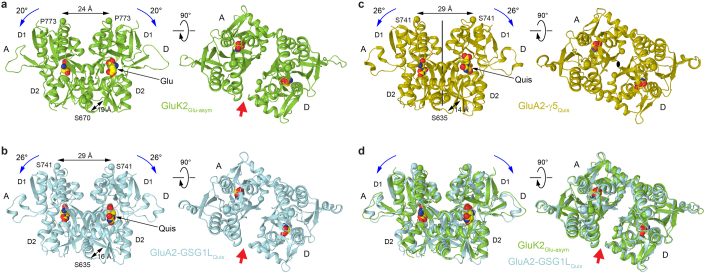
Extended Data Fig. 9. Comparison of desensitized-state dimers in KARs and AMPARs.
a-d, LBD dimers from structures of GluK2Glu-asym (a, green), GluA2-GSG1LQuis (b, PDB ID: 7RYZ, cyan), GluA2-γ5Quis (c, PDB ID: 7RZ8, olive) and superposition of GluK2Glu-asym and GluA2-GSG1LQuis (e) viewed from the side (left) or top (right). The agonist molecules Glu and quisqualate (Quis) are shown as space-filling models. Distances between Cα atoms of S670 and P773 in KARs and S635 and S741 in AMPARs are indicated. Rotation of the lobes D1 towards D2 upon agonist binding is illustrated by blue arrows. Red arrows point to the cleft between the LBD protomers signifying the loss of the dimer 2-fold rotational symmetry.