Abstract
Background
The prognosis of patients with Relapsed/Refractory Osteosarcoma (R/R OS) remains dismal without an agreement on systemic therapy. The use of High-Dose Ifosfamide (14 g/sqm) with an external pump in outpatient setting (14-IFO) in R/R OS patients is limited. This study represents the first retrospective cohort analysis focused on evaluating the activity and toxicity of 14-IFO in this setting.
Patients and methods
The study investigated 14-IFO activity, in terms of tumour response according to RECIST 1.1 criteria, as well as survival rates and toxicity, according to CTCAE v.5.
Results
The trial enrolled 26 patients with R/R OS. The Overall Response Rate (ORR) and Disease Control Rate (DCR) obtained was 23% and 57.5%, respectively. Patients with relapsed OS showed a higher ORR (45%) and DCR (82%) compared to refractory patients, irrespective of the number of prior treatment lines received. The achievement of disease control with 14-IFO administration enabled 27% of patients to undergo new local treatment. Four-month Progression-Free Survival (PFS) was 54% for all patients and 82% for the relapsed OS sub-group. Median Overall Survival (OSurv) was 13.7 months, with 1-year OSurv of 51% for all patients and 71% for relapsed patients. Age over 18 years and the presence of refractory disease were identified as negative prognostic factors for this patient cohort. A total of 101 cycles were evaluated for toxic assessment, demonstrating a tolerable profile without grade 3–4 non-haematological toxicities.
Conclusions
14-IFO should be considered a viable treatment option for R/R OS, particularly due to its well tolerated toxicity profile and the potential for home-administration, which can improve patient quality of life without compromising efficacy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-12498-x.
Keywords: Osteosarcoma, Chemotherapy, Ifosfamide, Relapse
Introduction
Osteosarcoma (OS), a rare and aggressive mesenchymal tumour, is the most common primary malignant bone tumour, predominantly afflicting individuals in the second decade of life, particularly adolescents and young adults [1, 2]. Despite the introduction of a first-line multidisciplinary treatment approach improved the 5-year Overall Survival (OSurv) from 10% to 60–70% [3–6], there has been a notable absence of further improvements in cure rates over the past four decades [7, 8] and approximately 30% of patients with OS experience local or systemic recurrence [7–9].
Treatment of patients with a recurrent or refractory OS (R/R OS) remains an unmet clinical need, as evidenced by a 5-year OSurv rate of less than 30% [2, 9–11] and a lack of consensus regarding an effective systemic treatment [2, 7].
Prognostic factors influencing the survival of patients with R/R OS include the length of the relapse-free interval (RFI), the site of recurrence, and the feasibility of a new complete surgical remission, although the latter remains achievable for only few patients [1, 2, 10]. Patients with inoperable lesions receive systemic treatment with the aim to improve their Disease Control Rate (DCR) and prolong survival [10, 11], nonetheless the benefit of chemotherapy for these patients is still debated [2, 10–11].
Ifosfamide is an alkylating chemotherapy agent that has been widely used in R/R OS treatment [12–18], either alone or in combination with other agents (Table 1), demonstrating a role in improving both Disease Control Rate (DCR) and Progression Free Survival (PFS) [12–19].
Table 1.
List of published chemotherapy regimens for patients with R/R OS
| Total pts enrolled (n°) | OS pts enrolled (n°) | OS Disease Status | Drugs and Schedule | In/Outpatient | PFS or EFS reported for OS pts | OSurv reported for OS pts | OS ORR (CR + PR) | OS DCR (CR + PR + SD) | |
|---|---|---|---|---|---|---|---|---|---|
| Harris et al., 1995 | 63 | 63 |
- Metastatic and/or unresectable primary OS (stratum 1: 33 pts) - Relapsed OS (stratum 2: 30 pts) |
IFO: 2,400 mg/sqm/day x 5 days | NR | NR | NR |
- Stratum 1: 27,3% (1CR + 8PR) - Stratum 2: 10% (1 CR + 2 PR) |
- Stratum 1: 72,7% (1 CR + 8 PR + 4 mixed + 11 NR/SD) - Stratum 2: 53,3% (1 CR + 2 PR + 1 mixed + 12 NR/SD) |
| Berrak et al., 2005 | 16 | 16 | Previously treated pediatric patients with locally recurrent and/or metastatic osteosarcoma | IFO: 2 g/sqm/dose administered intravenously at 12 hr interval x 7 days = total dose 14g/sqm | Inpatient | 15-month EFS: 43,7% | 15 month OSurv: 44 ± 12,4% | − 62,5% (6 CR + 4 PR) | − 62,5% (6 CR + 4 PR + 0 SD) |
| Patel et al., 1997 | 74 | 19 | NR | IFO: 14 g/sqm (bolus or continuos infusion) | Inpatient | NR | NR | -42% | NR |
| Verschoor et al., 2020 | 62 | 62 | Recurrent/metastatic | IFO 5 g/sqm as bolus infusion (26 patients) or IFO 3 g/sqm x 3 days continuos (36 patients) | NR |
Median PFS: 2,6 months (whole cohort) Median PFS: 2,1 months (dose 5g/sqm) Median PFS: 3,8 months (dose 9g/sqm) 4-month PFS: 12% (dose 5g/sqm) 4-month PFS: 44% (dose 9g/sqm) |
Median OSurv: 9,1 months (whole cohort) Median OSurv: 6,7 months (dose 5g/sqm) Median OSurv: 10,9 months (dose 9g/sqm) 9-month OSurv: 35% (dose 5g/sqm) 9-month OSurv: 69% (dose 9g/sqm). 12-month OSurv: 19% (dose 5g/sqm) 12-month OSurv: 44% (dose 9g/sqm) |
− 23% (dose: 5g/sqm) (6 CR, PR, clinical benefit*) - 36% (dose: 9g/sqm) (13 CR, PR, clinical benefit*) |
− 42% (dose: 5g/sqm) (6 CR, PR, clinical benefit* + 5 SD) - 78% (dose: 9g/sqm) (13 CR, PR, clinical benefit* + 15 SD) |
| Palmerini et al., 2020 | 51 | 51 | Relapsed and unresectable OS | IFO 3g/sqm/day x 5 days | NR |
Median PFS: 6,1 months 4-month PFS: 61% 6-month PFS: 51% |
Median OSurv: 14,5 months 1-year OSurv: 62% 2-year OSurv: 30% |
− 20% (1 CR + 9 PR) | . 77% (1 CR + 9PR + 29 SD) |
| Gaspar et al., 2021** | 81 pts enrolled and randomized | 81 | R/R OS | Arm A: Lenvatinib 14 mg/sqm/day x 21 days orally + IFO 3g/sqm/day iv x 3 days + ETOPOSIDE 100 mg/sqm/ day iv x 3 days Arm B: IFO 3g/sqm/day iv x 3 days + ETOPOSIDE 100 mg/sqm/day iv x 3 days | IFO + ETOPOSIDE inpatient |
