Abstract
Background/Objective
Observational real‐world study to analyze the clinical effects of alemtuzumab (ALEM) and subsequent disease‐modifying therapy (DMT) usage in multiple sclerosis (MS).
Methods
Data retrieved from the Austrian MS treatment registry (AMSTR) included baseline (BL) characteristics (at ALEM start), annualized relapse rate (ARR), 6‐month confirmed progression independent of relapse activity (PIRA; ≥ 0.5‐point Expanded Disability Status Scale (EDSS) score increase), 6‐month confirmed disability improvement (CDI; ≥ 0.5‐point EDSS decrease), and safety outcomes until initiation of a subsequent DMT. The EDSS was re‐baselined at 30 days from ALEM start (BL EDSS).
Results
Eighty‐seven ALEM‐treated patients (median age: 32 years, 72% female, 14% treatment‐naïve) were followed for a median of 55 (interquartile range 31–68) months. We found significant reductions in the ARR from 1.16 before ALEM to 0.15 throughout Years 1–9 (p < 0.001). Subsequent DMTs were initiated in 19 patients (22%, 74% anti‐CD20 monoclonal antibodies). At Year 5 (n = 53), more patients achieved CDI (58%, 95% confidence interval (CI) 45%–71%) than had experienced PIRA (14%, CI 7.5%–24%), and 58% remained relapse‐free. Shorter MS duration (p < 0.001, hazard ratio (HR) 0.86 (CI 0.80–0.93)) and no previous high‐efficacy treatment (p < 0.001, HR 5.16 (CI 2.66–10.0)) were the best predictors of CDI, while PIRA was associated with a higher number of previous DMTs (p = 0.04, HR 3.06, CI 1.05–8.89). We found no new safety signals.
Interpretation
ALEM had long‐lasting beneficial effects on the ARR and disability improvement, especially when initiated early in the course of the disease. Only a subset of patients received subsequent DMTs.
Introduction
Multiple sclerosis (MS) is an immune‐mediated disorder of the central nervous system (CNS) and a leading cause of disability among young adults. 1 During the relapsing phase of MS, the disease pathogenesis is driven by focal immune cell infiltration, leading to damage of the myelin sheaths with partial axonal preservation and reactive glial scar formation in the white and gray matter of the CNS. Eventually, irreversible axonal degeneration plays a pivotal role in the accumulation of disability during the progressive phase of the disease. The temporal dichotomy between relapsing and progressive MS courses has been challenged by evidence indicating that progression independent of relapse activity (PIRA) occurs from early in the MS course, 2 , 3 and ongoing inflammation and demyelination are observed throughout all stages of MS. 4 While available immunotherapies successfully reduce relapse rates, slowing disability progression remains a challenge. 5
Alemtuzumab (ALEM; Lemtrada®; Sanofi, Cambridge, MA, USA) is a disease‐modifying therapy (DMT) that was licensed by the European Medicines Agency (EMA) in 2013 for patients with highly active relapsing–remitting MS (RRMS). 6 ALEM is a monoclonal IgG1 kappa antibody that targets the leucocyte surface protein CD52 and induces a profound but transient depletion of circulating T and B cells. 7 , 8 Pivotal trials have consistently demonstrated that two intermittent ALEM treatment courses (12 mg/day for 5 consecutive days at baseline and additional 3 days after 12 months) significantly reduce relapse rates and improve magnetic resonance imaging (MRI) outcomes. 9 , 10 , 11 , 12 Moreover, about two‐thirds of the patients from the pivotal trials required no further treatment course(s) throughout 5 years. 13 Furthermore, the open‐label extension studies indicate that the clinical effects of two treatment cycles may be maintained for up to 13 years and support the benefits of ALEM by impacting pre‐existing disability. 14 , 15 However, the ability of ALEM to halt clinical progression and reverse disability in the long term needs to be further corroborated in independent cohorts. Moreover, major adverse events (AEs) in the pivotal trials included infusion‐associated reactions, autoimmune disorders (thyroid, hematological and renal disorders), and infections. Of note, several rare but potentially fatal autoimmune conditions, opportunistic infections, and acute cerebrovascular disorders have been reported in postmarketing studies. 16 , 17 , 18 While ALEM provides positive clinical benefits regardless of age, there are age‐related increases of serious infections, malignancies, and deaths. 19
Our study aimed to investigate disease course, safety aspects, and long‐term outcomes of patients with MS treated with ALEM in Austria. Moreover, we analyzed demographics and disease characteristics at baseline to predict treatment response to ALEM and the use of subsequent DMTs.
Materials and Methods
Data acquisition
The Austrian MS Treatment Registry (AMSTR) is a nationwide registry established in 2006 that collects data on the safety and real‐world effectiveness of all DMTs except interferon‐beta and glatiramer acetate. Austrian MS centers must prospectively document patient treatment data through a secure web‐based platform. The AMSTR requires the documentation of relapses, EDSS, adverse events (according to the MeDRA classification, including infusion‐associated reactions (IARs), cardiovascular side effects, infections, and secondary autoimmune events), and pregnancy. The registry also captures the usage and timing of subsequent DMTs.
A keyword search within the AMSTR identified individuals treated with ALEM by April 2023. We collected baseline (BL; at the time of ALEM start) information that consisted of demographics (age and sex), onset and duration of MS, previous DMTs, and relapses in the prior 12 months. The EDSS was re‐baselined at 30 days from ALEM start (BL EDSS) to minimize the chance of higher baseline EDSS values related to delayed recovery from the most recent relapse. We defined the observation period as starting from the initiation of ALEM until the last follow‐up available in the AMSTR or until the commencement of a subsequent DMT. Once patients started subsequent DMTs, their clinical course was no longer within the scope of this study.
