Based on these findings, we cannot assume that decreased activity translates to increased compliance in the veterinary clinic.
During examinations, there were no significant reductions in stress scores or aggression, and no differences in measures of heart rate and respiratory rate of cats that received trazodone vs placebo. Possible explanations for these findings include an inadequate scoring system, premature timing of examination relative to dosing, small number of feline subjects or limited drug effectiveness. Without an observable effect on aggression, the data do not provide evidence that the use of trazodone prior to veterinary visits would be beneficial.
Further studies are needed to explore the efficacy of single-dose oral trazodone in reducing anxiety associated with carrier confinement, transportation and examination in a clinical setting. If trazodone helped to lower the stress associated with veterinary visits it could increase owners’ willingness to present their cats for veterinary care.
Supplementary material The following files are available:
Appendix 1: Enclosure activity score
Appendix 2: Stress score
Supplemental Material
Appendix 1: Enclosure activity score
Appendix 2: Stress score
Acknowledgments
We thank Dr Lola Hudson who provided cats for use in this study and subsequently arranged for their adoption to pet homes; Dr Duncan Lascelles who provided accelerometers; and Dr Margaret Gruen for discussion on experimental design.
Footnotes
Funding: This study was supported by the North Carolina Veterinary Medical Foundation Animal Behavior Fund.
The authors do not have any potential conflicts of interest to declare.
Accepted: 23 April 2015
References
- 1. American Veterinary Medical Association (AVMA). Cat-owning households. In: AVMA (ed). U.S. pet ownership & demographics sourcebook. Schaumberg, IL: AVMA, 2012, pp 75–87. [Google Scholar]
- 2. Volk JO, Felsted KE, Thomas JG, et al. Executive summary of the Bayer veterinary care usage study. J Am Vet Med Assoc 2011; 238: 1275–1282. [DOI] [PubMed] [Google Scholar]
- 3. Rodan I. Understanding feline behavior and application for appropriate handling and management. Top Companion Anim Med 2010; 25: 178–188. [DOI] [PubMed] [Google Scholar]
- 4. Quimby JM, Smith ML, Lunn KF. Evaluation of the effects of hospital visit stress on physiologic parameters in the cat. J Feline Med Surg 2011; 13: 733–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rand JS, Kinnaird E, Baglioni A, et al. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med 2002; 16: 123–132. [DOI] [PubMed] [Google Scholar]
- 6. Rodan I. Understanding the cat and feline-friendly handling. In: Little S. (ed). The cat: clinical medicine and management. St Louis, MO: Elsevier Saunders, 2012, pp 2–19. [Google Scholar]
- 7. Carney HC, Little S, Brownlee-Tomasso D, et al. AAFP and ISFM feline-friendly nursing care guidelines. J Feline Med Surg 2012; 14: 337–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodan I, Sundahl E, Carney H, et al. AAFP and ISFM feline-friendly handling guidelines. J Feline Med Surg 2011; 13: 364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaultier E, Pageat P, Tessier Y. Effect of a feline facial pheromone analogue (Feliway®) on manifestations of stress in cats during transport. Proceedings of the 32nd Congress of the International Society for Applied Ethology; 1998 July 21-25; Clermont-Ferrand, France. Versailles: INRA, 1998, pp 198. [Google Scholar]
- 10. Griffith C, Steigerwald E, Buffington C. Effects of a synthetic facial pheromone on behavior of cats. J Am Vet Med Assoc 2000; 217: 1154–1156. [DOI] [PubMed] [Google Scholar]
- 11. Kronen PW, Ludders JW, Erb NH, et al. A synthetic fraction of feline facial pheromones (Feliway®) calms but does not reduce struggling in cats before venous catheterisation. Vet Anaesth Analg 2006; 33: 258–265. [DOI] [PubMed] [Google Scholar]
