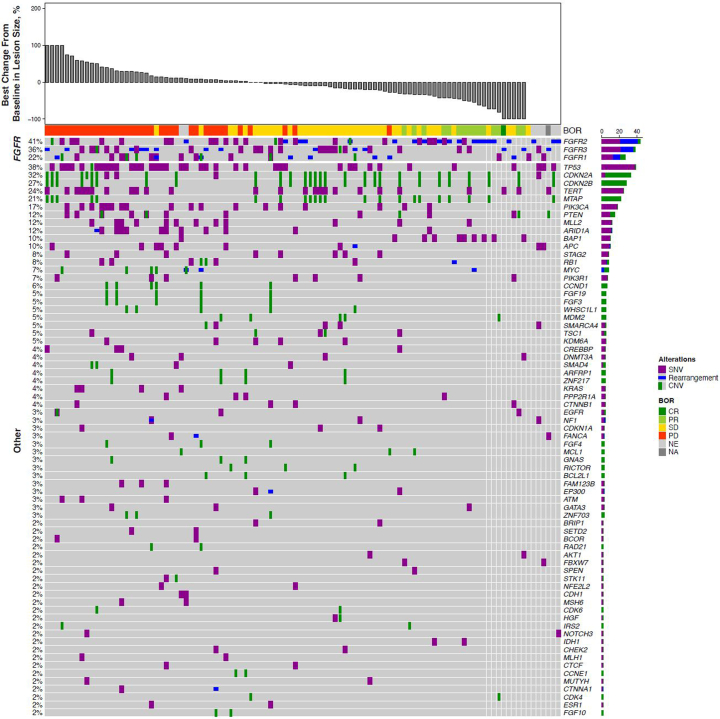
Extended Data Fig. 3. Across-Indication Analysis of Baseline Co-alterations.
Analysis of tumor tissue samples includes all evaluable patients from cohorts A, B, and C and central tissue next-generation sequencing (Foundation Medicine, Inc.) reporting. Known or likely pathogenic somatic gene alterations occurring in ≥2% of patients are shown. Patients are arranged by best percent change from baseline per RECIST or RANO. BOR, best overall response; cnv, copy number variation; CR, complete response; FGFR, fibroblast growth factor receptor; IRC, independent review committee; NA, not applicable; NE, not evaluable; PChg, percent change from baseline; PD, progressive disease; PR, partial response; SD, stable disease; snv, single-nucleotide variant.