Primary outcome measures
We compared the mean annualized relapse rate (ARR) throughout the observation period with the ARR 12 months before ALEM started. We focused on disability changes independent of overt relapses and evaluated the occurrence of PIRA and the achievement of confirmed disability improvement (CDI) during the follow‐up period. According to the revised Lublin criteria for secondary progressive MS (SPMS), 20 changes in the EDSS to explore PIRA and CDI rates were retrospectively studied. PIRA was defined as an increase of ≥0.5 points on the EDSS scale, persisting over 6 months and without evidence of relapses within the range of ±30 days). 3 , 21 If relapses with incomplete recovery occurred during the observation period, the reference EDSS was reset ≥90 days after the relapse onset for PIRA‐event assessment. We defined CDI as a 6‐month confirmed improvement of ≥0.5 points on the EDSS. We calculated the proportions of patients throughout the follow‐up that clinically improved, stabilized, or worsened according to EDSS changes. Lastly, we analyzed whether these BL parameters were associated with the following outcomes: occurrence and number of relapses, EDSS at the last follow‐up, the occurrence of PIRA, achievement of CDI, and commencement of a subsequent DMT.
Comparison with available real‐world evidence
In August 2023, we searched PubMed concerning the available literature on outcomes after ALEM treatment in real‐world studies. Studies that did not include key variables and readouts of our study were not respected.
Statistical analysis
We present descriptive data as mean ± standard deviations or 95% confidence intervals (CI) or median and interquartile range (IQR) and percentages. Fisher's exact test or Pearson's (2 tests were used to analyze cross‐tabulation tables). Spearman's correlations were computed and tested. Wilcoxon–Mann–Whitney and Hodges–Lehmann 95% CI based on Monte‐Carlo simulations were done to compare medians. Generalized estimation equation models (GEE) with Gamma or Poisson distribution were used for continuous or binomial distribution of discrete variables and logit as a link function. Least statistical difference (LSD) tests were used for pairwise comparisons. T‐tests and bootstrap t‐tests based on 5000 Monte‐Carlo simulations were used for dependent comparisons. Logistic regression models with odds ratios (OR) and 95% CI, Cox‐regression models for various covariates with corresponding hazard ratios (HR), stratified Cox model analysis for paired samples and 95% CI, and Kaplan–Meier analyses with log‐rank tests were applied. Two‐sided p‐values < 0.05 were considered statistically significant, and the confidence level was 95%. A heuristic approach was used to compare two dependent Kaplan–Meier curves (CDI and PIRA) at Year 5: 95% confidence intervals were computed and interpreted as significant if the 95% CI did not overlap. All statistical analyses in this report were performed by use of NCSS (NCSS 2022, NCSS, LLC. Kaysville, UT), STATISTICA 13 (Hill, T. & Lewicki, P. Statistics: Methods and Applications. StatSoft, Tulsa, OK), and SPSS Statistics for Windows, Version 29.0., Armonk, NY).
The Austrian MS Treatment Registry is approved by the Ethics Committee of the Medical University of Vienna, Austria (approval number 296/2013). This study was conducted according to the ethical principles of the Declaration of Helsinki and did not interfere with the care received by patients.
Results
Eighty‐eight individuals were treated with ALEM. Since one patient had to be excluded due to insufficient documentation, 87 patients were available for this study (Table 1). All patients had RRMS at the time of ALEM initiation. The cohort had a median age of 32 (IQR 26–36) years, a median disease duration of 5 (IQR 1–11) years, and a median EDSS of 2.5 (IQR 1.8–3.5). The median follow‐up time was 55 months (IQR 31–68), the longest individual observational period since the start of ALEM treatment was 97 months.
Table 1.
Demographics, disease characteristics, and follow‐up of the Austrian alemtuzumab cohort.
| n = 87 | % | |
|---|---|---|
| Female; No. | 63 | 72 |
| Age at MS diagnosis, y; median (IQR) | 26 (21–31) | |
| Age at ALEM start, y; median (IQR) | 32 (26–36) | |
| Disease duration, y; median (IQR) | 5 (1–11) | |
| RRMS; No. | 87 | 100 |
| ARR in the 12 months before ALEM; mean (95% CI) | 1.2 (0.8–1.8) | |
| EDSS; median (IQR) | 2.5 (1.8–3.5) | |
| Naïve to DMTs; No. | 12 | 14 |
| No. of previous DMTs; median (range) | 1 (0–3) | |
| No. of patients with prior high‐efficacy DMTs | 50 | 58 |
| No. patients switched from NAT | 16 | 18 |
| No. patients switched from FTY | 31 | 36 |
| No. of patients throughout the follow‐up | ||
| At month 24 | 81 | 93 |
| At month 48 | 62 | 71 |
| At month 72 | 31 | 36 |
| At month 96 | 7 | 8 |
ALEM, alemtuzumab; ARR, annualized relapse rate; CI, confidence interval; DMTs, disease‐modifying therapies; EDSS, expanded disability status scale; FTY, fingolimod; IQR, interquartile range; MS, multiple sclerosis; NAT, natalizumab; No., number of; RRMS, relapsing–remitting multiple sclerosis; y, years.
At the time of ALEM initiation, 12 patients (14%) had not been treated previously with DMTs (DMT‐naïve). Of the remaining individuals, 50 (58%) had received one DMT prior to ALEM, 24 (28%) had been treated with two DMTs, and one patient had had three DMTs before ALEM initiation. Fifty patients (58%) had undergone prior treatment with high‐efficacy DMTs: 16 were switched from natalizumab, 31 from fingolimod, two from daclizumab, and one from cyclophosphamide.
The first pwMS was treated with ALEM in 2014. In 2017, the absolute number of individuals treated with ALEM peaked to 25. Thereafter, the number of individuals in whom ALEM was started dropped, with only one additional patient receiving ALEM since 2020 (Fig. S1).
