Abstract
Objective:
To compare the rate of postoperative 30-day complications between laparoscopic pancreaticoduodenectomy (LPD) and robotic pancreaticoduodenectomy (RPD).
Background:
Previous studies suggest that minimally invasive pancreaticoduodenectomy (MI-PD)—either LPD or RPD—is noninferior to open pancreaticoduodenectomy in terms of operative outcomes. However, a direct comparison of the two minimally invasive approaches has not been rigorously performed.
Methods:
Patients who underwent MI-PD were abstracted from the 2014 to 2019 pancreas-targeted American College of Surgeons National Sample Quality Improvement Program (ACS NSQIP) dataset. Optimal outcome was defined as absence of postoperative mortality, serious complication, percutaneous drainage, reoperation, and prolonged length of stay (75th percentile, 11 days) with no readmission. Multivariable logistic regression models were used to compare optimal outcome of RPD and LPD.
Results:
A total of 1540 MI-PDs were identified between 2014 and 2019, of which 885 (57%) were RPD and 655 (43%) were LPD. The rate of RPD cases/year significantly increased from 2.4% to 8.4% (P = 0.008) from 2014 to 2019, while LPD remained unchanged. Similarly, the rate of optimal outcome for RPD increased during the study period from 48.2% to 57.8% (P < 0.001) but significantly decreased for LPD (53.5% to 44.9%, P < 0.001). During 2018–2019, RPD outcomes surpassed LPD for any complication [odds ratio (OR) = 0.58, P = 0.004], serious complications (OR = 0.61, P = 0.011), and optimal outcome (OR = 1.78, P = 0.001).
Conclusions:
RPD adoption increased compared with LPD and was associated with decreased overall complications, serious complications, and increased optimal outcome compared with LPD in 2018–2019.
Keywords: laparoscopic, minimally invasive, optimal outcome, pancreaticoduodenectomy, robotic
Pancreaticoduodenectomy (PD) is the mainstay of surgical therapy for patients with resectable pancreatic ductal adenocarcinoma and other periampullary pathologies.1 This surgical procedure is technically demanding and has historically been associated with significant morbidity and mortality.2 The adoption of minimally invasive pancreaticoduodenectomy (MI-PD) has been slow due to the complexity of the surgical dissection, the need to perform several anastomoses and the initial uncertainty about the added benefits offered by the minimally invasive (MI) platforms over the established open approach (OPD).3 Retrospective series and randomized trials have highlighted some key advantages of MI-PD which include a decrease in intraoperative blood loss, wound complications, and postoperative pain coupled with quicker recovery time and shorter length of stay (LOS) compared with a laparotomy approach.4–6 However, selection bias typically favors MI approaches. In addition, laparoscopic pancreaticoduodenectomy (LPD) remains a considerable technical challenge, as highlighted by the LEOPARD-2 trial that demonstrated increased 90-day mortality compared with OPD, which ultimately lead to its early termination.7 Three other randomized controlled trials also have failed to show a clinically meaningful advantage for LPD.8–10
Robotic pancreaticoduodenectomy (RPD) is considered by many as a natural evolution of LPD providing surgeons with increased dexterity from endo-wristed instruments, filtration of tremors, 3-dimensional stereoscopic views of the field, and the ability to perform complex dissection, sutures and knots with unprecedented precision and accuracy.11,12 Recently, multiple conflicting reports have been published on the safety and feasibility of MI-PD, yet the superiority of one MI-platform over the other has not been established.13,14 Therefore, the primary aim of this study was to compare RPD and LPD with respect to trends over time, 30-day complication rate, and optimal outcome using a large national database.
METHODS
Study Design
The study utilized the 2014–2019 American College of Surgeons National Sample Quality Improvement Program (ACS NSQIP) pancreas-targeted participant user files (PT-PUF) database to perform a retrospective cohort study comparing RPD and LPD trends and outcomes. The ACS NSQIP collects > 150 variables from 700 participating hospitals, including preoperative, intraoperative, and 30-day postoperative mortality and morbidity outcomes.15 The PT-PUF files benefits from 26 additional variables specific to pancreatectomy in comparison to the general NSQIP database. The ACS NSQIP database is maintained by trained and certified surgical clinical reviewers who collect and verify the accuracy of the data. Due to the nature of the database used, no institutional review board approval was required for this study.
Patient Selection and Definitions
All patients who underwent elective, nonemergent PD were identified using the procedural terminology (CPT) codes of 41850, 48152 (classic PD procedure with and without pancreatojejunostomy, respectively), 48153, and 48154 (pylorus-preserving PD with and without pancreatojejunostomy).
Patients who underwent other procedures performed concomitantly were excluded. The flow diagram for the final cohort included in the study is presented in Supplemental Digital Content 1 (http://links.lww.com/SLA/E192).
Outcomes and Trends
The primary outcomes of this study were measured at 30 days and included: (a) any complication rate, (b) serious complication rate, (c) optimal outcome, and (d) trends of both LPD and RPD. The data were classified into 3 distinct time periods: 2014–2015, 2016–2017, 2018–2019. Serious complication included any of the following events: pneumonia, unplanned intubation, pulmonary embolism, requiring ventilator support for > 48 hours, deep surgical site infection, organ space surgical site infection, wound dehiscence, deep vein thrombosis/thrombophlebitis, cerebrovascular accident, cardiac arrest, myocardial infarction, sepsis/septic shock, or renal failure.16 Optimal pancreatic surgery outcome was defined as absence of postoperative mortality, serious complication, percutaneous drainage, reoperation, and prolonged LOS (75th percentile, 11 days) with no readmission.17,18
Statistical Analysis
Categorical values were shown as counts and proportions, while continuous variables were reported as medians with interquartile ranges. Univariate linear regression for continuous variables and a Pearson χ2 or Fisher exact tests or univariate logistic regression were performed, when appropriate, for categorical variables. To determine the association of the type of MI approach with complications and optimal outcome, preoperative and intraoperative factors were adjusted using forward multivariable logistic regression. Variables with P value <0.2 on univariate analysis were carried on to the multivariate analysis. The significance level was set at <0.05 for all analyses. Statistical analyses were performed using the IBM SPSS statistical package (Version 26; IBM Corp., Armonk, NY).