Arm A: 4-month PFS: 76,3% Arm B: 4 month PFS: 66% |
Arm A: 12-month OSurv: 49,2% Arm B: 12 month OSurv: 72,1% |
Arm A: 15% Arm B: 9,8% |
NR |
| Palmerini et al., 2016 | 51 | 40 (35 pts evaluable) | R/R OS | GEMCITABINE: 675–900 mg/sqm oon Day 1 and 8 + DOCETAXEL 75 mg/sqm Day 8. | Outpatient | 4-month PFS: 56% | 12-month OSurv: 30% (whole cohort) | − 17% (6 PR) | − 57% (6 PR + 14 SD) |
| Song et al., 2014 | 28 | 28 (17 pts evaluable) | R/R OS | GEMCITABINE: 675–900 mg/sqm oon Day 1 and 8 + DOCETAXEL 100 mg/sqm Day 8. | NR | NR |
Median OSurv: 9 months 12-month OSurv: 35,3% |
− 11,8% (1 CR + 1 PR) | − 41,2% (3CR including 2 metabolic CR + 1 PR + 3 SD) |
| Navid et al., 2008 | 22 | 17 (10 pts evaluable) | R/R OS | GEMCITABINE: 675 mg/sqm oon Day 1 and 8 + DOCETAXEL 75 mg/ sqm Day 8. | Outpatient | NR | NR | − 30% (3 PR) | − 40% (3 PR + 1 SD) |
| Fox et al., 2012 | 53 | 14 | Relapsed OS | GEMCITABINE: 675 mg/sqm oon Day 1 and 8 + DOCETAXEL 75 mg/ sqm Day 8. | NR | NR | NR | − 7% (1 PR) | NR |
| Berger et al., 2009 | 26 | 26 | Relapsed OS | CYCLOPHOSPHAMIDE: 4 g/sqm on Day 1 + ETOPOSIDE 200 mg/sqm on Days 2, 3, and 4. | Inpatient | 4-month PFS: 42% |
4-month OSurv: 93% 12-month OSurv: 50% |
− 19% (2 CR + 3 PR) | − 54% (2 CR + 3 PR + 9 SD) |
| Rodriguez-Galindo et al., 2002 | 14 | 14 | R/R OS | CYCLOPHOSPHAMIDE: 500 mg/sqm/day x 5 days + ETOPOSIDE: 100 mg/sqm/day x 5 days + GCSF | NR | NR | NR | − 28,5% (1 CR + 3 PR) | − 64,2% (1 CR + 3 PR + 5 SD) |
| Miser et al., 1987 | 124 | 17 (8 pts evaluable) | Relapsed OS | IFO 1,8 g/sqm/day x 5 days + ETOPOSIDE: 100 mg/sqm/day x 5 days | Inpatient | NR | NR | − 37,5% (3 PR) | NR |
| Kung et al., 1993 | 311 | 32 | R/R OS | IFO: 2 g/sqm x 3 + ETOPOSIDE:100 mg/sqm x 3 | NR | NR | NR | − 15,6% (2 CR + 3 PR) | NR |
(NR = not reported; OS = Osteosarcoma; R/R = Relapsed/Refractory; pts = patients; PFS = Progression Free Survival; EFS = Event Free Survival; OSurv = Overall Survival; ORR = Overall Response Rate; DCR = Disease Control Rate; CR = Complete Response; PR = Partial Response; SD = Stable Disease. *the authors reported an overall ORR including patients with clinical benefit, not more details are reported; **the results reported in this table for this trial are published on clinicaltrial.gov)
However, previous administration schedules have been associated with significant toxicity, adversely affecting the quality of life of patients with R/R OS [17, 18]. Furthermore the timing of ifosfamide infusion has been shown to correlate with tolerability [20–22].
The administration of high-dose Ifosfamide in a prolonged 14-day continuous infusion using an external pump in an outpatient setting (14-IFO) demonstrated an excellent tolerability and toxicity profile, compared to previously reported schedules (Table 1, Supplementary File) [12, 14–17, 23−30], even among young patients with R/R sarcoma, including bone sarcomas [20]. A prolonged continuous infusion has been shown to be feasible and correlates with reduced incidence of adverse events alongside an improved therapeutic index [20, 31–32].
However, the use of 14-IFO in patients with R/R OS remains limited. This is the first retrospective cohort analysis focused on evaluating the activity and toxicity of 14-IFO within this patient cohort.
Methods
Patients and methods
Clinical data from patients diagnosed with OS R/R and treated with 14-IFO were retrospectively analyzed, across five sarcoma centres within the national network of the Italian Paediatric Onco-Haematology Association (AIEOP) and Italian Sarcoma Group (ISG). The following eligibility criteria were required for the present analysis: (i) patients with OS at first or subsequent relapse, defined as the return of the disease following complete surgical tumor remission in either first or subsequent treatment lines or (ii) patients with refractory OS, defined as the persistence of the disease despite surgical and/or chemotherapeutic interventions; (iii) patients aged younger than 40 years at the time of their first dose of 14-IFO; (iv) at least one cycle of 14-IFO received ; (v) radiological tumour assessments according to Response Evaluation Criteria in Solid Tumours (RECIST 1.1) [33].
The primary objective of this trial was to describe the 14-IFO anti-tumour activity in patients with R/R OS, the secondary objective was to assess the treatment’s toxicity profile.
The primary endpoint was PFS, at 4 and 6-months, defined as the ratio between patients achieving Complete Response (CR), Partial Response (PR) or Stable Disease (SD) and those progressing after four and six months from the first dose of 14-IFO. PFS was calculated from the date of the first 14-IFO cycle until either the occurrence of tumour progression or the most recent follow-up. Patients who achieved a surgical complete remission after the treatment with 14-IFO were censored at the time of surgery procedure.
The secondary endpoints were: DCR [defined as the percentage of patients achieving CR + PR + SD], Overall Response Rate (ORR) [defined as CR + PR], both according to RECIST 1.1, and OSurv at 1 and 2-years. OSurv was calculated from the date of the first dose of 14-IFO to the date of death or last follow-up. Patients were censored at the date of last follow-up in the absence of death or progression. Additional secondary endpoint was to assess the toxicity profile of 14-IFO. Treatment-related adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 5.0.
An additional exploratory endpoint was the estimation of the growth modulation index (GMI). This index was calculated as the ratio of time to progression with 14-IFO (TTPn) to the most recent prior line of therapy (TTPn-1) for each patient with available progression data prior to 14-IFO initiation. A GMI value of ≥ 1.33 was considered indicative of meaningful clinical activity, as previously defined [38].