Efficacy
In total, 30 patients experienced a cumulative count of 52 relapses, leaving 57 individuals relapse‐free during the observation period. Among the patients with ongoing relapse activity, the larger portion had either one (n = 17, 20%) or two relapses (n = 9, 10%), whereas four patients (5%) had three or more relapses during the follow‐up period. The ARR significantly declined from 1.16 (95% CI 0.95–1.42) before ALEM to 0.15 (95% CI 0.09–0.21) across Years 1 to 9 (p < 0.001, Fig. 1A,B). The subgroup analysis of patients with ongoing relapse activity (n = 30) revealed a drop of the ARR from 1.20 (95% CI 0.81–1.79) to 0.44 (95% CI 0.29–0.59) (p = 0.008, Fig. 1C,D).
Figure 1.
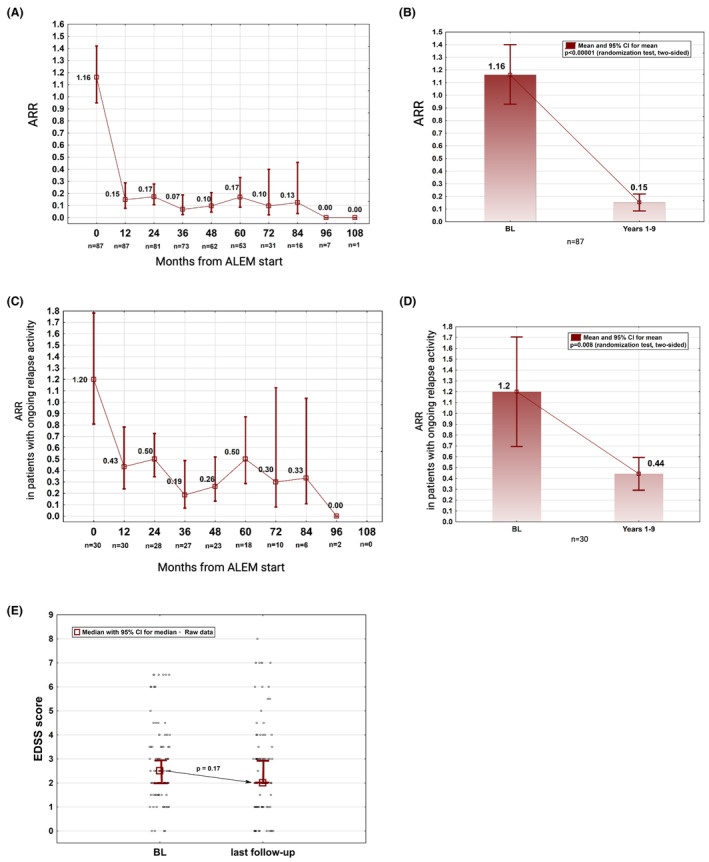
Long‐term effects of ALEM on the annualized relapse rate (ARR) and on the EDSS. (A, B) ARR among the whole cohort. (C, D) ARR among patients with ongoing relapse activity throughout the observation period. (E) Median EDSS scores at baseline (BL) and at the last available follow‐up; (A, C) data are shown as means with 95% CI; (E) The BL EDSS refers to the EDSS at 30 days from ALEM start. ALEM, alemtuzumab; BL, baseline; CI, confidence interval; EDSS, Expanded Disability Status Scale.
The median EDSS score at BL and at the end of the follow‐up period were 2.5 (IQR 1.8–3.5) and 2.0 (IQR 1.0–3.5), respectively (Fig. 1E). The EDSS score improved in 45 (52%), remained stable in 15 (17%), and worsened in 27 (31%) individuals. At Year 5 (n = 53), significantly more patients achieved CDI (58%, 95% CI 45%–71%) than had experienced PIRA (14%, 95% CI 7.5%–24%) and 58% (95% CI 46%–70%) remained relapse‐free (Fig. 2A–C).
Figure 2.
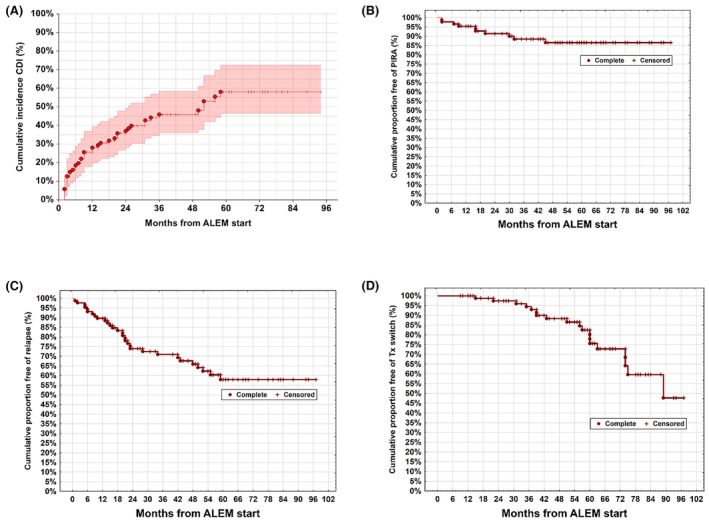
Time‐to‐event analyses on the achievement of CDI (A), the occurrence of PIRA (B) and relapses (C) and subsequent DMT usage (D). CDI, confirmed disability improvement; PIRA, progression independent of relapse activity; Tx switch, disease‐modifying therapy (DMT) after ALEM treatment.
Two patients received a third ALEM course, while no individual had four or more cycles. A subsequent DMT was started in 19 (22%) patients after a median of 56 (range 15–89) months from initiating ALEM (Fig. 2D, Table S1). The details for starting a new DMT were available in 15 patients: seven received subsequent immunotherapy due to a combination of clinical relapse and MRI activity, five due to isolated MRI activity, one following a clinical relapse without signs of MRI activity, and one due to relapse‐independent EDSS progression. One individual started a new DMT upon personal request in Year 7. Most of these patients (74%) received anti‐CD20 monoclonal antibodies, and all but one patient switched to high‐efficacy DMTs after ALEM discontinuation.