RESULTS
Baseline Characteristics
A total of 1540 MI-PD cases met inclusion criteria of which 885 (55.5%) were RPD and 655 (44.5%) were LPD. Patients who underwent RPD were older (67 vs 65 years, P < 0.05), more likely to be White (82.4% vs 71.6%, P < 0.001), have preoperative biliary stenting (55.2% vs 45.8%, P = 0.013), receive neoadjuvant chemotherapy (24.5% vs 17.4%, P < 0.001), have pancreatic duct size <3 mm (33.3% vs 24.6%, P < 0.001), and soft pancreatic gland texture (48.2% vs 31.1%, P < 0.001) compared with LPD (Table 1). Nonetheless, neoadjuvant radiotherapy utilization [7.8% (n = 51/655) vs 3.4% (30/885), P < 0.001] and vein resection (12.1% vs 6.1%, P < 0.001) were recorded with higher frequency in the LPD compared with RPD group, respectively (Table 1). The two cohorts were similar for the remaining examined variables (Table 1, Supplemental Digital Content 2, http://links.lww.com/SLA/E193).
TABLE 1.
Characteristics of Patients Undergoing MI-PD From 2014 to 2019
| n (%)/Median (IQR) |
P | ||
|---|---|---|---|
| Robotic (N = 885) | Laparoscopic (N = 655) | ||
|
| |||
| Age (y) | 67 (59, 73) | 65 (57, 72) | 0.045 |
| Sex | 0.764 | ||
| Male | 462 (52.2) | 347 (53.0) | |
| Female | 423 (47.8) | 308 (47.0) | |
| Race | < 0.001 | ||
| White | 729 (82.4) | 469 (71.6) | |
| African American | 69 (7.8) | 57 (8.7) | |
| Other | 87 (9.8) | 129 (19.7) | |
| BMI | 27.1 (23.7, 31.1) | 26.95 (23.7, 30.4) | 0.363 |
| Jaundice | 310 (35.0) | 237 (36.2) | 0.83 |
| Biliary stenting | 462 (52.2) | 300 (45.8) | 0.013 |
| ASA | 0.112 | ||
| I | 2 (0.2) | 5 (0.8) | |
| II | 211 (23.8) | 174 (26.6) | |
| III | 641 (72.4) | 445 (67.9) | |
| IV | 31 (3.5) | 31 (4.7) | |
| Neoadjuvant chemotherapy | 217 (24.5) | 114 (17.4) | 0.001 |
| Neoadjuvant radiotherapy | 30 (3.4) | 51 (7.8) | 0.001 |
| Pancreatic duct size | < 0.001 | ||
| < 3 mm | 295 (33.3) | 161 (24.6) | |
| > 3 mm | 485 (54.8) | 314 (47.9) | |
| Unknown | 105 (11.9) | 180 (27.5) | |
| Pancreatic gland | < 0.001 | ||
| texture | |||
| Soft | 427 (48.2) | 204 (31.1) | |
| Hard | 328 (37.1) | 252 (38.5) | |
| Unknown | 130 (14.7) | 199 (30.4) | |
| Vessel resection | < 0.001 | ||
| None | 792 (89.5) | 524 (80.0) | |
| Vein | 54 (6.1) | 79 (12.1) | |
| Artery | 11 (1.2) | 19 (2.9) | |
| Both | 18 (2.0) | 19 (2.9) | |
| Unknown | 10 (1.1) | 14 (2.1) | |
| Histology | 0.809 | ||
| Malignant | 699 (79.0) | 514 (78.5) | |
| Benign | 186 (21.0) | 141 (21.5) | |
| Malignant histology | 0.632 | ||
| PDAC | 449 (64.2) | 337 (65.6) | |
| Non-PDAC | 250 (35.8) | 177 (34.4) | |
ASA indicates American Society of Anesthesiology; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; PDAC, pancreatic ductal adenocarcinoma.
Univariate Outcomes of RPD Versus LPD
For the entire cohort, LPD had significantly more perioperative transfusions (18.6% vs 9.3%, P < 0.001), a longer operative time [421 vs 405 (median, minutes), P = 0.012], and a higher rate of serious complication (40.2% vs 33.4%, P = 0.006), conversion to laparotomy (31.3% vs 13.3%, P < 0.001), and pneumonia (4.4% vs 1.8%, P = 0.003) compared with RPD. On the other end, RPD had a significantly higher rate of readmission [21.7% (n = 192/885) vs 16.6% (n = 109/665), P = 0.013] compared with LPD (Supplemental Digital Content 3, http://links.lww.com/SLA/E194).
In 2014–2015, RPD had a significantly higher rate of any complication (62.9% vs 47.5%, P = 0.008), serious complication (46.8% vs 35%, P = 0.028), and superficial surgical site infection (10.6% vs 3%, P = 0.004) compared with LPD. As a result, the rate of optimal outcome was lower for RPD compared with LPD (48.2% vs 53.5%, P = 0.518); however, this difference was not statistically significant. Nonetheless—even in the early years (2014–2015)—the conversion rate was significantly higher in the LPD (26.7% vs 13.5%, P = 0.003) compared with RPD (Table 2). In 2018–2019, RPD had a significantly higher rate of optimal outcome (57.8% vs 44.9%, P = 0.002) compared with LPD. Moreover, LPD demonstrated a significantly higher rate of conversion (36.4% vs 15%, P < 0.001), perioperative transfusion (22.2% vs 9.4%, P < 0.001), any complication (61% vs 47.7%, P = 0.001), serious complication (45.3% vs 32.3%, P = 0.001), pneumonia (7.1% vs 2%, P = 0.001), and prolonged LOS (28.7% vs 20.3%, P = 0.016) compared with RPD (Table 3).
TABLE 2.