Patient and tumour characteristics at initial diagnosis, pattern of recurrence, treatment details, outcome and adverse events were recorded through specific Case Report Forms (CRFs) collected in a retrospective way.
All patients received a total dose of Ifosfamide of 14 g/sqm per cycle, administered over a period of 14 days within a 21-days cycle, mixed with Mesna 14 g/sqm (at a ratio of 1:1), in normal saline solution (total volume up to 275 ml) intravenously via an external pump in an outpatient setting. The external pump was replaced either after 3 or 7 days, depending on local institutional practices. Therefore, patients received Ifosfamide at a dose of 7 g/sqm with Mesna 7 g/sqm during week 1 and week 2 for a total of 14 days or Ifosfamide at a dose of 3 g/sqm with Mesna 3 g/sqm every 3 days for a total of 14 days. No hyperhydration or additional Mesna were administered, but adequate oral hydration (1500 ml/day) was recommended, and antiemetic treatments were provided as needed based on local clinical practices. Antibiotic prophylaxis was not required during chemotherapy infusion and the prophylactic use of G-CSF was not mandatory at the end of chemotherapy infusion, but administered only if deemed necessary.
Clinical and laboratory assessments were performed concurrently with elastomer pump replacement, thus every 3 or 7 days, in accordance with local institutional practices, and one week following the completion of the 14-IFO infusion. Additional assessments were carried out as per local clinical protocols.
Laboratory evaluations comprised full blood count test, liver and kidney function tests, electrolytes and urine analysis. Radiological assessments were scheduled every two or three 14-IFO cycles, following institutional clinical practices, using Computed Tomography scans in adherence to the RECIST criteria version 1.1. Additional radiological assessments were performed as clinically warranted.
The study protocol was approved by the local ethical and regulatory committee of each institute and registered with ClinicalTrials.gov, number NCT04651569. All study procedures were carried out in accordance with the International Council for Harmonisation guidelines on good clinical practice and the STROBE Statement for observational studies.
Statistical analysis
The distribution of clinical and demographic characteristics of the patients is described using median and inter-quantile ranges for continuous variables and frequencies, and percentages for categorical items. Comparisons of qualitative variables were conducted using the χ² test and Fisher’s exact test as appropriate.
The analysis of OSurv and PFS were conducted using the Kaplan-Meier method with a 95% confidence interval (95% CI). Differences between survival curves were tested through Log-rank tests. The level of statistical significance is set at a value of 0.05. Statistical analyses were performed using R software version 4.2.1.
Results
Between January 2012, and December 2021, 26 patients with R/R OS were treated with at least one complete 14-IFO cycle in 5 Italian comprehensive sarcoma centres. All patients were evaluable for safety and efficacy. Overall, the mean follow-up period was 16,3 months (range: 5–83).
The median age of patients was 19 years (range: 9–37) at the beginning of 14-IFO; eleven patients (42%) were younger than 18 years old.
Most patients had metastatic disease (23 patients – 88%) and, as expected with OS, metastases were mainly in lungs and bone. Fifteen patients (58%) had refractory disease to two or more previous treatments, whilst the remaining (42%) had relapsed OS.
All patients received the three most common drugs used for treating OS in first line treatment: Methotrexate, Doxorubicin and Cisplatin. Sixteen patients (61%) had previously received Ifosfamide (at a dose of either 10 g/sqm or 15 g/sqm administered over five days) and eight patients (31%) received Mifamurtide during their first-line treatment.
In the subset of patients with relapsed disease, the majority (10/11 patients) relapsed following their first treatment line, with a median disease-free interval (DFI) of 18 months (range: 7.0–33.1) before starting 14-IFO. Only one patient experienced disease relapse after second line treatment with a DFI of 6.8 months before commencing 14-IFO.
Clinical characteristics are described in Table 2.
Table 2.
Patients’ characteristics:
| n° | % | |
|---|---|---|
| All | 26 | 100 |
| Age median, range ( years ) | 19 (9–37) | |
| < 18 years | 11 | 42 |
| ≥ 18 years | 15 | 58 |
| Sex | ||
| Male | 18 | 69 |
| Female | 8 | 31 |
| Histological Response for primary tumour at first diagnosis | ||
| ≥ 90% | 6 | 23 |
| < 90% | 15 | 58 |
| Not Available | 5 | 19 |
| Disease Status at 14-IFO | ||
| Relapsed OS | 11 | 42 |
| Refractory OS | 15 | 58 |
| Disease staging at 14-IFO | ||
| Localized Disease | 3 (1 femur, 1 hip, 1 orbit) | 12 |
| Metastatic Disease (only lung) | 12 | 46 |
| Metastatic (only bone) | 4 | 15 |
| Metastatic (lung + bone) | 2 | 7,5 |
| Metastatic (lung + other) | 3 | 12 |
| Metastatic (lung + bone + other) | 2 | 7,5 |
| Disease staging at 14-IFO according to American Joint Commission on Cancer (AJCC) TNM system | ||
| Stage IIB | 2 | 7,5 |
| Stage III | 23 | 88,5 |
| Data not available | 1 | 4 |
| Ifosfamide in pre-treatment | ||
| Yes | 16 | 61 |
| No | 10 | 39 |
| n° of treatment lines before 14-IFO | ||
| 1 line | 13 | 50 |
| 2 lines | 5 | 19 |
| 3 lines | 5 | 19 |
| ≥ 4 lines | 3 | 12 |
Response
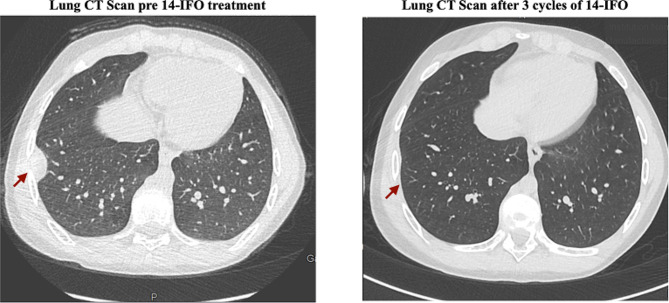
1 patient achieved a CR (Figs. 1) and 5 patients achieved a PR leading to an ORR of 23%. The median duration of response was 9 months (range: 2–31). ORR was 6.7% and 45% for refractory and relapsed patients, respectively (p = 0.054) (Table 3).
Fig. 1.
CT Scan images showing complete response according to RECIST Criteria v1.1 in a patient with relapses OS after 3 14-IFO cycles
Table 3.