We found no significant association between the investigated BL parameters and the occurrence or number of relapses after ALEM initiation. An EDSS increase at the end of the observation period was associated with a longer disease duration at ALEM start (p < 0.001, r = 0.35), a higher BL EDSS (p < 0.001, r = 0.76), and usage of a higher number of DMTs before ALEM (p = 0.014, r = 0.26), especially with high‐efficacy DMTs (p = 0.004, median EDSS differences 1.0 (95% CI 0.0–2.0), Table 2)). In comparison, treatment‐naïve patients at the time of ALEM initiation had lower EDSS scores at the last follow‐up visit (p = 0.017, median EDSS difference 1.0 (95% CI 0.0–2.2)). Occurrence of PIRA was associated with a higher number of DMTs prior to ALEM start (p = 0.04, HR 3.06 [95% CI 1.05–8.89], Fig. S2). Achievement of CDI was associated with a shorter disease duration at the time of ALEM start (p < 0.001, HR 0.86 [95% CI 0.80–0.93]). The usage of high‐efficacy DMTs prior to ALEM was a strong predictor for not achieving CDI (p < 0.001; HR = 5.2 [95% CI 2.66–10.0]). Moreover, pretreatment with natalizumab (p = 0.025, HR 3.9 [95% CI 1.18–12.9]) and fingolimod (p = 0.016, HR 2.3 [95% CI 1.16–4.58]) were both associated with not achieving CDI. No investigated BL parameter could predict the necessity of subsequent DMT usage following ALEM.
Table 2.
Analysis of BL characteristics and outcome following ALEM treatment.
| Baseline variables | p‐value | Correlation |
|---|---|---|
| EDSS increase at last follow‐up | ||
| MS duration at ALEM initiation | <0.001 | r = 0.35 |
| BL EDSS | <0.001 | r = 0.76 |
| Treatment naïve | 0.017 a | Median EDSS difference = 1.0 (95% CI 0.0–2.20) b |
| No. DMTs prior ALEM | 0.014 a | r = 0.26 |
| High‐efficacy pretreatment | 0.004 a | Median EDSS differences = 1.0 (95% CI 0.0–2.0) |
| Switch from NAT | 0.004 a | Median EDSS differences = 1.0 (95% CI 0.5–2.20) b |
| PIRA “yes/no” | ||
| No. DMTs prior to ALEM | 0.04 c | HR 3.06 (95% CI 1.05–8.89) |
| Achievement of CDI “yes/no” | ||
| Age at MS diagnosis | 0.04 c | HR 1.96 (1.03–3.74) |
| MS duration at ALEM initiation | 0.00009 c | HR 0.86 (0.80–0.93) |
| High‐efficacy DMTs prior to ALEM | <0.001 c | HR 5.16 (95% CI 2.66–10.0) |
| Switch from NAT | 0.025 c | HR 3.9 (95% CI: 1.18–12.9) |
| Switch from FTY | 0.016 c | HR 2.3 (95% CI: 1.16–4.58) |
ALEM, alemtuzumab; BL, baseline; CDI, confirmed disability improvement; CI, confidence interval; DMT, disease‐modifying therapy; EDSS, Expanded Disability Status Scale; FTY, fingolimod; HR, hazard ratio (Cox‐regression model); MS, multiple sclerosis; NAT, natalizumab; No., number of; OR, odds ratio (logistic regression); PIRA, progression independent of relapse activity; r, Spearman correlation coefficient.
Wilcoxon–Mann–Whitney based on 10,000 Monte‐Carlo simulations.
Hodges‐Lehmann 95% CI.
Cox‐regression model.
Safety
Infusion‐related reactions were common and observed in 31 (36%) patients. These adverse events most frequently consisted of rash or urticaria (81%), flu‐like symptoms (13%), and arterial hypertension (6%). One patient experienced transient bradycardia and another transient tachycardia during the administration of ALEM. Noninfusion‐related events were reported in 39 (45%) patients, ranging from one (n = 30) to three (n = 1) per patient. Twenty‐six patients (30%) developed secondary autoimmune thyroid disorders, peaking at Years 2 to 4 (Fig. 3). No thyroid‐related adverse events occurred during Years 6 to 9. One patient developed immune thrombocytopenic purpura (ITP) 28 months from the initiation of ALEM. The thrombocyte counts recovered after intravenous treatment with steroids, immunoglobulins (IVIG), and rituximab. This single rituximab infusion administered due to ITP was not considered as subsequent DMT, and consequently, this individual remained in the study. Another patient was diagnosed with macrophage activation syndrome at Month 25 and an additional patient with psoriasis at Month 27. Infectious adverse events were reported in 16 patients (18%); all were categorized as nonsevere and urinary tract infections prevailed among. Four patients were diagnosed with localized herpetic infections at a median of 35 (range 26–44) months after starting ALEM. All four patients had thoracic herpes zoster. No opportunistic CNS infections and no vascular side effects were observed, and no patient died.
Figure 3.
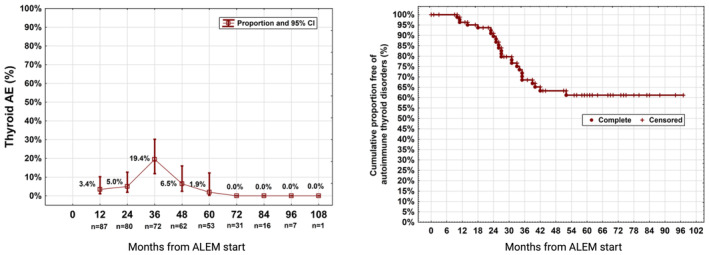
Thyroid‐related adverse events (AE) following ALEM treatment.
