Abstract
Sickle cell disease (SCD) is a severe monogenic hereditary hemoglobinopathy that is characterized by repeated clinical and biological manifestations able to generate stress erythopoiesis. A clonal hematopoiesis involving mainly variants of TP53, DNMT3A, ASXL1, and/or TET2 may be more prevalent in patients with SCD, suggesting that mutations in these genes may lead to an increased risk of leukemia. An increased prevalence of leukemia in patients with SCD has been confirmed by an increasing number of acute myeloid leukemia cases with myelodysplastic features reported in this patient population even in the absence of disease-modifying treatments. This leads to the hypothesis of a mechanism involving multifactorial causes through the pathophysiologic manifestations of SCD, in which cells are undergoing constant hematopoietic hyperplasia, inducing genomic damage and somatic mutations.
Keywords: Sickle cell disease, Acute leukemia, Clonal hematopoiesis, Mutations
Key Summary Points
| Clinical manifestations occurring among patients with sickle cell disease (SCD) are known to generate stress erythropoiesis, which may have, via a higher cell turnover, an impact on the quality of bone marrow hematopoietic stem cells (HSC) and progenitor cells and promote mutations and clonal hematopoiesis. |
| The probability to develop clonal hematopoiesis in SCD, leading to a higher risk of malignant cell development, increases with the repetition of crises and therefore with patient age. |
| The risk of myeloid malignancies in the SCD patient population is believed to be five- to ten-folds higher than that in the general population, confirmed by the occurrence of acute leukemia in many cases, with a high proportion of patients displaying genetic markers and clinical features of underlying myelodysplastic syndrome, tending to present some SCD subsets as potential pre-leukemic diseases. |
Sickle cell anemia is an autosomal recessive disorder involving sickle hemoglobin (HbS). It is one of the most common and severe monogenic diseases worldwide [1, 2], and is the result of the replacement of a glutamine acid residue by valine. It is well known that the substitution of a single amino acid can alter the function of a gene product sufficiently to produce widespread clinical effects [3]. The homozygous state (SS disease) is the most severe of sickle cell diseases (SCD), with hemoglobin (Hb) SC disease and sickle cell β thalassemia (Sβ+or Sβ0) tending to be somewhat milder, and Hb SC disease being the mildest of the group.
Wide variation in the severity of clinical manifestations occurs among patients with SCD due to the influence of various factors including, in addition to the nature of the Hb, the activity of red cell enzymes and levels of metabolites. Many patients with SCD do achieve a steady-state level of fitness, but this state is periodically interrupted by crises, including pain events, stroke, and organ damage, which may have potentially a fatal outcome. Infarctive crises are the most common of such crises and the hallmark of patients with SCD. They result from the polymerization of HbS under hypoxic conditions, which exacerbates sickle red blood cell rigidity and poor deformability, shortening red cell survival and increasing erythropoietic turnover. Among other types of crises, aplastic and megaloblastic crises are generally associated with infections observed in the context of a deficiency of folic acid; sequestration crises can occur particularly in children and adults with splenomegaly. The red cell lifespan is shortened in all SCD. An increased rate of hemolysis can be observed in hemolytic crises, leading to falling Hb level and an elevated reticulocyte count [2].
All of these clinical manifestations are known to generate stress erythropoiesis, which results from the short lifespan of erythrocytes and ineffective erythropoiesis from the apoptosis of erythroid precursors. Chronic stress erythropoiesis and the activation of inflammatory mediators may have an impact on the quality of the bone marrow hematopoietic stem cells (HSCs) and progenitor cells [4, 5]. In SCD, the marrow generally shows erythroid hyperplasia, with a higher cell turnover potentially promoting mutations that in turn lead to a higher risk of malignant cell development. Studies have demonstrated increased proportions of bone marrow CD34+CD38− cells co-expressing glycophorin A compared to normal progenitor cells in SCD [6, 7], with the authors of one of these studies also observing a depletion of megakaryocyte-erythroid progenitors and an increase in HSCs in SCD patients compared to controls [7]. Red blood cell transfusions can also lead to increased iron levels and non-specific immunomodulation that could increase the risk of malignancy. Chronic inflammation may increase the proliferation rate of HSCs, resulting in accelerated clonal hematopoiesis [8]. Inflammation can cause chronic organ damage and generate cellular defects with subsequent malignant transformation. In this setting, inflammasomes behave as pro-tumorigenic agents associated with the promotion of cell proliferation, inhibition of apoptosis, and an immunosuppressive effect on the immune cells [9]. Constant hematopoietic hyperplasia, stimulated by a hemolysis-induced cytokine storm, may also increase the risk of somatic mutations, resulting in a potential transformation of myeloid precursors [10].
