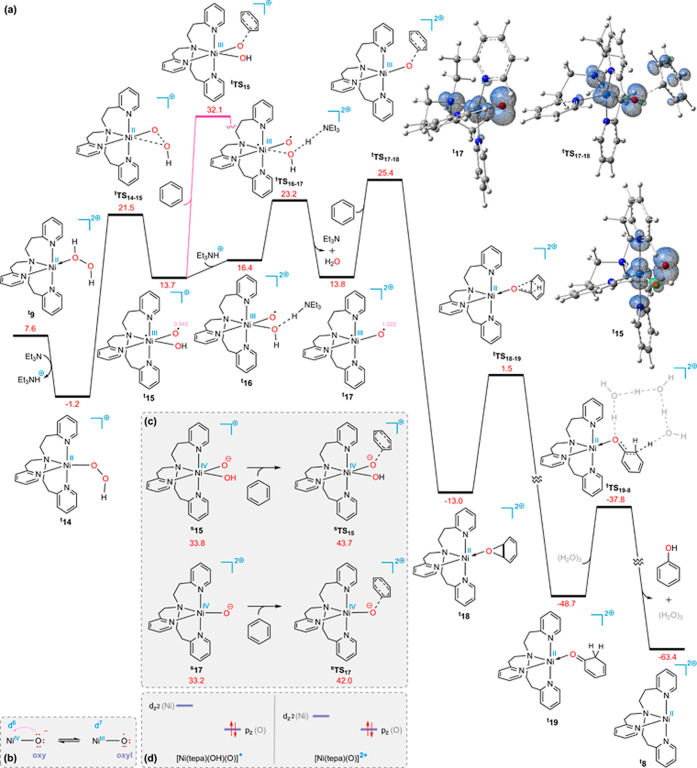
Figure 3.
(a) Calculated energy profile for [NiII(tepa)]2+-catalyzed hydroxylation of benzene; spin density distribution plots for t15, t17, and tTS17–18 at an isosurface value of 0.006. (b) Equilibria between oxy and oxyl forms. (c) Calculated energy barrier for the nucleophilic attack of benzene to the oxy group of s15 and s17. (d) Schematic illustration of the effect of the formal charge of the nickel complex on the ease of conversion from oxy to oxyl. The superscripts “s” and “t” represent the singlet and triplet ground states, respectively. The relative Gibbs free energy values obtained from the SMD/B3LYP-D3/def2-TZVP//SMD/B3LYP-D3/6-31G(d),SDD calculations are given in kcal/mol (in red) and spin density values in e/Å3 (in pink).