Univariate 30-day Outcomes in 2014–2015
| n (%)/Median (IQR) |
P | ||
|---|---|---|---|
| Robotic (N = 141) | Laparoscopic (N = 202) | ||
|
| |||
| Operative time (min) | 411 (336, 518) | 411 (239, 496) | 0.239 |
| Conversion | 19 (13.5) | 54 (26.7) | 0.003 |
| Perioperative transfusion | 20 (14.2) | 29 (14.4) | 0.964 |
| Mortality | 6 (4.3) | 8 (4) | 0.892 |
| Any complication | 78 (62.9) | 87 (47.5) | 0.008 |
| Serious complication | 66 (46.8) | 70 (35) | 0.028 |
| Superficial SSI | 15 (10.6) | 6 (3) | 0.004 |
| Deep SSI | 4 (2.8) | 2 (1) | 0.199 |
| Organ space SSI | 20 (14.2) | 22 (10.9) | 0.407 |
| Dehiscence | 1 (0.7) | 4 (2) | 0.334 |
| Pneumonia | 1 (0.7) | 8 (4) | 0.064 |
| Reintubation | 9 (6.4) | 9 (4.5) | 0.431 |
| Failure to wean off ventilator | 3 (2.1) | 2 (1) | 0.387 |
| Pulmonary embolism | 4 (2.8) | 10 (5) | 0.330 |
| Renal insufficiency | 0 (0) | 1 (0.5) | 0.403 |
| Acute renal failure | 2 (1.4) | 2 (1) | 0.716 |
| UTI | 8 (5.7) | 3 (1.5) | 0.030 |
| Cerebrovascular accident | 2 (1.4) | 0 (0) | 0.090 |
| Cardiac arrest | 4 (2.8) | 5 (2.5) | 0.837 |
| Myocardial infarction | 2 (1.4) | 0 (0) | 0.090 |
| Deep vein thrombosis | 4 (2.8) | 8 (4) | 0.577 |
| Sepsis | 10 (7.1) | 10 (5) | 0.405 |
| Septic shock | 4 (2.8) | 6 (3) | 0.942 |
| CR-POPF | 27 (19.1) | 25 (12.4) | 0.085 |
| Delayed gastric emptying | 22 (15.6) | 32 (15.8) | 0.702 |
| Drain POD30 | 14 (9.9) | 15 (7.4) | 0.412 |
| Prolonged LOS | 34 (24.1) | 44 (21.8) | 0.612 |
| Readmission | 30 (21.3) | 34 (16.8) | 0.298 |
| Optimal outcome | 68 (48.2) | 108 (53.5) | 0.084 |
CR-POPF indicates clinically relevant postoperative pancreatic fistula; IQR, interquartile range; POD, postoperative day; SSI, surgical site infection; UTI, urinary tract infection.
TABLE 3.
Univariate 30-day Outcomes in 2018–2019
| n (%)/Median (IQR) |
P | ||
|---|---|---|---|
| Robotic (N = 446) | Laparoscopic (N = 225) | ||
|
| |||
| Operative time (min) | 404 (342, 491) | 431 (365, 515) | 0.061 |
| Conversion | 67 (15) | 82 (36.4) | < 0.001 |
| Perioperative transfusion | 42 (9.4) | 50 (22.2) | < 0.001 |
| Mortality | 7 (1.6) | 8 (3.6) | 0.100 |
| Any complication | 200 (47.7) | 133 (61) | 0.001 |
| Serious complication | 144 (32.3) | 101 (45.3) | 0.001 |
| Superficial SSI | 25 (5.6) | 16 (7.1) | 0.442 |
| Deep SSI | 1 (0.2) | 3 (1.3) | 0.078 |
| Organ space SSI | 70 (15.7) | 46 (20.4) | 0.125 |
| Dehiscence | 0 (0) | 2 (0.9) | 0.046 |
| Pneumonia | 9 (2) | 16 (7.1) | 0.001 |
| Reintubation | 11 (2.5) | 9 (4) | 0.270 |
| Failure to wean off ventilator | 6 (1.3) | 4 (1.8) | 0.662 |
| Pulmonary embolism | 9 (2) | 9 (4) | 0.134 |
| Renal insufficiency | 3 (0.7) | 2 (0.9) | 0.758 |
| Acute renal failure | 4 (0.9) | 5 (2.2) | 0.159 |
| UTI | 4 (0.9) | 3 (1.3) | 0.599 |
| Cerebrovascular accident | 2 (0.4) | 0 (0) | 0.314 |
| Cardiac arrest | 3 (0.7) | 3 (1.3) | 0.391 |
| Myocardial infarction | 2 (0.4) | 4 (1.8) | 0.084 |
| Deep vein thrombosis | 13 (2.9) | 10 (4.4) | 0.304 |
| Sepsis | 28 (6.3) | 16 (7.1) | 0.681 |
| Septic shock | 12 (2.7) | 13 (5.8) | 0.046 |
| CR-POPF | 82 (18.4) | 50 (22.2) | 0.238 |
| Delayed gastric emptying | 78 (17.5) | 39 (17.3) | 0.453 |
| Drain POD30 | 38 (8.5) | 25 (11.1) | 0.277 |
| Prolonged LOS | 88 (20.3) | 62 (28.7) | 0.016 |
| Readmission | 93 (20.9) | 42 (18.7) | 0.505 |
| Optimal outcome | 257 (57.8) | 101 (44.9) | < 0.001 |
CR-POPF indicates clinically relevant postoperative pancreatic fistula; IQR, interquartile range; POD, postoperative day; SSI, surgical site infection; UTI, urinary tract infection.
Multivariate Analysis of Perioperative Outcomes: RPD Versus LPD
For the entire study cohort—between 2014 and 2019—the odds of any complication, serious complication, and optimal outcome were similar between RPD and LPD on multivariate analysis. In 2014–2015, compared with RPD, LPD had significantly lower odds of any complication [odds ratio (OR) = 0.50, P = 0.008] and serious complication (OR = 0.53, P = 0.008) (Table 4). However, in 2018–2019, LPD had significantly higher odds of any complication (OR = 1.73, P = 0.004) and serious complication (OR = 1.62, P = 0.011), while it had significantly lower odds of optimal outcome (OR = 0.56, P = 0.001) compared with RPD (Table 5).
TABLE 4.
