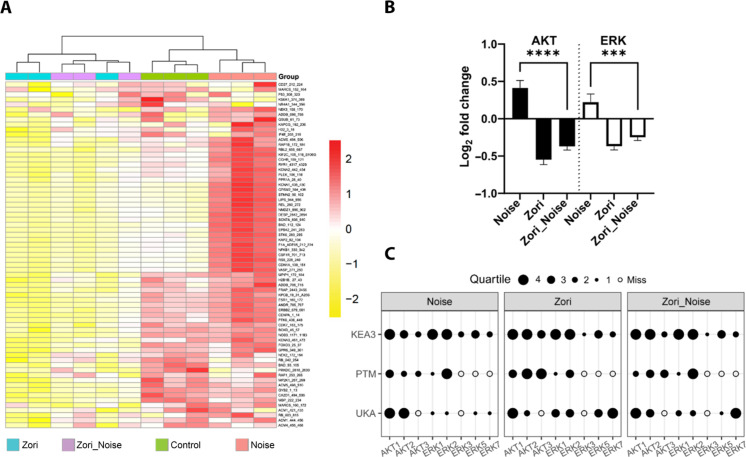
Fig. 8. Zorifertinib suppresses noise-activated EGFR signaling pathways in mouse cochleae.
(A) Heatmap of phosphorylation activity at the reporter peptides on the kinome panel (STK PamChip; n = 3 chips run in technical triplicate). Cochlear sensory epithelial lysates were collected 30-min after noise exposure from control, noise-exposed, zorifertinib-treated (Zori), and zorifertinib-treated plus noise-exposed (Zori_Noise) adult FVB mice. Each row represents a peptide. The protein kinase activity measured as phosphorylation of reported peptides on the chip is scaled with red being higher activity and yellow indicating lower activity. (B) Log2 fold activity changes of reporter peptides “mapped” as putative targets of AKT and/or ERK kinase families (24 and 25 peptides, respectively) expressed as means ± SD. An average of triplicates was used for each peptide per condition. N/C, noise versus control; Z/C, zorifertinib versus control; N + Z/C, noise + zorifertinib versus control. Bars, SEM; ****P < 0.0001 and ***P < 0.001, two-way ANOVA. (C) Identification of specific protein kinases using complementary software packages (details in the Supplementary Materials). Selected AKT and ERK family members were identified as candidate “hits” using deconvolution strategies that specify protein kinases that are contributing to the phosphorylation signal seen across all reporter peptides on the STK chip. Upstream kinase analysis (UKA), posttranslational modification signature enrichment analysis (PTM-SEA), and kinase enrichment analysis 3 (KEA3) were used to identify specific kinases within the AKT and ERK families using online phosphosite mapping databases. Data were normalized and grouped as quartiles (1 to 4 black dots) for visualization using our bespoke Creedenzymatic R package.