- 12. Pageat P, Tessier Y. Usefulness of the F3 synthetic pheromone, Feliway, in preventing behavior problems in cats during holidays. Proceedings of the First International Conference on Veterinary Behavioural Medicine; 1997 April 1-2; Birmingham, UK. Universities Federation for Animal Welfare: Great Britain, 1997, pp 231. [Google Scholar]
- 13. Landsberg GM. Pharmacologic intervention in behavioral therapy. In: Behavior problems of the dog and cat. 3rd ed. Philadelphia, PA: Saunders Elsevier, 2013, pp 113–138. [Google Scholar]
- 14. Center S, Elston T, Rowland P, et al. Fulminant hepatic failure associated with oral administration of diazepam in 11 cats. J Am Vet Med Assoc 1996; 209: 618–625. [PubMed] [Google Scholar]
- 15. Hughes D, Moreau RE, Overall KL, et al. Acute hepatic necrosis and liver failure associated with benzodiazepine therapy in six cats, 1986–1995. J Vet Emerg Crit Care 1996; 6: 13–20. [Google Scholar]
- 16. Plumb DC. Diazepam. In: Plumb DC. (ed). Plumb’s veterinary drug handbook. 7th ed. Stockholm, WI: PharmaVet Inc, 2011, pp 304–308. [Google Scholar]
- 17. Stahl SM. Mechanism of action of trazodone: a multifunctional drug. CNS Spectr 2009; 14: 536–546. [DOI] [PubMed] [Google Scholar]
- 18. Gruen ME, Sherman BL. Use of trazodone as an adjunctive agent in the treatment of canine anxiety disorders: 56 cases (1995–2007). J Am Vet Med Assoc 2008; 233: 1902–1907. [DOI] [PubMed] [Google Scholar]
- 19. Gruen ME, Sherman BL. Animal behavior case of the month. J Am Vet Med Assoc 2012; 241: 1293–1295. [DOI] [PubMed] [Google Scholar]
- 20. Gruen ME, Roe SC, Griffith E, et al. Use of trazodone to facilitate postsurgical confinement in dogs. J Am Vet Med Assoc 2014; 245: 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jay AR, Krotscheck U, Parsley E, et al. Pharmacokinetics, bioavailability, and hemodynamic effects of trazodone after intravenous and oral administration of a single dose to dogs. Am J Vet Res 2013; 74: 1450–1456. [DOI] [PubMed] [Google Scholar]
- 22. Gruen ME, Thomson AE, Clary GP, et al. Conditioning laboratory cats to handling and transport. Lab Anim 2013; 42: 385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Indrawirawan Y, McAlees T. Tramadol toxicity in a cat: case report and literature review of serotonin syndrome. J Feline Med Surg 2014; 16: 572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jauch R, Kopitar Z, Prox A, et al. Pharmacokinetics and metabolism of trazodone in man. Arzneimittelforschung 1976; 26: 2084–2089. [PubMed] [Google Scholar]
- 25. Kessler MR, Turner DC. Stress and adaptations of cats (Felis silvestris catus) housed singly, in pairs, and in groups in boarding catteries. Anim Welf 1997; 6: 243–254. [Google Scholar]
- 26. Good PI, Hardin JW. Collecting data. In: Good PI, Hardin JW. (eds). Common errors in statistics (and how to avoid them). Hoboken, NJ: John Wiley, 2012, pp 31–56. [Google Scholar]
- 27. Grap MJ, Borchers CT, Munro CL, et al. Actigraphy in the critically ill: correlation with activity, agitation, and sedation. Am J Crit Care 2005; 14: 52–60. [PubMed] [Google Scholar]
- 28. Weinbroum AA, Abraham RB, Ezri T, et al. Wrist actigraphy in anesthesia. J Clin Anesth 2001; 13: 455–460. [DOI] [PubMed] [Google Scholar]
- 29. Carregaro MV, Luna SP, Mataqueiro, et al. Effects of buprenorphine on nociception and spontaneous locomotor activity in horses. Am J Vet Res 2007; 68: 246–250. [DOI] [PubMed] [Google Scholar]
- 30. Kissin I, Brown PT, Bradley EL. Locomotor activity after recovery from hypnosis: midazolam-morphine versus midazolam. Anesth Analg 1992; 75: 929–931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Enclosure activity score
Appendix 2: Stress score