Multivariate Analysis for Any Complication, Serious Complication, and Optimal Outcome in 2014–2015
| 2014–2015 | Any Complication |
Serious Complication |
Optimal Outcome |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% of OR |
P | OR | 95% of OR |
P | OR | 95% of OR |
P | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
|
| ||||||||||||
| Sex (female) | 0.659 | 0.416 | 1.042 | 0.075 | ||||||||
| Race | ||||||||||||
| White | Reference | Reference | ||||||||||
| African American | 2.404 | 0.921 | 6.276 | 0.073 | 0.458 | 0.195 | 1.074 | 0.072 | ||||
| Other | 0.937 | 0.425 | 2.063 | 0.871 | 1.318 | 0.596 | 2.913 | 0.495 | ||||
| BMI | 1.024 | 0.989 | 1.061 | 0.188 | ||||||||
| Diabetes | ||||||||||||
| None | Reference | 0.291 | ||||||||||
| Non–insulin dependent | 1.949 | 0.844 | 4.496 | 0.118 | ||||||||
| Insulin dependent | 1.044 | 0.458 | 2.381 | 0.919 | ||||||||
| History of smoking | 0.700 | 0.388 | 1.265 | 0.238 | ||||||||
| History of COPD | 1.361 | 0.383 | 4.834 | 0.634 | 0.313 | 0.096 | 1.025 | 0.055 | ||||
| History of hypertension | 1.494 | 0.892 | 2.501 | 0.127 | 0.567 | 0.362 | 0.887 | 0.013 | ||||
| Weight loss > 10% | 2.605 | 1.036 | 6.549 | 0.042 | 1.837 | 0.852 | 3.959 | 0.121 | ||||
| History of bleeding disorder | 2.237 | 0.568 | 8.810 | 0.250 | ||||||||
| History of obstructive jaundice | 0.648 | 0.393 | 1.069 | 0.089 | 0.596 | 0.370 | 0.960 | 0.033 | ||||
| Vessel involvement | ||||||||||||
| None | Reference | 0.145 | Reference | 0.064 | Reference | |||||||
| Vein | 1.925 | 0.868 | 4.269 | 0.107 | 2.170 | 1.076 | 4.375 | 0.030 | 0.605 | 0.302 | 1.212 | 0.156 |
| Artery | 2.600 | 0.634 | 10.669 | 0.185 | 3.410 | 0.955 | 12.169 | 0.059 | 0.658 | 0.174 | 2.483 | 0.537 |
| Both | 0.459 | 0.128 | 1.641 | 0.231 | 0.536 | 0.138 | 2.085 | 0.368 | 0.286 | 0.075 | 1.093 | 0.067 |
| Approach | ||||||||||||
| Robotic | Reference | Reference | ||||||||||
| Laparoscopic | 0.506 | 0.306 | 0.836 | 0.008 | 0.535 | 0.336 | 0.851 | 0.008 | ||||
BMI indicates body mass index; COPD, chronic obstructive pulmonary disease.
TABLE 5.
Multivariate Analysis for Any Complication, Serious Complication, and Optimal Outcome in 2018–2019
| 2018–2019 | Any Complication |
Serious Complication |
Optimal Outcome |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Multivariate |
Multivariate |
Multivariate |
||||||||||
| OR | 95% of OR |
P | OR | 95% of OR |
P | OR | 95% of OR |
P | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | |||||||
|
| ||||||||||||
| Age | 1.02 | 1.01 | 1.04 | 0.024 | 1.01 | 0.99 | 1.02 | 0.506 | ||||
| Sex (female) | 1.24 | 0.90 | 1.71 | 0.176 | ||||||||
| Race | ||||||||||||
| White | Reference | Reference | ||||||||||
| African American | 1.04 | 0.57 | 1.91 | 0.898 | 1.24 | 0.68 | 2.28 | 0.468 | ||||
| Other | 1.46 | 0.91 | 2.34 | 0.117 | 1.43 | 0.91 | 2.24 | 0.119 | ||||
| BMI | 1.04 | 1.08 | 1.07 | 0.015 | ||||||||
| Diabetes | ||||||||||||
| None | Reference | Reference | ||||||||||
| Non–insulin dependent | 0.75 | 0.45 | 1.23 | 0.250 | 0.86 | 0.54 | 1.38 | 0.551 | ||||
| Insulin dependent | 1.65 | 0.97 | 2.83 | 0.067 | 0.63 | 0.38 | 1.05 | 0.078 | ||||
| History of dyspnea | 3.17 | 1.17 | 8.54 | 0.023 | 2.76 | 1.12 | 6.82 | 0.027 | ||||
| History of COPD | 1.83 | 0.72 | 4.65 | 0.204 | 0.53 | 0.20 | 1.37 | 0.193 | ||||
| History of hypertension | 1.01 | 0.69 | 1.45 | 0.995 | 1.18 | 0.82 | 1.68 | 0.357 | 0.88 | 0.63 | 1.23 | 0.474 |
| History of biliary stenting | 0.76 | 0.54 | 1.07 | 0.117 | 0.76 | 0.54 | 1.07 | 0.125 | 1.99 | 1.36 | 2.92 | 0.000 |
| Neoadjuvant chemotherapy | 0.78 | 0.51 | 1.21 | 0.280 | ||||||||
| Neoadjuvant radiotherapy | 0.63 | 0.28 | 1.43 | 0.276 | ||||||||
| Duct size > 3 mm | 0.48 | 0.32 | 0.72 | 0.000 | 0.46 | 0.31 | 0.69 | 0.000 | 1.99 | 1.36 | 2.92 | 0.000 |
| Hard gland texture | 0.83 | 0.55 | 1.25 | 0.367 | 0.76 | 0.49 | 1.17 | 0.215 | 1.64 | 0.95 | 2.82 | 0.075 |
| Vessel involvement | ||||||||||||
| None | Reference | Reference | ||||||||||
| Vein | 1.35 | 0.77 | 2.37 | 0.294 | 1.68 | 0.95 | 2.96 | 0.074 | ||||
| Artery | 2.89 | 0.69 | 12.01 | 0.145 | 6.01 | 1.49 | 24.14 | 0.011 | ||||
| Both | 16.70 | 2.07 | 135.07 | 0.008 | 4.12 | 1.39 | 12.23 | 0.011 | ||||
| Approach | ||||||||||||
| Robotic | Reference | Reference | Reference | |||||||||
| Laparoscopic | 1.74 | 1.19 | 2.53 | 0.004 | 1.62 | 1.11 | 2.36 | 0.011 | 0.56 | 0.39 | 0.78 | 0.001 |
BMI indicates body mass index; COPD, chronic obstructive pulmonary disease.
Trends Over Time
The relative percentage of RPDs—over all PD recorded in the ACS NSQIP database—has significantly increased from 2014–2015 to 2018–2019 (2.4% vs 8.4%, P = 0.006), while no substantial change was observed in the relative percentage of LPD cases (Fig. 1). Notably, the rate of optimal outcome significantly increased for RPD (48.2% vs 52.9%, P < 0.001) from 2014–2015 to 2018–2019, respectively. Conversely, any complication (47.5% vs 57.8%, P < 0.001) and serious complication (35% vs 45.3%, P < 0.001) significantly increased from 2014–2015 to 2018–2019 among LPD cases (Supplemental Digital Content 4, http://links.lww.com/SLA/E195). Not surprisingly, the increase in LPDs complication rate was accompanied by a significant decrease in optimal outcome (53.5% vs 44.9%, P < 0.001) from 2014–2015 to 2018–2019 (Fig. 2).
FIGURE 1.
