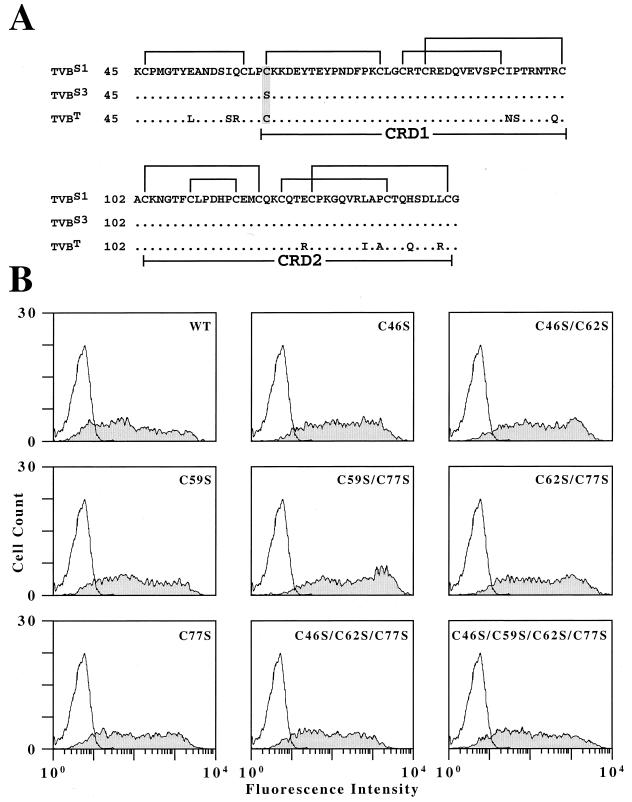
FIG. 3.
Mutational analysis of the first four cysteine residues of TVBS1. (A) TVBS3 and TVBS1 differ by a serine-to-cysteine substitution at residue 62 (shaded). The regions of TVBS1, TVBS3, and TVBT that encompass all extracellular cysteine residues (amino acids 45 to 144) are shown aligned, and the predicted intrachain disulfide bonds in TVBS1 are indicated. (B) All of the mutant receptors bearing cysteine mutations were expressed at the cell surface and bind to SUB-rIgG. Human 293 cells transfected with no DNA or with plasmids encoding wild-type TVBS1(ΔDD) (WT) or with mutant forms of this receptor were incubated with extracellular supernatant containing SUB-rIgG and with a fluorescein isothiocyanate-conjugated antibody specific for rabbit Igs. The cells were then analyzed by flow cytometry; the results shown were obtained with nonreceptor-expressing cells (open histograms) and receptor-expressing cells (shaded histograms).