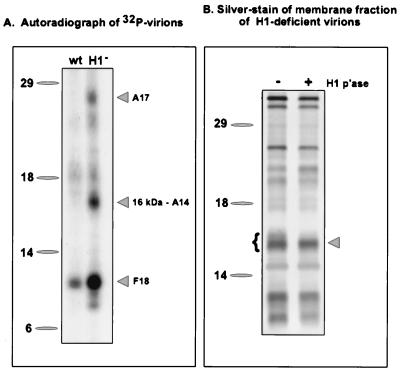
FIG. 1.
Identification of the A14 protein as a 16-kDa component of the virion membrane that is hyperphosphorylated in H1-deficient virions. (A) Sucrose-banded 32P-labeled virions were prepared from cells infected with wt virus or vindH1 in the absence of IPTG. Equivalent quantities of virions were analyzed by electrophoresis on an SDS–17% polyacrylamide gel and visualized by autoradiography. Three hyperphosphorylated proteins of 25, 16, and 11 kDa were seen in H1-deficient virions. The identification of the 16-kDa protein as the product of the A14 gene is reported in this study. For both panels, the electrophoretic migrations of protein standards are shown at the left, with their molecular masses indicated in kilodaltons. (B) H1-deficient virions (5 μg) were permeabilized with NP-40 plus DTT, and the membrane fraction was prepared. Half of the material was analyzed directly (lane −), and half was treated with recombinant H1 phosphatase (p'ase) (35) for 3 h at 37°C (lane +). The samples were then fractionated on an SDS–17% polyacrylamide gel and visualized by silver staining. The brace at the left indicates the multiple species of the 16-kDa protein seen in H1-deficient virions; these bands are converted to the most rapidly migrating form upon treatment with the H1 phosphatase. This dephosphorylated species was subjected to microsequencing analysis and found to be the product of the A14 gene.