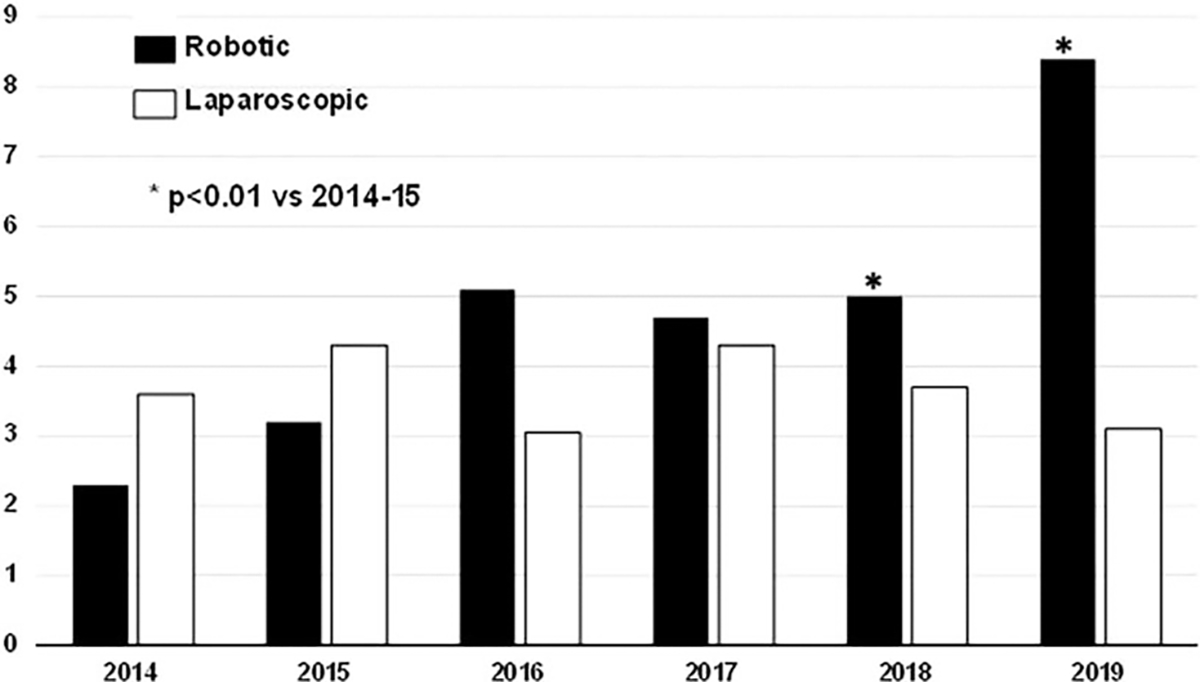
Change in percentage of MI-PD approaches from 2014 to 2019.
FIGURE 2.
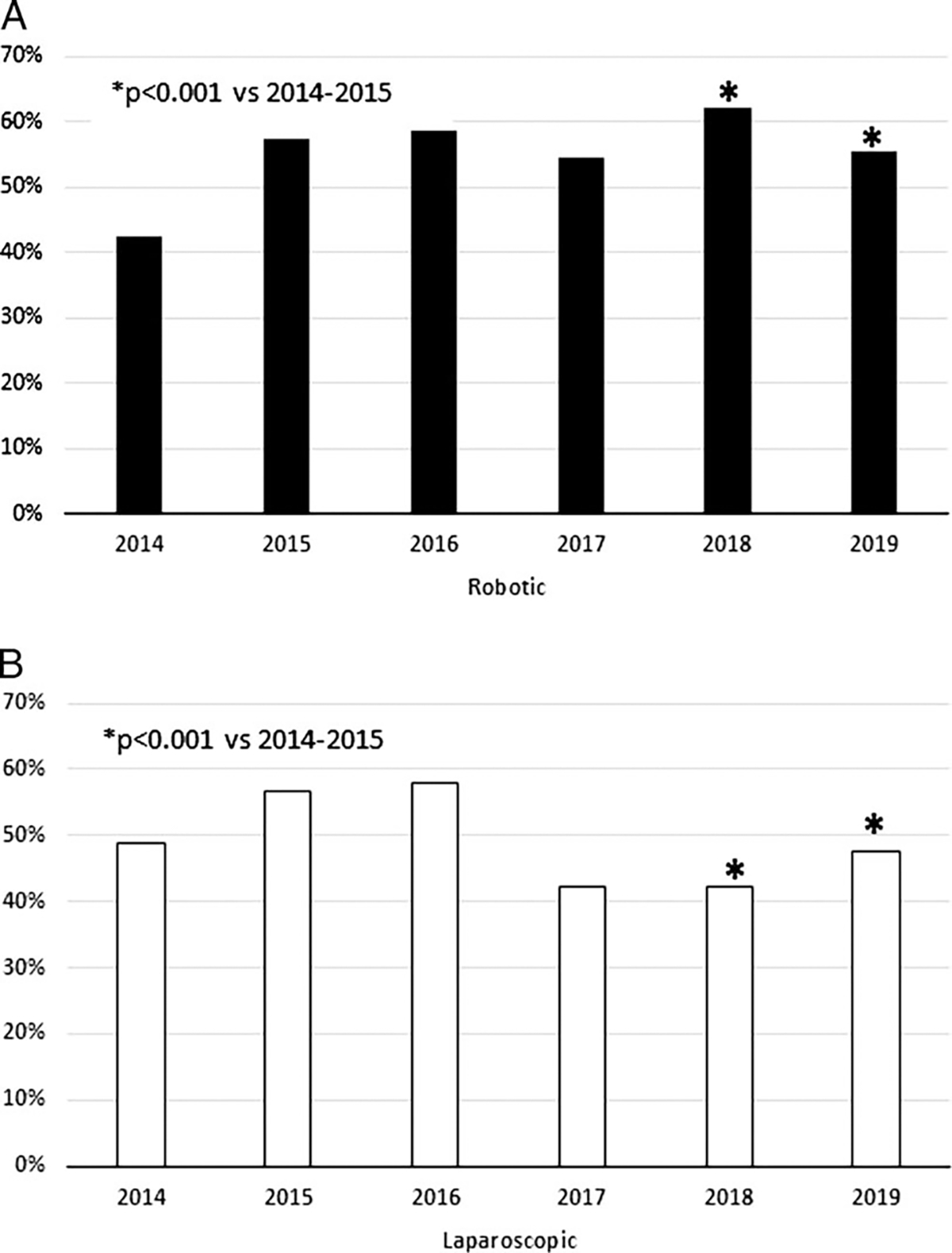
Trends of optimal outcome between RPD (A) and LPD (B).