Comparison with other real‐world cohorts
Table 3 summarizes the outcomes of 20 real‐world cohorts after ALEM treatment. These studies encompass a total of 2605 individuals, primarily from multicenter studies. 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 The number of patients reported ranged from 19 to 883. The follow‐up duration in most of these studies covered 2 to 3 years of observation after ALEM start. The demographic characteristics, including age, disease duration, and gender, were comparable for most studies. However, the treatment history at the time of ALEM start varied across the cohorts, with a range of 0% to 73% of patients being treatment naïve at BL, which suggests different approaches to the usage of ALEM. The available studies consistently demonstrate a significant reduction in the ARR, in line with the findings of our study. However, conflicting evidence exists regarding the efficacy of ALEM on the EDSS course. Some studies reported improvements in the EDSS compared to baseline values, whereas most studies including our own showed no statistically significant changes 20 , 26 , 30 , 32 , 39 or an increase in the EDSS score. 35 We found a lower rate of infusion‐related side effects in our retrospective cohort analysis compared to other studies. However, rates of infectious events (18%) and secondary autoimmune disorders (30%) align with findings from available real‐world and pivotal studies.
Table 3.
ALEM usage in multiple sclerosis: An overview of real‐world studies.
| Design | Study design, demographics, and baseline characteristics | EDSS | ARR | NEDA‐3 | Common AE | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Follow‐up period (mean*/SD or median**/IQR/R) | Sex (%female) | Age (years, mean*/SD or median**/IQR/R) | DD (years, mean*/SD or median**/IQR/R) | Prior DMT | Baseline EDSS (mean*/SD or median**/IQR/R) | ΔEDSS | ARR pre‐ALEM (SD) | ARR post‐ALEM (SD) | ||||
| Alroughani et al. 2023 (Kuwait) | Multicenter (n = 73) | 4 y* ± 1.7 | 73% | 34* ± 8 | 9* ± 6 | 56%; 27% FTY, 19% NAT | 2.4* ± 1.9 | ↓EDSS | 0.9 ± 0.5 | 0.2 ± 0.5 | 58% | 75% IRR, 16% AT, 6% infection |
| Bónitto et al. 2022 (Colombia) | Single‐center (n = 23) | 22.8 m* ± 9.9 | 48% | 42* ± 11 | 11* ± 6 | 100% | 3.0** | ↓EDSS | NR | NR | NR | 30% AE; 13% Herpes virus infection, 4% thyroid dysfunction |
| R 0.0–8.0 | ||||||||||||
| Bose et al. 2021 (Canada) | Single‐center (n = 46) | 3.2 y**; R1.0–10.8 | 83% | 36**; IQR 31–42 | 5**; IQR 3–10 | 94% | 3.0** | NR | NR | NR | 80% | 80% IRR, 22% shingles, 56% secondary autoimmunity |
| IQR 2–4 | ||||||||||||
| Jakob et al. 2021 (Slovenia, Croatia) | Multicenter (n = 71) | 3.2 y* ± 1.1 | 72% | 28* ± 9 | 4* ± 3 | 72% | 3.0** (0–6) | ↓EDSS 27%; stable 56% | 1.7 ± 0.8 | 1y: 0.2 ± 0.4 | 25%, Clinical NEDA 60% | 84% AE; 59% IRR, 32 AT, 28% infections |
| 2y: 0.2 ± 0.4 | ||||||||||||
| 3y: 0.3 ± 0.5 | ||||||||||||
| Eichau et al. 2023 (Spain) | Single‐center | 29.7 m* ± 13.8 | 78% | NR (age at MS diagnosis 40 ± 9) | 14* ± 7 | 91%; 51% FTY, 13% DMF, 12% NAT, 5% GA | 4.5** | ↓EDSS | 1.5 (SD NR) | 0.1 (95% CI 0.0–0.1) | NR (relapse‐ free 90%) | 79% IRR, 37% infections, 22% autoimmune diseases |
| (n = 123) | IQR 3.5–6.0 | |||||||||||
| López‐Real et al. 2023 (Spain) | Multicenter (n = 195) | 2.4 y* ± 1.1 | 69% | 39* ± 8 | 9* ± 7 | 90%; 35% FTY, 23% NAT | 3.1* ± 1.5 | NC | 1y: 1.2 ± 1.0 | 1y: 0.2 ± 0.4 | 1y: 61% | IRR (1y: 83%; 2y: 69%; 3y: 46%); Infections (1y: 36%; 2y: 29%; 3y: 19%); secondary autoimmunity 19% |
| 2y: 0.2 ± 0.5 | 2y: 49% | |||||||||||
| 3y: 0.2 ± 0.4 | 3y: 42% | |||||||||||
| Rauma et al. 2021 (Finnland) | Multicenter (n = 121) | 30.3 m**; IQR 20.9–42.5 | 74% | 32**; IQR 28–38 | 5**; IQR 1–9 | 83%; 41% FTY, 17% NAT | Median 3.0** | NR | NR | NR | NR | IRR (y1: 84%; y2: 57%; y3:57%); serious AE 32%; autoimmune AE 31%, Infections 25% |
| IQR 2.0–5.0 | ||||||||||||
| Russo et al. 2021 (Italy) | Multicenter (n = 322) | 1.9 y** | 71% | 38* ± 10 | 9**; IQR 4–15 | 89%; 33% FTY, 25% NAT, 14% DMF | 3.0** | After 1y ↓EDSS; improvement in 14% after 1 year; 21% after 2 years | 1.0 ± 1.0 | 0.1 (SD NR) | 1y: 72% | 58% AE; 43% IRR; 11% AT |
| IQR 2.0–5.0 | 2y: 59% | |||||||||||