We can therefore hypothesize that clonal hematopoiesis can be more common and can occur earlier in patients with SCD due to accelerated biological aging of the hematopoietic system, as compared to the general population. Although for most people the consequences of clonal hematopoiesis are minimal, the accumulation of multiple genetic abnormalities over years, due to a high degree of proliferative activity of bone marrow cells, may be theoretically responsible for an increased risk of hematologic malignancy. Furthermore, this high risk of clonal hematopoiesis could be strengthened by SCD treatment that could incite mutant clones to expand and potentially transform as a result. Although the leukemic risk of hydroxyurea (HU), which remains a therapeutic mainstay in the treatment of SCD, has never been confirmed in large series of patients [11–13] and has not been associated with an increased risk of clonal hematopoiesis [14], the potential risk following a conditioning regimen for HSC transplantation remains more questionable. There is a real possibility that the medley of high-risk clonal hematopoiesis and HSC transplantation incites mutant clones to expand and subsequently transform [15].
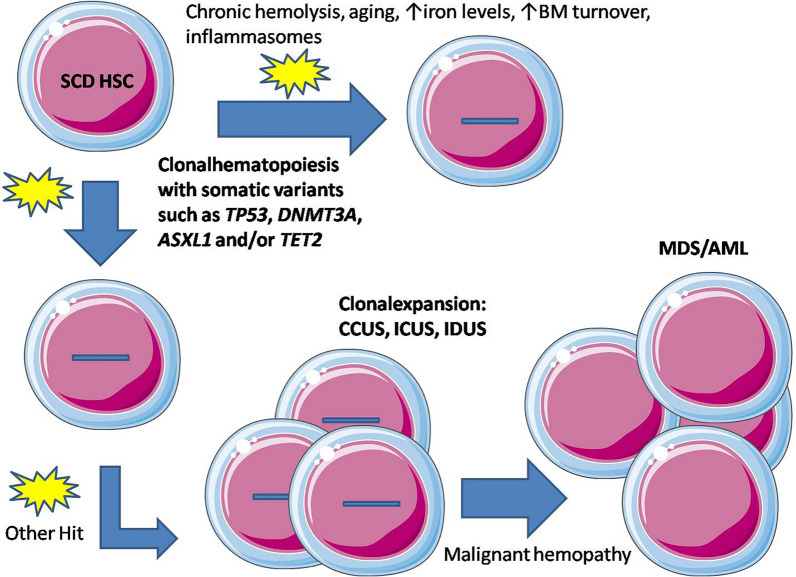
It can also be hypothesized that the probability of individuals with SCD developing clonal hematopoiesis increases with the repetition of crises and therefore with patient age (Fig. 1), possibly resulting from increased patient survival. In high-income countries, improved newborn screening has resulted in an important decrease in childhood mortality [2]. Furthermore, antibiotic prophylaxis and effective therapy with HU have led to large gains in life expectancy over the last decades [16–18]. A potential increase in clonal hematopoiesis should therefore be observed in the years to come in high-income countries, but also in low-income countries where significant improvements in the management of SCD are expected.
Fig. 1.
Repetitive chronic clinical and biological manifestations may lead to patients with SCD developing clonal hematopoiesis over time that (following further hits) may or may not evolve to clonal expansion and potentially to malignant disease. AML Acute myeloid leukemia, BM bone marrow, CCUS clonal cytopenia of undetermined significance, HSC hematopoietic stem cell, ICUS idiopathic cytopenia of undetermined significance, IDUS idiopathic dysplasia of undetermined significance, MDS myelodysplastic syndrome, SCD sickle cell disease
The documented development of malignancies in pediatric and adult patients with SCD tend to confirm our assumptions. The risk of myeloid malignancies in patients with SCD has not been well studied, but is believed to be five- to ten-fold higher than that in the general population [4, 19, 20]. This risk is potentially as high as in patients with recognized leukemia predisposition syndromes, including many bone marrow disorders. Since the first description of SCD coexisting with acute myeloid leukemia (AML) [21], the occurrence of acute leukemia has been reported in many cases, confirming the increased risk of leukemia in individuals with SCD [22]. While a coincidental event between acute leukemia and SCD could be evocated in some of these cases, a high proportion of the patients presented with complex structural rearrangements that involved complete or partial loss of chromosome 5 and/or chromosome 7, 17p deletions, 11q23, and/or chromosome 3 abnormalities, and/or molecular abnormalities associated with a poor prognosis (mutations in TP53, KMT2A, RAS, RUNX1, PTPN11). The presence of these significant underlying myelodysplastic syndrome (MDS) features, comparable to those associated with therapy-related myeloid neoplasms, was abnormally high in the SCD patients compared to leukemia patients of the same age from the general population. These findings suggest that a clonal hematopoiesis may be more prevalent in patients with SCD and may expand prior to leukemia development. Convincing evidence comes from a series of recipients of allogeneic HSC transplantation who developed AML after graft failure [23]. In two reported cases, TP53 mutations were present before transplantation and evolved within 3 years post-transplant. In both cases, TP53 mutant clones accounted for < 1% of the total hematopoietic output, supporting the notion that the nature of mutations and biological context, rather than clone size, determines the oncologic potential of clonal hematopoiesis.