DISCUSSION
The present study utilized the ACS NSQIP pancreatectomy-targeted PUFs to compare 30-day outcomes of MI-PD performed over a 6-year period. Using a large sample size of 1540 patients, this analysis showed a significantly increasing trend in RPD utilization, as well as significantly improving outcomes for the robotic approach. Conversely, LPD utilization has remained relatively unchanged, with a trend toward increased conversion rates and decreasing optimal outcomes. Importantly, this analysis suggests that in recent years (2018–2019) the robotic approach was significantly superior to the laparoscopic approach with respect to any complication, serious complications, and optimal outcome.
To date, pancreaticobiliary surgeons choose freely—within the limits of their training and MI platform availability—between the 2 MI-PD approaches, namely LPD or RPD. The one for LPD is a sound utilitarian argument, suggesting that laparoscopy platforms are readily available within hospital systems and are already integrated into formal surgical training thus making it a natural transition—for trainees and surgeons alike—to learn how to perform a LPD.19,20 Moreover, a considerable cost-saving opportunity exists in pursuing laparoscopy as the MI-PD platform of choice—which requires minimal capital investment—compared with the acquisition, per-use cost and overall maintenance of a robotic platform.20,21 In contrast, supporters of the robotic approach note that the improved ergonomic, visual perception, and wider range of motions offered by articulating instruments allows surgeons and trainees to simplify what would have otherwise been complex MI maneuvers, ultimately leading to increased reproducibility and possibly better outcomes.
The robotic platform has been shown to be at least equivalent to laparoscopic approaches for several organ-specific procedures such as colorectal, urological, gastric, gynecological, and distal pancreatic resection.22–27 However, no large single-institutional comparisons have been performed for RPD and LPD—since only a limited number of institutions perform MI-PD28—and, most surgeons who perform MI-PD have adopted one approach over the other, which further hinders direct comparison via clinical trials or even retrospective analyses.29
The current study suggests that any complication and serious complication rates have improved significantly over time for RPD, an observation that is in line with recent reports on robotic surgery for other surgical procedures.30–32 Indeed, the largest published series on RPD outcomes reported on 500 consecutive RPD cases and suggested that the robotic approach was associated with a temporal trend toward improving operating time, perioperative blood loss, conversion, and clinically relevant postoperative pancreatic fistula despite an increasing complexity of cases over time.33 Similar findings were replicated by Shi et al34—who described their experience with 450 consecutive RPD performed at the Shanghai Ruijin Hospital, China—noting progressive improvement of operative and oncologic outcomes. The results obtained at the University of Pittsburgh Medical Center and Ruijin Hospital need to be interpreted with caution, as they represent retrospective analysis of 2 high-volume independent single-center experiences where surgical procedures were performed by a dedicated surgical team beyond the learning curve for RPD, which limits generalizability.
Nonetheless, when comparing 2 relatively recent technologies it is paramount to evaluate their performance on multiple parameters over a definitive period and, ideally, with data generated from multiple different centers. Although individual metrics of perioperative performance—such as mortality and morbidity—are crucial to understanding surgical quality, they ultimately lack the ability to provide a wholesome understanding of the patient’s total experience. Several composite variables have been developed over the years, each with its merits and limitations, yet most recently “optimal outcome” was defined for pancreatic surgery as a compositive variable able to concisely capture the most satisfactory outcome following pancreatic resection.18 In our cohort, patients undergoing RPD had significantly higher rates of optimal outcome compared with LPD. In addition, the percentage of optimal outcome among RPD cases significantly increased from 2014 to 2019, while no change was noted among the LPD cohort.
Improvements in optimal outcome in robotic pancreas surgery have been previously driven—at least in part—by a reduced postoperative hospital stay and the need for percutaneous drainage.18,35 Multiple studies have demonstrated a decrease in LOS among robotic-assisted gastrointestinal procedures when compared with laparoscopic ones.36,37 This difference in LOS remains true even among patients who received enhanced recovery after surgery protocol.38,39 However, the true driver of improved optimal outcome—within our study cohort—was the decreasing rate of serious complication (46.8%–32.3%). With continued improvement in the RPD technique and the optimization and diffusion of robotic curriculum and training programs, adverse outcomes will likely continue to decrease.
The opposite trend identified among LPD cases, one characterized by lower optimal outcome and higher complication rates and a stalling rate of national utilization certainly raises questions regarding the long-term prospect of the laparoscopic approach. Several reasons might explain these contrasting temporal trends between RPD and LPD. First, validated curricula have been developed for RPD, and are being integrated into both residency and fellowship training programs.40,41 These curricula might be preferentially steering new adopters of MI-PD toward the robotic platform which is perhaps less technically demanding on surgeons compared with laparoscopy. Moreover, the negative results of the LEOPARD-2 trial and the minimal clinical benefits observed in the recent Chinese randomized controlled trial reported by Wang and colleagues might be also contributing to a general decreasing interest toward LPD.42,43 In contrast to the LEOPARD-2 trial, which is often criticized for the heterogeneity of the procedural expertise of the participating surgeons—which led to inadequate procedures in 22% of cases based on video review—the surgeons participating in the Wang and colleagues’ trial had extensive procedural expertise and yet the benefit of LPD over a classic laparotomy approach were minimal and restricted to 1 day benefit in LOS (16 vs 17).
Previous randomized trials, such as the PADULAP and PLOT, which showed a similar if not superior postoperative morbidity and mortality compared with laparotomy PD were single-institutional analyses often of a single experienced surgeon which makes for difficult generalizability of those results to a wider population of new adopters.9,10 LPD—when performed by very experienced surgeons in high-volume centers—can achieve remarkable outcomes, as many retrospective studies have shown.19,44,45 Yet, considering that ~80% of all MI-PDs are still being performed at low volume centers (< 20 MI-PD cases/year) the analysis of a large national database like ACS NSQIP is likely to give a more realistic representation of the national trend again supporting centralization of care for procedures of high complexity.46,47 Second, the current analysis revealed a significantly lower rate of conversion among RPD as compared with LPD, despite increased utilization. This finding is similar to previous reports, suggesting an increased capacity of the robotic platform to facilitate recovery from intraoperative complications—such as bleeding and difficult dissections—and assist with high-risk anastomoses such as in the setting of soft glands and small pancreatic ducts.48,49 Conversion from a MI approach to open laparotomy has been linked with increased morbidity. Therefore, that new adopters would gravitate toward a platform perceived to have lower rates of conversion is not surprising.35,49
The current study has several limitations. First, the severity of complications as described by the Clavien-Dindo classification could not be determined as this detail is not available in the ACS NSQIP database. However, the classification of complication severity as recorded in the ACS NSQIP represents a valuable surrogate to the Clavien-Dindo classification. Also, procedure-targeted pancreatectomy in NSQIP does define clinically relevant postoperative pancreatic fistulas. Second, the procedure-targeted PUFs do not contain any institutional data; and as such, trends in participating hospitals (volume/surgeon experience) could not be reported. Consequently, this study was unable to determine if the overall increase in RPD volume was a result of established minimally invasive surgery (MIS) surgeons converting to the robotic platform as opposed to new adopting surgeons transitioning part of their volume from OPD to RPD.
