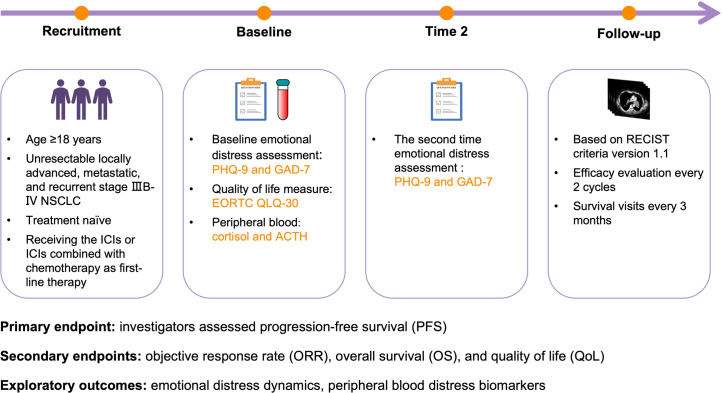
Extended Data Fig. 1. Study design of STRESS-LUNG-1.
Stage IIIB-IV NSCLC patients who are about to receiving ICIs or combination therapy with chemotherapy as first-line treatment are screened for enrollment in this study. Emotional distress at baseline is assessed using the PHQ-9 and GAD-7 scales, while QoL is evaluated based on the EORTC QLQ-C30 questionnaire; A second assessment (Time 2) of emotional distress is conducted during follow-up. The efficacy of ICIs is evaluated every two cycles. Abbreviations: NSCLC, non-small-cell lung cancer; ICIs, immune checkpoint inhibitors; PHQ-9, Patient Health Questionnaire-9; GAD-7, Generalized anxiety disorder 7-item; EORTC QLQ-C30, European organization for research and treatment of cancer core quality of life questionnaire; ACTH, adrenocorticotropic hormone; RECIST, Response Evaluation Criteria in Solid Tumors.