Patient’s characteristic and outcomes according to disease status at the beginning of 14-IFO:
| Refractory pts (tot: 15) | Relapsed pts (tot: 11) | p | |
|---|---|---|---|
| Median Age (years), IQR | 21 (17,31) | 17 (14, 19) | 0.077 |
| Sex | > 0.9 | ||
| Female | 5 (33%) | 3 (27%) | |
| Male | 10 (67%) | 8 (73%) | |
| n° of treatment lines before 14-IFO | 0.003 | ||
| 1 line | 3 (20%) | 10 (91%) | |
| 2 lines | 4 (27%) | 1 (9%) | |
| 3 lines | 5 (33%) | 0 (0%) | |
| ≥ 4 lines | 3 (20%) | 0 (0%) | |
| Previous Ifosfamide | 0.10 | ||
| Yes | 11 (73%) | 5 (45%) | |
| No | 3 (20%) | 6 (55%) | |
| Data Not Available | 1 (6.7%) | 0 (0%) | |
| Staging at 14-IFO | 0.063 | ||
| Localized Disease | 0 (0%) | 3 (27%) | |
| Metastatic Disease | 15 (100%) | 8 (73%) | |
| n° of 14-IFO cycles received | 0.013 | ||
| 1 cycle | 0 (0%) | 1 (9%) | |
| 2 cycles | 7 (47%) | 0 (0%) | |
| 3 cycles | 2 (13%) | 0 (0%) | |
| 4 cycles | 2 (13%) | 5 (45,5%) | |
| ≥ 5 cycles | 4 (27%) | 5 (45,5%) | |
| Best Response post 14-IFO | 0.062 | ||
| Complete Response | 0 (0%) | 1 (9.1%) | |
| Partial Response | 1 (6.7%) | 4 (36%) | |
| Stable Disease | 5 (33%) | 4 (36%) | |
| Progression Disease | 9 (60%) | 2 (18%) | |
| Timing of Best Response achievement | |||
| Complete Response | / | 1 pt after 3 cycles | |
| Partial Response | 1 pt after 4 cycles |
2 pts after 2 cycles 2 pts after 4 cycles |
|
| Stable Disease | 5 pts after 2 cycles | 4 pts after 2 cycles | |
| DCR | 0.051 | ||
| 6 (40%) | 9 (82%) | ||
| ORR | 0.054 | ||
| 1 (6.7%) | 5 (45%) | ||
| PFS | 0.02 | ||
| 4-month | 33% | 82% | |
| 6-month | 20% | 64% | |
| 12-month | 0% | 18% | |
| OSurv | 0.1 | ||
| 4-month | 100% | 100% | |
| 6-month | 93% | 91% | |
| 1-year | 36% | 71% | |
| 2-year | 15% | 30% | |
The DCR was 57.5% for the entire patient cohort (1 CR + 5 PR + 9 SD) and 40% and 82% for refractory and relapsed patients, respectively (p = 0.05) (Table 3).
In the cohort of relapsed or refractory patients, no statistically significant differences in DCR or ORR were observed when comparing patients previously treated with High-Dose Ifosfamide (10–15 g/sqm over 5 days) with those who had not received prior treatment with High-Dose Ifosfamide (Table 4).
Table 4.
Disease Control Rate (DCR) and Overall Response Rate (ORR) in relapsed and refractory patients by previous treatment type. Data about previous treatment with High Dose Ifosfamide were not available for one patient
| Relapsed OS patients | |||
|---|---|---|---|
| Pretreatment with | |||
| High Dose Ifosfamide | |||
| No | Yes | P | |
| n | 6 | 5 | |
| ORR (%) | 4 (66.7) | 1 (20.0) | 0.347 |
| DCR (%) | 5 (83.3) | 4 (80.0) | 1 |
| Refractory OS patients | |||
| Pretreatment with | |||
| High Dose Ifosfamide | |||
| No | Yes | p | |
| n | 3 | 11 | |
| ORR (%) | 1 (33.3) | 0 (0.0) | 0.47 |
| DCR (%) | 2 (66.7) | 3 (27.3) | 0.56 |
For seven patients (27%), local treatment was feasible following 14-IFO administration. Specifically, five patients underwent surgery, including two patients who underwent bilateral thoracic procedures (one individual at different times following the second and third cycles, and one individual after the fourth cycle); two patients who underwent lateral thoracic procedures after 4 and 8 cycles, respectively; one patient who underwent a local maxillary procedure after 5 cycles. One patient received local Carbon-Ion radiotherapy for vertebral metastases after 4 cycles, and another patient underwent photon-radiotherapy for vertebral metastases after 4 cycles.
Notably, one patient with a localised orbital OS achieved a PR according to RECIST 1.1, and a complete metabolic response confirmed by Positron Emission Tomography/Computed Tomography (PET-CT) Total Body after 4 cycles. Subsequently, the patient underwent local surgery, achieving a macroscopic CR.
GMI was assessable for 13 patients (all of them in the relapsed OS cohort). Only one patient showed a GMI greater than 1.33.
Survival
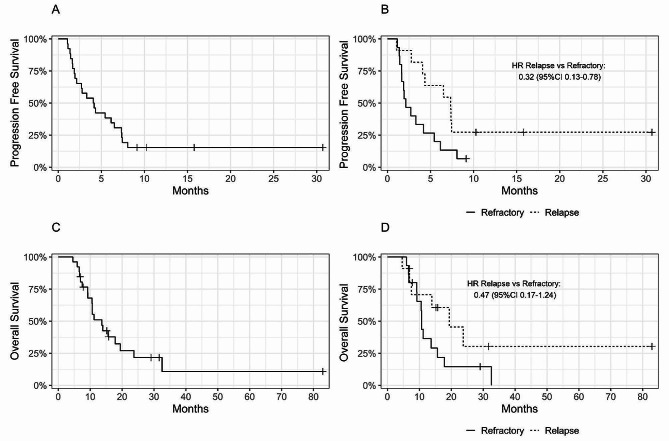
The median PFS was 4.1 months [95% CI 2.13, 7.37] for the whole cohort. Four-month and 6-month PFS were 54% [95% CI 38–77] and 38% [95% CI 24–63], respectively (Fig. 2A). PFS was significantly better in the group of patients with relapsed OS compared to patients with refractory disease (HR: 0.32, [95% CI 0.13, 0.78]). Median PFS was 7.33 months for relapsed patients vs. 2.13 months for refractory patients (p = 0.02). Four and 6-month PFS were 33% [95% CI 16–68] and 20% [95% CI 7.33-55], respectively, for refractory patients compared to 82% [95% CI 62–100] and 64% [95% CI 41–99] for relapsed patients. (p = 0.02) (Fig. 2B).
Fig. 2.