| Theodorsdottir et al. 2021 (Denmark) | Nationwide (n = 209) | 3.1y* ± 1.4 | 78% | 38**; IQR 33–44 | 9**; IQR 5–16 | 98%; 45% FTY, 28% NAT, 12% DMF | 3.8* ± 1.8 | EDSS improved or stable in 81% | 0.8 (SD NR) | 0.3 (SD NR) | NR (24 m: 75% relapse‐free) | 45% AE; 32% IRR, 16% infections, 14% autoimmune adverse events |
| Zmira et al. 2021 (Israel) | Single‐center (n = 35) | 2 y | 19 / 35 | 37* ± 11 | 10* ± 6 | 100%; 11/35 FTY, 9/35 IFN | 4.0** | NC | 2.0 (IQR 1.0–3.0) | 0.0 | 33% (at 2nd year follow‐up) | 35/35 urticaria (IRR), UTI 7/35, thyroid dysfunction 3/35 |
| IQR 2.5–6.0 | ||||||||||||
| Ziemssen et al. 2021 (Germany) | Multicenter (n = 883) | NR | 72% | 36* ± 9 | 8* ± 7 | 82%; 52% IFN, 35% FTY, 35% NAT, 29% GA | 2.5** | NR | NR | NR | NR | NR |
| R 0.0–7.5 | ||||||||||||
| Kim et al. 2019 (Korea) | Single‐center (n = 19) | 1.5* y ± 0.4 | 79% | NR (at disease onset 25* ± 9) | 8* ± 4 | 100%; 89% IFN, 11% TERI | 3.1* ± 1.3 | NC | 1.2 (SD NR) | 0.3 (SD NR) | 53% | 90% IRR, 3/19 herpes zoster |
| Hyun et al. 2019 (Korea) |
Single‐center (n = 23) |
17 m**; R 1–24 | 16 / 23 | 36* (SD NR) | 10*; Range 2–20 | 100% | 4.0** | ↓EDSS in 25%, stable in 58%, worsening in 17% | 1.5 (SD NR) | 0.2 (SD NR) | 2y: 33% | 83% IRR, 43% infections, 3/23 herpes zoster infection, 2/23 thyroid dysfunction |
| R 0.0–7.5 | ||||||||||||
| Herman et al. 2020 (USA) | Single‐center (n = 60) | 2.6 y* ± 1.4 | 78% | 45* ± 11 | 8* ± 5 | 100%; 61% RTX/OCV | 2.5** | ↓EDSS | NR | 1y: 0.1 | 2y: 61% | 85% adverse events, 58% IRR, 20% AT, 13% infections |
| IQR 2.5–6.0 | 2y: 0.1 | |||||||||||
| 3y: 0.1 | ||||||||||||
| 4y: 0.0 | ||||||||||||
| (SD NR) | ||||||||||||
| Tuohy et al. 2015 (UK) | Multicenter (n = 87) | 86.1 m* ± 23.9 m | 70% | 33* ± 8 | 36 m**; R 5–144 | 39% | 3.8* ± 1.9 | NC | 1.8 ± 0.8 | 0.2 ± 0.3 | NR | 48% clinical autoimmune disease, 41% AT, 4% ITP |
| Willis et al. 2016 (UK) | Single‐center (n = 100) | 6.1 y* | 67 / 100 | NR (at disease onset 28* ± 9) | 4 ± 4 | 27 / 100 | 4.0* ± 1.9 | EDSS↑ | 2.1 (SD NR) | 0.2 (SD NR) | NR | 87% IRR, 47/100 acquired autoimmune disease, 3/100 ITP |
| Huhn et al. 2018 (Germany) | Multicenter (n = 50) | 64 weeks | 30 / 50 | 36*; Range 20–56 | 13*; R 1–35 | 100% FTY | 3.0** | NC | 2.2 ± 1.8 | 0.3 ± 0.7 | NR | NR |
| R 1.0–6.5 | ||||||||||||
| Prosperini et al. 2018 (Italy) | Multicenter (n = 40) | 3 y | 82% | 34* ± 8 | 12* ± 6 | 98% | 4* ± 1.8 | ↓EDSS | 2.0 ± 1.1 | Numbers NR | 3y: 45% | 38/40 IRR, 8/40 AT, 7/40 herpes virus infection |
| Frau et al. 2019 (Italy) | Single‐center (n = 90) | 27 m* ± 23 | 74% | NR (at disease onset 26* ± 7) | 36* ± 8 | 92%; 32% NAT, 22% FTY, 16% DMF | 2.5** | ↓EDSS | NR | NR | 2y: 44% | 96% IRR, 11% pneumonitis, 10/90 AT, 3% thrombocytopenia |
| IQR 1.5–4.0 | ||||||||||||
| di Ioia et al. 2020 (Italy) | Single‐center (n = 35) | 36 m | 17 / 35 | 44* ± 8 | 17* ± 6 | 26/35 NAT, 7/35 FTY | 4.0** | NC | NR | NR | 3y: 67% | 29/36 IRR, 26% thyroid dysfunction, 3% ITP |
| R 1.0–7.5 | ||||||||||||
AE, adverse events; ALEM, alemtuzumab; ARR, Annualized Relapse Rate; AT, autoimmune‐related thyroiditis; CI, confidence interval; DD, disease duration; DMF, dimethylfumarate; EDSS, Expanded Disability Status Scale; FTY, fingolimod; GA, glatirameracetate; IFN, interferon beta; IQR, interquartile range; IRR, infusion‐related reactions; ITP, immune thrombocytopenia; m, months; n, number of patients enrolled in the respective studies; NAT, natalizumab; NC, no change; NEDA‐3, No Evidence of Disease Activity (iteration 3); NR, not reported; R, range; SD, standard deviation; TERI, teriflunomide; UK, united kingdom; UTI, urinary tract infections; y, years.
Mean.
Median.
Discussion
This nationwide observational study corroborates the findings of the pivotal trials of ALEM in relapsing–remitting MS, which revealed a profound reduction of the ARR over the period of 5 years. Our long‐term results expand the evidence for the efficacy and safety of ALEM in real‐world, 34 , 36 , 37 , 38 , 40 and show a favorable effect of ALEM on the ARR that could last for up to 9 years. In our cohort, the mean ARR declined from 1.16 (95% CI 0.95–1.42) in the 12 months before ALEM start to 0.15 (95% CI 0.09–0.21) throughout Years 1 to 9, with most patients remaining relapse‐free. Half of the patients were switched to ALEM from prior high‐efficacy DMTs, underscoring that a substantial number of patients in this cohort had a highly active MS course.