Nonetheless, our personal observation, based on physician-to-physician interaction, participation in national and international meetings, and recently published literature, is that the increase in RPD is mostly driven by new adopters of the MIS platform rather than established LPD surgeons transitioning to the RPD approach. The reasons are likely multifactorial—as shown by the data reported in this manuscript, the LPD volume has remained relatively constant with only a marginal decrease—which potentially suggests that providers who have adopted and mastered the LPD platform continue to utilize this technique. Yet, the significant volume increase in RPD argues for either increasing utilization by a few centers or, most likely, increased adoption throughout North America—which we favor based on our personal experience.
Last, outcomes are followed up for only 30 days instead of 90 days, which is usually recommended for pancreatic surgeries.
However, despite these limitations, the statistically significant analysis demonstrating improved optimal PD using the robotic platform and better outcomes of RPD versus LPD in recent years is a testament of the ongoing efforts toward optimization and safe implementation of MI robotic-assisted pancreatic surgery.
CONCLUSIONS
Between 2014 and 2019, the utilization of RPD has significantly increased and at a faster pace compared with the laparoscopic approach. Overall morbidity, serious complications, and optimal outcome have significantly improved for robotic cases, while having worsened or stayed the same for laparoscopic procedures. RPD is positioned to become the preferred MI-PD approach as suggested by an increasing adoption rate accompanied by a concomitant improvement in perioperative outcome measures.
Supplementary Material
Acknowledgments
This study did not receive any grant from funding agencies in the public, commercial, or non-profit sector.
Footnotes
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website, www.glaucomajournal.com.
REFERENCES
- 1.Jiang Y-L, Zhang R-C, Zhou Y-C. Comparison of overall survival and perioperative outcomes of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. BMC Cancer. 2019;19:781–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lyu HG, Sharma G, Brovman E, et al. Risk factors of reoperation after pancreatic resection. Dig Dis Sci. 2017;62:1666–1675. [DOI] [PubMed] [Google Scholar]
- 3.Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220:530–536. [DOI] [PubMed] [Google Scholar]
- 4.Lefor AK. Robotic and laparoscopic surgery of the pancreas: an historical review. BMC Biomed Eng. 2019;1:2–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou J, Xiong L, Miao X, et al. Outcome of robot-assisted pancreaticoduodenectomy during initial learning curve versus laparotomy. Sci Rep. 2020;10:9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zureikat AH, Beane JD, Zenati MS, et al. 500 minimally invasive robotic pancreatoduodenectomies: one decade of optimizing performance. Ann Surg. 2021;273:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Hilst J, de Rooij T, Bosscha K, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours (LEOPARD-2): a multicentre, patient-blinded, randomised controlled phase 2/3 trial. Lancet Gastroenterol Hepatol. 2019;4:199–207. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, Li D, Chen R, et al. Laparoscopic versus open pancreatoduodenectomy for pancreatic or periampullary tumours: a multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6:438–447. [DOI] [PubMed] [Google Scholar]
- 9.Poves I, Burdío F, Morató O, et al. Comparison of perioperative outcomes between laparoscopic and open approach for pancreatoduodenectomy: the PADULAP randomized controlled trial. Ann Surg. 2018;268:731–739. [DOI] [PubMed] [Google Scholar]
- 10.Palanivelu C, Senthilnathan P, Sabnis SC, et al. Randomized clinical trial of laparoscopic versus open pancreatoduodenectomy for periampullary tumours. Br J Surg. 2017;104:1443–1450. [DOI] [PubMed] [Google Scholar]
- 11.Zeh H, Bartlett DL, Moser AJ. Robotic-assisted major pancreatic resection. Adv Surg. 2011;45:323–340. [DOI] [PubMed] [Google Scholar]
- 12.Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg. 2004;239:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dokmak S, Ftériche FS, Aussilhou B, et al. Laparoscopic pancreaticoduodenectomy should not be routine for resection of periampullary tumors. J Am Coll Surg. 2015;220:831–838. [DOI] [PubMed] [Google Scholar]
- 14.Nassour I, Wang SC, Christie A, et al. Minimally invasive versus open pancreaticoduodenectomy: a propensity-matched study from a national cohort of patients. Ann Surg. 2018;268:151–157. [DOI] [PubMed] [Google Scholar]
- 15.American College of Surgeons. National Surgical Quality Improvement Program. 2008. Accessed February, 2021. https://www.facs.org/Quality-Programs/ACS-NSQIP
- 16.American College of Surgeons (ACS). ACS Surgical Risk Calculator. 2013. Accessed February 28, 2022. https://riskcalculator.facs.org/RiskCalculator/about.html
- 17.Pitt HA. Benchmark, textbook or optimal pancreatic surgery? Ann Surg. 2019;270:219–220. [DOI] [PubMed] [Google Scholar]
- 18.Beane JD, Borrebach JD, Zureikat AH, et al. Optimal pancreatic surgery: are we making progress in North America? Ann Surg. 2021;274:e355–e363. [DOI] [PubMed] [Google Scholar]
- 19.Croome KP, Farnell MB, Que FG, et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Ann Surg. 2014;260:633–638; discussion 638–640. [DOI] [PubMed] [Google Scholar]
- 20.Rosemurgy A, Ross S, Bourdeau T, et al. Cost analysis of pancreaticoduodenectomy at a high-volume robotic hepatopancreaticobiliary surgery program. J Am Coll Surg. 2021;232:461–469. [DOI] [PubMed] [Google Scholar]
- 21.Mesleh MG, Stauffer JA, Bowers SP, et al. Cost analysis of open and laparoscopic pancreaticoduodenectomy: a single institution comparison. Surg Endosc. 2013;27:4518–4523. [DOI] [PubMed] [Google Scholar]