Kaplan-Meier curve for Progression Free Survival (A), Progression Free Survival according to status pre 14-IFO (B), Overall Survival (C), Overall Survival according to status pre 14-IFO (D)
Moreover, patients younger than 18 years old had a higher PFS rate compared to older patients. Four- and 6-month PFS were both 73% [95% CI 51–100] for patients younger than 18 years old at the beginning of 14-IFO treatment, compared to 40% [95% CI 22–74] and 13% [95% CI 3.7–48] for patients older than 18 (HR: 2.87 95% CI (1.13–7.29), p = 0.03).
The median OSurv was 13.7 months [95% CI 10.6–23.7] for the whole cohort. One-year and 2-year OSurv were 51% [95% CI 35–75] and 22% [95% CI 9.5–49], respectively (Fig. 2C).
Median OSurv was longer for relapsed patients than refractory patients (19.4 vs. 10.7 months (p = 0.1). One-year OSurv was 71% [95% CI 48–100] and 36% [95% CI 18–73], for relapsed and refractory patients, respectively. Meanwhile, 2-year OSurv was 30% [95% CI 10–91] vs. 15% [95% CI 4–52], for relapsed and refractory patients, respectively (p = 0.1) (Fig. 2D).
No significant PFS and OSurv differences were observed according to sex, staging, previous histological response, and previous treatments. More interestingly, no statistically significant PFS and OSurv differences were detected comparing patients previously treated with High Dose Ifosfamide (10–15 g/sqm over 5 days) with patients without a prior treatment with High Dose Ifosfamide.
Six-month PFS was 56% [95% CI 31–100] for patients who didn’t receive High Dose Ifosfamide in a previous line vs. 25% [95% CI 11–58] for patients pretreated with High Dose Ifosfamide (Log-rank p = 0,2).
One- and 2-year OSurv were 53% [95% CI 28–100] and 27% [95% CI 8.3–86], respectively, for patients who didn’t receive High Dose Ifosfamide in a previous line vs. 54% [95% CI 34–86] and 20% [95% CI 6.1–63], for patients pretreated with High Dose Ifosfamide (Log-rank p = 0,7).
Treatment administration and toxicities
Treatment administration, with details regarding treatment delay, dose reductions and toxicities, is presented in Table 5.
Table 5.
14-IFO cycles administration and toxicities:
| n° | % | |
|---|---|---|
| All patients | 26 | 100 |
| Cycles received | ||
| 1 cycle | 1 | 4 |
| 2 cycles | 7 | 27 |
| 3 cycles | 2 | 8 |
| 4 cycles | 7 | 27 |
| 5 cycles | 4 | 15 |
| 6 cycles | 4 | 15 |
| 8 cycles | 1 | 4 |
| Delayed cycle | ||
| Yes (toxicities reason) | 6 | 23 |
| Yes (organizational reason) | 1 | 4 |
| No | 19 | 73 |
| n° of delayed cycles/patient | ||
| 1 cycle | 4 | 15 |
| 2 cycles | 1 | 4 |
| 3 cycles | 1 | 4 |
| 4 cycles | 1 | 4 |
| Dose Reduction | ||
| Yes | 5 | 19 |
| No | 21 | 81 |
| n° | % | |
| All Cycles | 101 | 100 |
| Leukopenia g.1–2 | 41 | 40.5 |
| Leukopenia g.3 | 18 | 19 |
| Leukopenia g.4 | 6 | 5.8 |
| Neutropenia g.1–2 | 37 | 36.6 |
| Neutropenia g.3 | 15 | 14.8 |
| Neutropenia g.4 | 9 | 8.9 |
| Thrombocytopenia g.1–2 | 16 | 15.8 |
| Thrombocytopenia g.3 | 3 | 2.9 |
| Thrombocytopenia g.4 | 1 | 0.9 |
| Febrile Neutropenia g.3 | 1 | 0.9 |
| Febrile Neutropenia g.4 | 0 | 0 |
| Nausea/Vomiting g.1–2 | 4 | 3.9 |
| Nausea/Vomiting g.3 | 0 | 0 |
| Nausea/Vomiting g.4 | 0 | 0 |
| Fatigue g.1–2 | 3 | 2.9 |
| Fatigue g.3 | 0 | 0 |
| Fatigue g.4 | 0 | 0 |
| Neurological g.1–2 | 0 | 0 |
| Neurological g.3 | 0 | 0 |
| Neurological g.4 | 0 | 0 |
| Renal g.1–2 | 0 | 0 |
| Renal g.3 | 2* | 1,9 |
| Renal g.4 | 0 | 0 |
*1 patient started 14-IFO treatment with an ongoing renal toxicity g.2 and during the 2nd course exhibited a worsening of the pre-existing toxicity up to g.3 (creatinine and eGFR increased)
Most patients (sixteen patients – 61%) received at least four 14-IFO cycles. A total of 101 cycles were administered and all evaluable for toxicity assessment.
No patient permanently discontinued the treatment because of adverse events. Treatment delay occasionally occurred in 7 (27%) out of 26 patients (6 patients for toxicity and 1 patient due to logistic reasons), however, most of these delays involved only one cycle throughout the entire treatment period.
Dose reductions were reported in five (19%) patients. These reductions were primarily attributed to adverse events, particularly hematological toxicities. Specifically, one patient received four cycles with a 50% dose reduction, three patients received one cycle each with a 25% dose reduction (equivalent to 75% of the full dose), and one patient received one cycle with a 15% dose reduction (equivalent to 85% of the full dose).
Overall, the most common grade 3 or worse treatment-related adverse events included haematological toxicities. Grade 3–4 haematological events were observed in 53 cycles (52%) as follows: (i) white-blood cell decrease in 24 cycles (23.7%); (ii) neutropenia in 24 cycles (23.7%); (iii) thrombocytopenia in 4 cycles (3.9%).
In addition, 12 patients (46%) experienced grade 3–4 leukopenia during at least one 14-IFO cycle, with 11 of them concurrently experiencing grade 3–4 neutropenia (42%). However, only five patients required G-CSF administration resulting in rapid blood count recovery. Two patients (7.6%) showed grade 3–4 thrombocytopenia in at least one cycle and only one patient (3.8%) experienced an episode of febrile neutropenia, requiring hospital admission. Otherwise, no other admissions related to 14-IFO treatment were reported. No grade 3–4 anaemia or non-haematological toxicities were reported. Moreover, throughout the whole treatment period, no patients exhibited neurological toxicities. Notably, one patient experienced deterioration of a pre-existing renal toxicity. This patient commenced 14-IFO treatment with an ongoing grade 2 renal toxicity according to CTCAE v.5.0 (chronic kidney disease with increased creatinine and reduced estimated Glomerular Filtration Rate [eGFR]) and after two 14-IFO cycles, the renal toxicity worsened compared to baseline, reaching grade 3.
