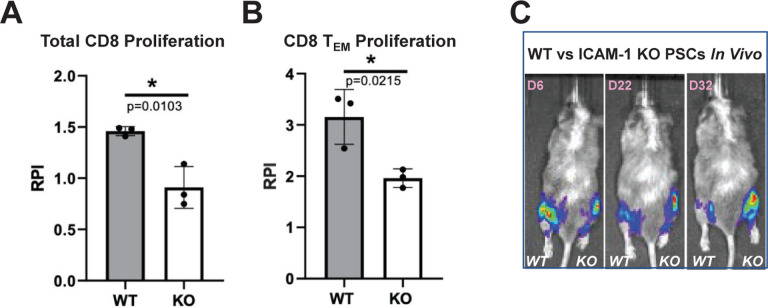
Figure 7. In Vitro and In Vivo Immune Responses to ICAM-1 Knock-out (KO) Cells.
To test in vitro immune responses to edited cells, highly pure (>85% cTNT+) PSC-derived CMs were made from wild type (WT) and ICAM-1 KO pluripotent stem cells (PSCs), similar to Figure 1C. Cells were co-cultured for 6 days with HLA-mismatched peripheral blood mononuclear cells (1:6 T:E ratio) labeled with VPD450 proliferation dye in a mixed lymphocyte reaction. Alloreactivity was assessed via proliferation (VPD450 dye dilution) of total viable cells and various T cell subpopulations by flow cytometry. Proliferation of (A) Total CD8+CD3+ T cells and (B) Effector memory T (TEM) cells (CD45RO+ CD45RA−CD62L−CCR7−) is shown. Relative proliferation index (RPI) is the ratio of % proliferating cells with cardiomyocyte targets/baseline proliferating cells without targets. N=3 biological replicates, error bars = standard deviation, analysis via 2-tailed unpaired t-test, Prism 9.3.1. N=3 repeat experiments. (C) To test in vivo immune responses to edited cells, NeoThy humanized mice with flow-confirmed hCD45+ and hCD3+ (both >10%) immune cells were engrafted with 1e6 ICAM-1 KO (right leg) vs. WT (left leg) isogeneic PSCs co-injected with Matrigel. Humanized immune systems were HLA-mismatched to the PSC grafts. Bioluminescence imaging (BLI) signal was monitored for 32 days at early (6 days), middle (22 days), and late/terminal (32 days) time points. A representative mouse is shown reflecting loss of WT graft, and retention of KO graft, which was seen in 4 of 5 mice BLI signal quantified by total flux (p/s).