Clonal hematopoiesis refers to the presence of clonal molecular genetic changes in blood or bone marrow cells in the absence of signs of hematological neoplasm. This is a common age-related condition, with an estimated incidence of 10% of persons from the age of 70 years onwards. Somatic mutation with an allelic frequency of at least 2% has been primarily identified in one of the following genes: DNMT3A, TET2, JAK2, SF3B1, ASXL1, TP53, CBL, GNB1, BCOR, U2AF1, CREBBP, CUX1, SRSF2, MLL2 (KMT2D), SETD2, SETDB1, GNAS, PPM1D, and BCORL1 [24]. Two recent large studies have addressed clonal hematopoiesis in SCD [14, 25]. Despite some discrepancies between the two studies, related to the technique used, the control cohort, and the value of low-variant allele frequency (VAF) chosen, only < 5% of cases were identified as having clonal hematopoiesis, with DNMT3A and TP53 reported as the most commonly mutated genes. In the former study [14] the prevalence of clonal hematopoiesis was not found to be significantly different between the SCD patient group and the control group, while in the latter study [25] clonal hematopoiesis appeared more prevalent in individuals with SCD. Although these two studies demonstrated high quality, there are a number limitations that deserve discussion. Study samples were not systematically provided from patients presenting a long past history of SCD complications. Samples could have also frequently been drawn at the time of diagnosis, namely during childhood or adolescence. Furthermore, VAF clonal hematopoiesis clones may have been undetectable by the methods used; as such, documentation of a clonal hematopoiesis could have been underestimated and should be reevaluated in further studies, with a higher risk being likely associated with patient age and in patients with prior history of severe disease, chronically high inflammatory markers, and/or particular therapies. In addition to its malignant potential, clonal hematopoiesis has also been associated with a high risk of vascular-occlusive events, known to be especially common in SCD, through an over-expression of pro-inflammatory cytokines and chemokines in monocytes/macrophages with mutated DNMT3A or TET2.
Many questions remain open about cancer risk in patients with SCD, especially those living well into adulthood. Although several mechanisms potentially involved in the leukemic transformation have been discussed [15, 22, 26], somatic mutations identified at the time of leukemia diagnosis appear likely to be related to a lifetime of erythropoietic stress and inflammation. New large studies involving SCD patients tested with a large panel of mutations frequently involved in clonal hematopoiesis and using highly sensitive assays are still required. It will be critical to understand the risk of clonal hematopoiesis in patients with SCD in order to identify patient subsets at higher risk. This should lead to a better understanding of this potential complication in SCD and to an accurate prophylactic management in patients at risk. Screening for clonal hematopoiesis should be addressed in order to optimize therapeutic strategies in SCD as such screening can notably reduce the risks associated with stem cell and gene therapies. A strategy of testing for high-risk clonal hematopoiesis prior to therapeutic intervention and subsequently deferring gene therapy if a high risk is found might abrogate the risk of leukemic transformation. Further studies should also specify the impact of current therapies. In a recent study, HU treatment appeared to be effective in normalizing the observed concentration of CD34+ cells and of all HSCs and therefore decrease premature aging and potentially hematologic malignancy risk compared to the other therapeutic modalities [7]. The demonstration of clonal hematopoiesis favored by aging, severe disease, repeated crises, and chronic inflammation should lead healthcare professionals to consider some subsets of SCD as potential pre-leukemic diseases.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author Contributions
Giovanna Cannas, Mohamed Elhamri, and Xavier Thomas wrote the manuscript. All authors gave final approval of the version to be submitted.
Funding
No funding or sponsorship was received for this study or publication of this article.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Declarations
Conflict of Interest
The authors do not have any competing financial interest in relation with the work described. Xavier Thomas is an Editorial Board member of Oncology and Therapy. Xavier Thomas was not involved in the selection of peer reviewers for the manuscript nor any of the subsequent editorial decisions.