Previous treatment with High-Dose Ifosfamide (10 g/sqm or 15 g/sqm delivered over five days infusion) did not increase the toxicity incidence rate, nor affected haematological and non-haematological toxicities.
Patients and their caregiver did not encountered difficulties in managing the elastomer-pump at home, and no patients required telehealth assistance or face-to-face support due to technical pump issues.
Discussion
Our results provided evidence of the anti-tumour activity of 14-IFO in heavily pre-treated paediatric and young adult patients with R/R OS, alongside a good quality of life.
The ORR and DCR obtained with 14-IFO was 23% and 57.5%, respectively. Notably, patients with relapsed OS showed a higher ORR (45%) and DCR (82%) compared to refractory patients, irrespective of the number of prior treatment lines received. The achievement of disease control with 14-IFO administration allowed 27% of patients to undergo subsequent local treatment, which is still considered the best treatment option for R/R OS [10]. For 71% of them, the local treatment was a new surgical procedure.
Four-month PFS was 54% for the whole cohort of patients and 82% for the relapsed OS sub-group, highlighting a positive outcome for patients with advanced OS according to the recent clinical trials outcomes recommendation [8]. Median OSurv was 13.7 months and 1-year OSurv was 51% for all patients and 71% for relapsed patients. Aside from age and disease status, where an age over 18 years old and refractory disease status were identified as negative prognostic factors, other clinical features did not significantly influence the outcome. Furthermore, no significant PFS and OSurv differences were observed according to previous treatment regimens. More interestingly, our analysis revealed that prior treatment with High-Dose Ifosfamide (10 g/sqm or 15 g/sqm delivered over five days infusion) did not influence the survival rate or the incidence of toxicity. While these findings warrant confirmation in a larger patient cohort, they suggest that 14-IFO could be a viable and well-tolerated therapeutic option also for patients with R/R OS previously treated with High-Dose Ifosfamide.
Compared to other chemotherapy or tyrosine kinase inhibitor regimens recommended by major International OS Guidelines [1, 34], this trial demonstrated that the14-IFO schedule is not only feasible but also exhibits a significant antitumour activity in this setting. While previously published results for R/R OS encompass a heterogeneous patient population, and randomized controlled trials are infrequent in this setting, the outcomes described here align with and are comparable to those reported in the literature including High-Dose Ifosfamide administered at a schedule of 3 g/sqm over 5 days [12]. In particular, in our analysis, we observed an ORR of 20%, consistent with the previously reported ORR of 23% with the use of High-Dose Ifosfamide delivered over 5 days [12]. Furthermore, in our analysis the 2-year OSurv was 22% for the overall cohort and 30% for the relapsed OS subgroup, compared to the previously reported 2-year OSurv of 30% for the different schedule infusion [12]. It is noteworthy that our cohort was more heavily pretreated, with 50% of patients having received more than one treatment before 14-IFO, compared to only 10% of patients in the previous study using the High dose Ifosfamide schedule over 5 days [12].
Regarding the GMI data, it is important to highlight that our GMI evaluation remains exploratory and is limited by the small number of patients assessed for this analysis. In a context where further studies are required to confirm the overall reliability of GMI in assessing the efficacy of experimental drugs in advanced OS [39], our findings contribute to this ongoing investigation.
Considerably, our results highlight the manageable toxic profile of the 14-IFO regimen. Patients were able to receive chemotherapy treatment at home with scheduled clinical visits (every 3 or 7 days, according to local practice) using an external elastomer-pump carefully charged with the optimal Ifosfamide dose and MESNA.
Patients treated with 14-IFO showed a tolerable toxic profile in terms of haematological and non-haematological events (including nausea, gastrointestinal, renal and neurological events). Ifosfamide-induced encephalopathy, although rare, is a well-known adverse event which can occur in 2–5% of patients who have received the drug either intravenous or orally [36, 37] It can occur 12 to 146 h after the start of the infusion with different clinical features (from mild to life-threatening symptoms) [36, 37]. Notably, the cohort of patients analyzed in this study didn’t show any neurological toxicities, consistent with the previously reported association with continuous infusion and fewer side-effects, including low incidence of neurological events [19–22]. In fact, a prolonged infusion induces a reduction in the half-life of the active metabolites of ifosfamide, increasing their clearance and giving rise to a lower plasma peak, without changing the drug’s alkylating activity [19–22].
Due to the low toxic profile, patients treated with 14-IFO, could pursue their routinely activities outside of hospital, preserving their quality of life. The daily and nocturnal management of elastomer pump was feasible at home with a little bag or backpack or in a large pyjama trouser pocket, without any unexpected technical issues at home.
In the context of a rare tumour with a poor prognosis, it is strongly encouraged that the treatment options are guided by a careful balance between the potential for cure, the toxicity profile of the intended treatment, and the patient’s quality of life [2, 35]. For this reason, therapeutic decisions should prioritize the least toxic treatment option, and 14-IFO should be regarded as a viable course of action. Our results suggest that the use of 14-IFO represents a promising alternative option to other chemotherapy regimen, especially for Relapsed OS patients. Patients with Refractory OS continue to represent an unmet clinical need, highlighting the necessity for further research efforts to enhance our understanding and counteract the aggressive biological behavior of this disease.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge Mrs. Victoria Clifford for assistance with the manuscript.
Author contributions
ET, FF: conceptualisation of the study, results interpretation, writing original draft and revised the manuscript. AC, SA: data collection, results interpretation, writing original draft and revised the manuscript. VS, PB: formal statistical analysis, results interpretation, writing original draft and revised the manuscript. CM, GS, AL, TI, AT, LC, FC, PQ, CC, EDL: data collection, validation, reviewing and editing of main draft. All authors provided critical feedback and helped shape the research and analysis, discussed the results and commented on the manuscript.
Funding
None declared.
Data availability
Anonymized data that support the findings of this study are available from the corresponding author, AC and VS, upon request.
Declarations
Ethics approval and consent to participate
The study protocol was approved by the local ethics committee (COMITATO ETICO INTERAZIENDALE A.O.U. CITTA’. DELLA SALUTE E DELLA SCIENZA A.O. MAURIZIANO A.S.L. CITTA’ DI. TORINO) and registered with ClinicalTrials.gov, number NCT04651569 on 12th March 2020. Informed consent for study participation and publication was obtained by all subjects and/or their legal guardian(s) according to each Institution guideline and local Ethic Committee. All study procedures were carried out in accordance with the International Council for Harmonisation guidelines on good clinical practice and the STROBE Statement for observational studies.
Consent for publication
Written informed consent for study participation and publication was obtained by all subjects and/or their legal guardian(s).