Various modes of action have been proposed to explain the durable effects of ALEM in MS. Among are the induction of immune tolerance, the profound depletion of memory B cells, and lasting effects on T helper 17 cells. 7 , 8 , 41 , 42 ALEM is considered to act as an immune reconstitution therapy (IRT) that aims to rebuild a healthy immune repertoire through pulsed immunosuppression. 8 In our cohort, only two patients received additional ALEM courses, and only a minority (22%) required a subsequent DMT (mostly in Years 4 to 6 and following relapses and MRI activity) throughout the study period, underpinning the durability of ALEM effects observed in the pivotal trials. 9 , 10 , 11 , 12 , 13 The number of patients from this cohort requiring subsequent DMTs will likely rise with prolonged follow‐up. Anti‐CD20 monoclonal antibodies were the most frequently used post‐ALEM treatment choices (14/19), and only one patient was switched to a low‐moderate efficacy DMT. The Austrian treatment sequencing contrasts the approach of the CARE‐MS I/II, extension, and open‐label extension study (TOPAZ) with 11–13 years of observation. 15 There, almost half of the patients received >2 and up to 8 ALEM cycles. In this regard, the EMA licensed the monoclonal anti‐CD20 antibody ocrelizumab in 2018. 5 The availability of another highly active treatment option with a different mode of action is likely to explain the advancement of the Austrian treatment strategy in patients with ongoing inflammatory disease activity despite treatment with ALEM.
For relapse‐independent disability outcomes, we found that the proportions of patients who achieved CDI (58%) were markedly higher than those with PIRA (14%). This observation indicates that ALEM may halt disability progression in the absence of overt relapses. In an Italian multicenter study, sustained disability improvement (SDI), defined as a sustained reduction of ≥1.0 points in EDSS, was achieved by 28% over 36 months of follow‐up, and most patients remained free of relapses and EDSS worsening. 37 A 6‐month confirmed disease improvement (defined as >1.0‐points decrease of the EDSS) was present in 37%–49% of patients from the open‐label extension study (TOPAZ) of the pivotal trials. 10 , 11 , 15 There, early ALEM usage increased the likelihood for not requiring additional treatment courses and to remain free from 6‐month confirmed EDSS worsening. 15 These findings are consistent with our results, as treatment‐naïve individuals and those with a shorter MS duration at the time of ALEM start had a favorable outcome regarding their EDSS at the last follow‐up while prior usage of high‐efficacy DMTs was significantly associated with not achieving CDI during the follow‐up. Also, more pretreatments and previous use of natalizumab were associated with higher EDSS scores at the end of the observation period. Moreover, a greater number of DMTs at BL was associated with higher PIRA‐rates. In conclusion, ALEM appears most effective to reverse disability and halt disease progression when started early in the disease.
Immune reconstitution therapies such as ALEM carry several advantages compared to continuously administered DMTs, including high adherence to treatment and lower risks of adverse events related to chronic immunosuppression. However, immune‐mediated adverse events targeting the thyroid, kidney, and platelets were not only observed during the core phase of the pivotal trials. Postmarketing studies also revealed new safety concerns, including fatal outcomes from severe vascular events such as myocardial infarction, intracerebral bleeding, and cervicocephalic arterial dissection, mostly occurring within days from ALEM administration. 43 , 44 While the majority of patients who developed acute coronary syndrome had cerebrovascular risk factors, no particular pattern of risk factors was identified among patients with cerebral stroke. 44 , 45 In this regard, ALEM administration is associated with increased blood pressure, and vital signs should be closely monitored before and during infusion. 44 , 45 , 46 These postmarketing safety issues led to EMA's Pharmacovigilance Risk Assessment Committee (PRAC) recommendation in 2019 that ALEM must no longer be used in patients with certain heart, circulation or bleeding disorders, or in patients who have autoimmune disorders other than MS. 47 , 48 In the Austrian cohort, no fatal vascular events were reported. A subset of our patients developed thyroid disorders, which are the most frequently associated autoimmune adverse events related to ALEM, and reported in about 40% of MS patients during long‐term follow‐up. 49 , 50 In contrast to the rare but potentially fatal vascular events and opportunistic infections, secondary autoimmune events are generally manageable when recognized early. Of note, a total of 11 deaths occurred during TOPAZ, including two from cancer. 15 Both cancer cases (metastatic rectal cancer, metastatic carcinoma of unspecified localization) were deemed related to ALEM.
Considering the growing availability of highly effective alternative DMTs, ALEM's updated safety profile makes it a lesser chosen treatment option, despite its undeniable effects on disease activity. Additionally, the COVID‐19 pandemic impacted the prescription rate of ALEM, as it was uncertain, whether the risk for unfavorable COVID‐19 outcomes would increase with during the phase of immune depletion and whether the immune responses to vaccination would be compromised. 51 , 52 In fact, only four patients have started ALEM in Austria from 2019 to 2022. In the meantime, the available evidence indicates that ALEM treatment does not interfere with humoral and T‐cell responses to vaccination or with COVID‐19 outcomes. 53 , 54
This observational study is limited by the lack of systematic MRI and laboratory data. Although achievement of CDI and occurrence of PIRA are clinically meaningful outcome measures, they may not capture discrete changes especially among patients with higher EDSS scores. Yet, the median EDSS scores in our cohort were relatively low and we have chosen a 6‐month confirmed 0.5‐points change for the definition of disability outcomes to avoid neglecting subtle changes in the patient's functional performance. Importantly, there is no uniform definition for neither CDI nor for PIRA, ranging from EDSS changes of ≥0.5 to ≥1.5 and confirmation periods of 12 weeks to several years. 2 Although several cohorts have reported benefits of ALEM in terms of EDSS and disability improvements (Table 3), we cannot exclude that the high CDI rates among our study are to some amount associated with the natural remission of the disease and with symptomatic therapies. 55 Lastly, a reporting bias concerning the collection of data in the registry might explain the lower incidence of infusion‐related side effects. Strengths of this work include the follow‐up duration and the nationwide coverage that enhances the generalizability of our findings.