Ethical Approval
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
References
- 1.Aygun B, Odame I. A global perspective on sickle cell disease. Pediatr Blood Cancer. 2012;59:386–390. doi: 10.1002/pbc.24175. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376:1561–1573. doi: 10.1056/NEJMra1510865. [DOI] [PubMed] [Google Scholar]
- 3.Alexy T, Sangkatumvong S, Connes P, et al. Sickle cell disease: selected aspects of pathophysiology. Clin Hemarheol Microcirc. 2010;44:155–166. doi: 10.3233/CH-2010-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunson A, Keegan THM, Bang H, et al. Increased risk of leukemia among sickle cell disease patients in California. Blood. 2017;130:1597–1599. doi: 10.1182/blood-2017-05-783233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol. 2015;12:584–596. doi: 10.1038/nrclinonc.2015.105. [DOI] [PubMed] [Google Scholar]
- 6.Luck L, Zeng L, Hiti AL, et al. Human CD34+ and CD34+CD38- hematopoietic progenitors in sickle cell disease differ phenotypically and functionally from normal and suggest distinct subpopulations that generate F cells. Exp Hematol. 2004;32:483–493. doi: 10.1016/j.exphem.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Tolu SS, Wang K, Yan Z, et al. Characterization of hematopoiesis in sickle cell disease by prospective isolation of stem and progenitor cells. Cells. 2020;9:2159. doi: 10.3390/cells9102159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heyde A, Rohde D, McAlpine CS, et al. Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell. 2021;184:1348–1361. doi: 10.1016/j.cell.2021.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomasik J, Basak GW. Inflammasomes—new contributors to blood diseases. Int J Mol Sci. 2022;23:8129. doi: 10.3390/ijms23158129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alves PM, Martins PRJ, Dias FDL, et al. Sensitivity to cisplatin-induced mutations and elevated chromosomal aberrations in lymphocytes from sickle cell disease patients. Clin Exp Med. 2008;8:31–35. doi: 10.1007/s10238-008-0153-3. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: a 17.5 year follow-up. Am J Hematol. 2010;85:403–408. doi: 10.1002/ajh.21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS) Blood. 2010;115:2354–2363. doi: 10.1182/blood-2009-05-221333. [DOI] [PubMed] [Google Scholar]
- 13.Castro O, Nouraie M, Oneal P. Hydroxycarbamide treatment in sickle cell disease: estimates of possible leukaemia risk and of hospitalization survival benefit. Br J Haematol. 2014;167:687–691. doi: 10.1111/bjh.13093. [DOI] [PubMed] [Google Scholar]
- 14.Liggett LA, Cato LD, Weinstock JS, et al. Clonal hematopoiesis in sickle cell disease. J Clin Invest. 2022;132:e156060. doi: 10.1172/JCI156060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stonestrom AJ, Levine RL. The hematopoietic saga of clonality in sickle cell disease. J Clin Invest. 2022;132:e158251. doi: 10.1172/JCI158251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med. 1995;332:1317–1322. doi: 10.1056/NEJM199505183322001. [DOI] [PubMed] [Google Scholar]
- 17.Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood. 1996;88:1960–1964. doi: 10.1182/blood.V88.6.1960.bloodjournal8861960. [DOI] [PubMed] [Google Scholar]
- 18.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomized, controlled trial (BABY HUG) Lancet. 2011;377:1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seminog OO, Ogunlaja OI, Yeates D, et al. Risk of individual malignant neoplasms in patients with sickle cell disease: English National Record Linkage Study. J R Soc Med. 2016;109:303–309. doi: 10.1177/0141076816651037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schultz WH, Ware RE. Malignancy in patients with sickle cell disease. Am J Hematol. 2003;74:249–253. doi: 10.1002/ajh.10427. [DOI] [PubMed] [Google Scholar]
- 21.Goldin AG, Kelty KC, Beard MF. Sickle cell anemia terminating in acute myeloblastic leukemia. Ann Intern Med. 1953;39:920–928. doi: 10.7326/0003-4819-39-4-920. [DOI] [PubMed] [Google Scholar]
- 22.Cannas G, Poutrel S, Heiblig M, et al. Sickle cell disease and acute leukemia: one case report and an extensive review. Ann Hematol. 2023;102:1657–1667. doi: 10.1007/s00277-023-05294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghannam JY, Xu X, Maric I, et al. Baseline TP53 mutations in adults with SCD developing myeloid malignancy following hematopoietic cell transplantation. Blood. 2020;135:1185–1188. doi: 10.1182/blood.2019004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steensma DP, Bejar R, Jaiswal S, et al. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pincez T, Lee SSK, Ilboudo Y, et al. Clonal hematopoiesis in sickle cell disease. Blood. 2021;138:2148–2152. doi: 10.1182/blood.2021011121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ribeil JA. Primary myelofibrosis in untreated sickle cell disease: Are adult patients at higher risk for developing hematological myeloid neoplasms? Am J Hematol. 2022;97:4–6. doi: 10.1002/ajh.26371. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.