- 22.Wright GP, Zureikat AH. Development of minimally invasive pancreatic surgery: an evidence-based systematic review of laparoscopic versus robotic approaches. J Gastrointest Surg. 2016;20:1658–1665. [DOI] [PubMed] [Google Scholar]
- 23.Xie W, Cao D, Yang J, et al. Robot-assisted surgery versus conventional laparoscopic surgery for endometrial cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2016;142:2173–2183. [DOI] [PubMed] [Google Scholar]
- 24.Leow JJ, Heah NH, Chang SL, et al. Outcomes of robotic versus laparoscopic partial nephrectomy: an updated meta-analysis of 4919 patients. J Urol. 2016;196:1371–1377. [DOI] [PubMed] [Google Scholar]
- 25.Kim CW, Kim CH, Baik SH. Outcomes of robotic-assisted colorectal surgery compared with laparoscopic and open surgery: a systematic review. J Gastrointest Surg. 2014;18:816–830. [DOI] [PubMed] [Google Scholar]
- 26.Park JM, Kim HI, Han SU, et al. Who may benefit from robotic gastrectomy?: a subgroup analysis of multicenter prospective comparative study data on robotic versus laparoscopic gastrectomy. Eur J Surg Oncol. 2016;42:1944–1949. [DOI] [PubMed] [Google Scholar]
- 27.Daouadi M, Zureikat AH, Zenati MS, et al. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128–132. [DOI] [PubMed] [Google Scholar]
- 28.Hoehn RS, Nassour I, Adam MA, et al. National trends in robotic pancreas surgery. J Gastrointest Surg. 2021;25:983–990. [DOI] [PubMed] [Google Scholar]
- 29.Nassour I, Wang SC, Porembka MR, et al. Robotic versus laparoscopic pancreaticoduodenectomy: a NSQIP analysis. J Gastrointest Surg. 2017;21:1784–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Dong X, Felsenreich DM, Gogna S, et al. Robotic pancreaticoduodenectomy provides better histopathological outcomes as compared to its open counterpart: a meta-analysis. Sci Rep. 2021;11:3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muaddi H, Hafid ME, Choi WJ, et al. Clinical outcomes of robotic surgery compared to conventional surgical approaches (laparoscopic or open): a systematic overview of reviews. Ann Surg. 2021;273:67–473. [DOI] [PubMed] [Google Scholar]
- 32.Sheetz KH, Claflin J, Dimick JB. Trends in the adoption of robotic surgery for common surgical procedures. JAMA Netw Open. 2020;3:e1918911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zureikat AH, Beane JD, Zenati MS, et al. 500 minimally invasive robotic pancreatoduodenectomies: one decade of optimizing performance. Ann Surg. 2021;273:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y, Wang W, Qiu W, et al. Learning curve from 450 cases of robot-assisted pancreaticoduocectomy in a high-volume pancreatic center: optimization of operative procedure and a retrospective study. Ann Surg. 2021;274:e1277–e1283. [DOI] [PubMed] [Google Scholar]
- 35.Beane JD, Pitt HA, Dolejs SC, et al. Assessing the impact of conversion on outcomes of minimally invasive distal pancreatectomy and pancreatoduodenectomy. HPB. 2018;20:356–363. [DOI] [PubMed] [Google Scholar]
- 36.Chen P, Zhou B, Wang T, et al. Comparative efficacy of robot-assisted and laparoscopic distal pancreatectomy: a single-center comparative study. J Healthc Eng. 2022;2022:7302222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asklid D, Ljungqvist O, Xu Y, et al. Short-term outcome in robotic vs laparoscopic and open rectal tumor surgery within an ERAS protocol: a retrospective cohort study from the Swedish ERAS database. Surg Endosc. 2022;36:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal V, Thomas MJ, Joshi R, et al. Improved outcomes in 394 pancreatic cancer resections: the impact of enhanced recovery pathway. J Gastrointest Surg. 2018;22:1732–1742. [DOI] [PubMed] [Google Scholar]
- 39.Morgan KA, Lancaster WP, Walters ML, et al. Enhanced recovery after surgery protocols are valuable in pancreas surgery patients. J Am Coll Surg. 2016;222:658–664. [DOI] [PubMed] [Google Scholar]
- 40.Mark Knab L, Zenati MS, Khodakov A, et al. Evolution of a novel robotic training curriculum in a complex general surgical oncology fellowship. Ann Surg Oncol. 2018;25:3445–3452. [DOI] [PubMed] [Google Scholar]
- 41.Rice MK, Hodges JC, Bellon J, et al. Association of mentorship and a formal robotic proficiency skills curriculum with subsequent generations’ learning curve and safety for robotic pancreaticoduodenectomy. JAMA Surg. 2020;155:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kamarajah SK, Bundred J, Saint Marc O, et al. Robotic versus conventional laparoscopic pancreaticoduodenectomy: a systematic review and meta-analysis. Eur J Surg Oncol. 2020;46:6–14. [DOI] [PubMed] [Google Scholar]
- 43.Liu R, Wakabayashi G, Palanivelu C, et al. International consensus statement on robotic pancreatic surgery. Hepatobiliary Surg Nutr. 2019;8:345–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Croome KP, Farnell MB, Que FG, et al. Pancreaticoduodenectomy with major vascular resection: a comparison of laparoscopic versus open approaches. J Gastrointest Surg. 2015;19:189–194; discussion 194. [DOI] [PubMed] [Google Scholar]
- 45.Paniccia A, Chapman B, Edil BH, et al. Total laparoscopic pancreaticoduodenectomy. J Vis Surg. 2016;2:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hoehn RS, Nassour I, Adam MA, et al. National trends in robotic pancreas surgery. J Gastrointest Surg. 2021;25:983–990. [DOI] [PubMed] [Google Scholar]
- 47.Conroy PC, Calthorpe L, Lin JA, et al. Determining hospital volume threshold for safety of minimally invasive pancreaticoduodenectomy: a contemporary cutpoint analysis. Ann Surg Oncol. 2022;29:1566–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zureikat AH, Borrebach J, Pitt HA, et al. Minimally invasive hepatopancreatobiliary surgery in North America: an ACS-NSQIP analysis of predictors of conversion for laparoscopic and robotic pancreatectomy and hepatectomy. HPB (Oxford). 2017;19:595–602. [DOI] [PubMed] [Google Scholar]
- 49.Hester CA, Nassour I, Christie A, et al. Predictors and outcomes of converted minimally invasive pancreaticoduodenectomy: a propensity score matched analysis. Surg Endosc. 2020;34:544–550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