Competing interests
The authors declare no competing interests.
Disclosure
The authors have declared no potential conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anna Campello, Email: anna.campello@unito.it.
Veronica Sciannameo, Email: veronica.sciannameo@unito.it.
References
- 1.Strauss SJ, Frezza AM, Abecassis N, Bajpai J, Bauer S, Biagini R, et al. Bone sarcomas: ESMO–EURACAN–GENTURIS–ERN PaedCan Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2021;32(12):1520–36. doi: 10.1016/j.annonc.2021.08.1995. [DOI] [PubMed] [Google Scholar]
- 2.van Ewijk R, Herold N, Baecklund F, Baumhoer D, Boye K, Gaspar N, et al. European standard clinical practice recommendations for children and adolescents with primary and recurrent osteosarcoma. EJC Pediatr Oncol. 2023;2:100029. doi: 10.1016/j.ejcped.2023.100029. [DOI] [Google Scholar]
- 3.Marina NM, Smeland S, Bielack SS, Bernstein M, Jovic G, Krailo MD, et al. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): an open-label, international, randomised controlled trial. Lancet Oncol. 2016;17(10):1396–408. doi: 10.1016/S1470-2045(16)30214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielack SS, Smeland S, Whelan JS, Marina N, Jovic G, Hook JM, et al. Methotrexate, Doxorubicin, and cisplatin (MAP) plus maintenance Pegylated Interferon Alfa-2b versus MAP alone in patients with Resectable High-Grade Osteosarcoma and good histologic response to preoperative MAP: first results of the EURAMOS-1 Good Response Randomized Controlled Trial. JCO. 2015;33(20):2279–87. doi: 10.1200/JCO.2014.60.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaspar N, Occean BV, Pacquement H, Bompas E, Bouvier C, Brisse HJ, et al. Results of methotrexate-etoposide-ifosfamide based regimen (M-EI) in osteosarcoma patients included in the French OS2006/sarcome-09 study. Eur J Cancer. 2018;88:57–66. doi: 10.1016/j.ejca.2017.09.036. [DOI] [PubMed] [Google Scholar]
- 6.Palmerini E, Meazza C, Tamburini A, Bisogno G, Ferraresi V, Asaftei SD, et al. Phase 2 study for nonmetastatic extremity high-grade osteosarcoma in pediatric and adolescent and young adult patients with a risk‐adapted strategy based on ABCB1/P‐glycoprotein expression: an Italian Sarcoma Group trial (ISG/OS‐2) Cancer. 2022;128(10):1958–66. doi: 10.1002/cncr.34131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jafari F, Javdansirat S, Sanaie S, Naseri A, Shamekh A, Rostamzadeh D, et al. Osteosarcoma: a comprehensive review of management and treatment strategies. Annals Diagn Pathol. 2020;49:151654. doi: 10.1016/j.anndiagpath.2020.151654. [DOI] [PubMed] [Google Scholar]
- 8.Lagmay JP, Krailo MD, Dang H, Kim A, Hawkins DS, Beaty O, et al. Outcome of patients with recurrent Osteosarcoma enrolled in seven phase II trials through children’s Cancer Group, Pediatric Oncology Group, and Children’s Oncology Group: learning from the past to Move Forward. JCO. 2016;34(25):3031–8. doi: 10.1200/JCO.2015.65.5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kempf-Bielack B, Bielack SS, Jürgens H, Branscheid D, Berdel WE, Exner GU, et al. Osteosarcoma Relapse after Combined Modality Therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) JCO. 2005;23(3):559–68. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari S, Briccoli A, Mercuri M, Bertoni F, Picci P, Tienghi A, et al. Postrelapse Survival in Osteosarcoma of the extremities: prognostic factors for long-term survival. JCO. 2003;21(4):710–5. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 11.Tirtei E, Asaftei SD, Manicone R, Cesari M, Paioli A, Rocca M, et al. Survival after second and subsequent recurrences in osteosarcoma: a retrospective multicenter analysis. Tumori. 2018;104(3):202–6. doi: 10.1177/0300891617753257. [DOI] [PubMed] [Google Scholar]
- 12.Palmerini E, Setola E, Grignani G, D’Ambrosio L, Comandone A, Righi A, et al. High dose Ifosfamide in Relapsed and Unresectable High-Grade Osteosarcoma patients: a Retrospective Series. Cells. 2020;9(11):2389. doi: 10.3390/cells9112389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chou AJ, Merola PR, Wexler LH, Gorlick RG, Vyas YM, Healey JH, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104(10):2214–21. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 14.Verschoor AJ, Speetjens FM, Dijkstra PDS, Fiocco M, Sande MAJ, Bovée JVMG, et al. Single-center experience with Ifosfamide Monotherapy as Second-Line treatment of Recurrent/Metastatic osteosarcoma. Oncologist. 2020;25(4):e716–21. doi: 10.1634/theoncologist.2019-0528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris MB, Cantor AB, Goorin AM, Shochat SJ, Ayala AG, Ferguson WS, et al. Treatment of osteosarcoma with ifosfamide: comparison of response in pediatric patients with recurrent disease versus patients previously untreated: a pediatric oncology group study. Med Pediatr Oncol. 1995;24(2):87–92. doi: 10.1002/mpo.2950240205. [DOI] [PubMed] [Google Scholar]
- 16.Patel SR, Vadhan-Raj S, Papadopolous N, Plager C, Burgess MA, Hays C, et al. High-dose ifosfamide in bone and soft tissue sarcomas: results of phase II and pilot studies–dose-response and schedule dependence. JCO. 1997;15(6):2378–84. doi: 10.1200/JCO.1997.15.6.2378. [DOI] [PubMed] [Google Scholar]
- 17.Berrak SG, Pearson M, Berberoğlu S. Ilhan Inci ErgüRhan, Jaffe N. High-dose ifosfamide in relapsed pediatric osteosarcoma: therapeutic effects and renal toxicity. Pediatr Blood Cancer. 2005;44(3):215–9. [DOI] [PubMed]
- 18.Ferrari S, Zolezzi C, Cesari M, Fasano MC, Lamanna G, Bacci G. Prospective evaluation of high-dose ifosfamide-related nephrotoxicity in young adult patients with recurrent osteosarcoma previously treated with cisplatin, methotrexate and standard-dose ifosfamide. Anticancer Drugs. 1999;10(1):25–32. doi: 10.1097/00001813-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Carter TJ, Milic M, McDerra J, McTiernan A, Ahmed M, Karavasilis V, et al. Continuous 14 day Infusional Ifosfamide for Management of Soft-tissue and bone sarcoma: a single Centre Retrospective Cohort Analysis. Cancers. 2020;12(11):3408. doi: 10.3390/cancers12113408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meazza C, Casanova M, Luksch R, Podda M, Favini F, Cefalo G, et al. Prolonged 14-day continuous infusion of high-dose ifosfamide with an external portable pump: feasibility and efficacy in refractory pediatric sarcoma. Pediatr Blood Cancer. 2010;55(4):617–20. doi: 10.1002/pbc.22596. [DOI] [PubMed] [Google Scholar]
- 21.Cerny T, Castiglione M, Brunner K, Küpfer A, Martinelli G, Lind M. Ifosfamide by continuous infusion to prevent encephalopathy. Lancet. 1990;335(8682):175. doi: 10.1016/0140-6736(90)90053-8. [DOI] [PubMed] [Google Scholar]
- 22.Boddy AV, Yule SM, Wyllie R, Price L, Pearson ADJ, Idle JR. Comparison of continuous infusion and bolus administration of ifosfamide in children. Eur J Cancer. 1995;31(5):785–90. doi: 10.1016/0959-8049(95)00090-6. [DOI] [PubMed] [Google Scholar]
- 23.Palmerini E, Jones RL, Marchesi E, Paioli A, Cesari M, Longhi A, et al. Gemcitabine and docetaxel in relapsed and unresectable high-grade osteosarcoma and spindle cell sarcoma of bone. BMC Cancer. 2016;16(1):280. doi: 10.1186/s12885-016-2312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song BS, Seo J, Kim DH, Lim JS, Yoo JY, Lee JA. Gemcitabine and docetaxel for the treatment of children and adolescents with recurrent or refractory osteosarcoma: Korea Cancer Center Hospital experience. Pediatr Blood Cancer. 2014;61(8):1376–81. doi: 10.1002/pbc.25035. [DOI] [PubMed] [Google Scholar]
- 25.Navid F, Willert JR, McCarville MB, Furman W, Watkins A, Roberts W, et al. Combination of gemcitabine and docetaxel in the treatment of children and young adults with refractory bone sarcoma. Cancer. 2008;113(2):419–25. doi: 10.1002/cncr.23586. [DOI] [PubMed] [Google Scholar]
- 26.Fox E, Patel S, Wathen JK, Schuetze S, Chawla S, Harmon D, et al. Phase II study of Sequential Gemcitabine followed by Docetaxel for Recurrent Ewing Sarcoma, Osteosarcoma, or unresectable or locally recurrent Chondrosarcoma: results of Sarcoma Alliance for Research through collaboration Study 003. Oncologist. 2012;17(3):321–e329. doi: 10.1634/theoncologist.2010-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berger M, Grignani G, Ferrari S, Biasin E, Brach del Prever A, Aliberti S, et al. Phase 2 trial of two courses of cyclophosphamide and etoposide for relapsed high-risk osteosarcoma patients. Cancer. 2009;115(13):2980–7. doi: 10.1002/cncr.24368. [DOI] [PubMed] [Google Scholar]
- 28.Rodríguez-Galindo C, Daw NC, Kaste SC, Meyer WH, Dome JS, Pappo AS, et al. Treatment of refractory osteosarcoma with fractionated Cyclophosphamide and Etoposide. J Pediatr Hematol Oncol. 2002;24(4):250–5. doi: 10.1097/00043426-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 29.Miser JS, Kinsella TJ, Triche TJ, Tsokos M, Jarosinski P, Forquer R, et al. Ifosfamide with mesna uroprotection and etoposide: an effective regimen in the treatment of recurrent sarcomas and other tumors of children and young adults. JCO. 1987;5(8):1191–8. doi: 10.1200/JCO.1987.5.8.1191. [DOI] [PubMed] [Google Scholar]
- 30.Kung FH, Pratt CB, Vega RA, Jaffe N, Strother D, Schwenn M, et al. Ifosfamide/etoposide combination in the treatment of recurrent malignant solid tumors of childhood. A pediatric oncology group phase II study. Cancer. 1993;71(5):1898–903. doi: 10.1002/1097-0142(19930301)71:5<1898::AID-CNCR2820710529>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Coriat R, Mir O, Camps S, Ropert S, Billemont B, Leconte M, et al. Ambulatory administration of 5-day infusion ifosfamide + mesna: a pilot study in sarcoma patients. Cancer Chemother Pharmacol. 2010;65(3):491–5. doi: 10.1007/s00280-009-1054-1. [DOI] [PubMed] [Google Scholar]
- 32.Loeffler TM, Weber FW, Hausamen TU. Ambulatory high-dose 5-day continuous-infusion ifosfamide combination chemotherapy in advanced solid tumors: a feasibility study. J Cancer Res Clin Oncol. 1991;117(S4):S125–8. doi: 10.1007/BF01613216. [DOI] [PubMed] [Google Scholar]
- 33.Schwartz LH, Seymour L, Litière S, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1 – standardisation and disease-specific adaptations: perspectives from the RECIST Working Group. Eur J Cancer. 2016;62:138–45. doi: 10.1016/j.ejca.2016.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.NCCN. Guidelines_Bone Sarcoma.pdf.
- 35.Wiener L, Zadeh S, Battles H, Baird K, Ballard E, Osherow J, et al. Allowing adolescents and young adults to Plan their end-of-Life Care. Pediatrics. 2012;130(5):897–905. doi: 10.1542/peds.2012-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Di Cataldo A, Astuto M, Rizzo G, Bertuna G, Russo G, Incorpora G. Neurotoxicity during ifosfamide treatment in children. Med Sci Monit. 2009;15(1):CS22–5. [PubMed] [Google Scholar]
- 37.Idle JR. Diren Beyoğlu,. Ifosfamide - History, efficacy, toxicity and encephalopathy. Pharmacol Ther. 2023;243:108–366. doi: 10.1016/j.pharmthera.2023.108366. [DOI] [PubMed] [Google Scholar]
- 38.Von Hoff DD. There are no bad anticancer agents, only bad clinical trial designs–twenty-first Richard and Hinda Rosenthal Foundation Award lecture. Clin Cancer Res. 1998;4:1079–86. [PubMed] [Google Scholar]
- 39.Italiano A, Mir O, Mathoulin-Pelissier S, Penel N, Piperno-Neumann S, Bompas E, Chevreau C, Duffaud F, Entz-Werlé N, Saada E, Ray-Coquard I, Lervat C, Gaspar N, Marec-Berard P, Pacquement H, Wright J, Toulmonde M, Bessede A, Crombe A, Kind M, Bellera C, Blay JY. Cabozantinib in patients with advanced ewing sarcoma or osteosarcoma (CABONE): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2020;21(3):446–55. doi: 10.1016/S1470-2045(19)30825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data that support the findings of this study are available from the corresponding author, AC and VS, upon request.