Conclusion
Functional improvement as reflected by the high CDI rates is an important benefit of immune reconstitution therapy with ALEM in our nationwide real‐world cohort. While potential mechanisms that may drive neurological recovery remain elusive, our analysis suggests that disability accumulation may be reversible or at least preventable. The efficacy data support a role for ALEM treatment early in the disease course, aligning with the hit‐hard‐and‐early concept. However, due to safety concerns, EMA's PRAC recommended restricting ALEM for use in adults with RRMS that is highly active despite adequate treatment with at least one DMT or if the disease is worsening rapidly with at least two disabling relapses in a year and brain‐imaging showing new damage.
Author Contributions
Conceptualization, data curation, investigation, methodology, project administration, validation, writing—original draft, and writing—review and editing: Tobias Moser. Conceptualization, formal analysis, investigation, methodology, writing—original draft, and writing—review and editing: Fabian Foettinger. Conceptualization, data curation, formal analysis, methodology, software, visualization, and writing—review and editing: Wolfgang Hitzl. Data curation, formal analysis, investigation, and writing—review and editing: Bianka Novotna. Methodology, project administration, resources, supervision, and writing—review and editing: Thomas Berger. Conceptualization, data curation, formal analysis, investigation, and writing—review and editing: Gabriel Bsteh. Investigation, methodology, and writing—review and editing: Franziska Di Pauli. Conceptualization, formal analysis, investigation, methodology, writing—original draft, and writing—review and editing: Harald Hegen. Investigation, methodology, supervision, and writing—review and editing: Barbara Kornek. Data curation, formal analysis, and writing—review and editing: Dieter Langenscheidt. Conceptualization, funding acquisition, investigation, methodology, project administration, supervision, validation, writing—original draft, and writing—review and editing: Johann Sellner (corresponding author).
Funding Information
This research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors.
Conflict of Interest
FF, WH, and BN declare that they have no conflict of interest. TM received travel support, honoraria for presentations or participation on advisory boards from Biogen, BMS, Novartis, Roche, Sanofi, Merck, and Teva. TB participated in meetings sponsored by and received honoraria (lectures, advisory boards, consultations) from pharmaceutical companies marketing treatments for MS: Allergan, Bayer, Biogen, Bionorica, Biologix, BMS, Eisai, Janssen‐Cilag, Jazz/GW, Horizon, MedDay, Merck, Novartis, Octapharma, Roche, Sandoz, Sanofi‐Genzyme, UCB, and Teva. His institution received financial support in the past 12 months by unrestricted research grants (Biogen, Bayer, BMS/Celgene, Merck, Novartis, Sanofi Aventis, and Teva and for participation in clinical trials in multiple sclerosis sponsored by Alexion, Bayer, Biogen, Merck, Novartis, Octapharma, Roche, Sanofi‐Genzyme, and Teva. GB participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Lilly, Merck, Novartis, Roche, Sanofi‐Genzyme, and Teva, and received honoraria for consulting Biogen, Celgene/BMS, Novartis, Roche, Sanofi‐Genzyme, and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis. FDP participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations) or travel funding from Bayer, BMS, Biogen, Merck, Novartis, Sanofi‐Genzyme, Teva, and Roche. HH participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Celgene, Janssen, Merck, Novartis, Sanofi, Siemens, and Teva and received honoraria for acting as consultant for Biogen, Celgene, Novartis, and Teva. BK received honoraria for speaking and for consulting from Biogen, BMS‐Celgene, Johnson & Johnson, Merck, Novartis, Roche, Teva, and Sanofi outside of the submitted work. DL received honoraria from Biogen, Novartis, Merck, and Roche. JS received honoraria for lectures, assembly of educational material and participation in advisory boards from Alexion/Astra Zeneca, Biogen, BMS, Fresenius, Gerot‐Lannach, GSK, Horizon/Amgen, Lundbeck, Immunic, Merck, Novartis, Pfizer, Sanofi, and Sandoz.
Supporting information
Supplemental Figure 1.
Supplemental Figure 2.
Supplemental Table 1.
Acknowledgements
We are grateful to all Austrian MS centers for contributing data to the registry. A full list of centers can be found at https://www.oegn.at/neurologie-in-oesterreich/ms-zentren. Open access funding was provided by the Department of Neurology, Christian Doppler Medical Center, Paracelsus Medical University, Salzburg, Austria.
Data Availability Statement
The data that support the findings of this study are available on reasonable request by qualified researchers from the corresponding author (JS).
References
- 1. Filippi M, Bar‐Or A, Piehl F, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43. [DOI] [PubMed] [Google Scholar]
- 2. Sharrad D, Chugh P, Slee M, Bacchi S. Defining progression independent of relapse activity (PIRA) in adult patients with relapsing multiple sclerosis: a systematic review. Mult Scler Relat Disord. 2023;78:104899. [DOI] [PubMed] [Google Scholar]
- 3. Kappos L, Wolinsky JS, Giovannoni G, et al. Contribution of relapse‐independent progression vs relapse‐associated worsening to overall confirmed disability accumulation in typical relapsing multiple sclerosis in a pooled analysis of 2 randomized clinical trials. JAMA Neurol. 2020;77(9):1132‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathol. 2018;135(4):511‐528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Findling O, Sellner J. Second‐generation immunotherapeutics in multiple sclerosis: can we discard their precursors? Drug Discov Today. 2021;26(2):416‐428. [DOI] [PubMed] [Google Scholar]
- 6. Willis MD, Robertson NP. Alemtuzumab for the treatment of multiple sclerosis. Ther Clin Risk Manag. 2015;11:525‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moser T, Akgun K, Proschmann U, Sellner J, Ziemssen T. The role of TH17 cells in multiple sclerosis: therapeutic implications. Autoimmun Rev. 2020;19(10):102647. [DOI] [PubMed] [Google Scholar]
- 8. Lunemann JD, Ruck T, Muraro PA, Bar‐Or A, Wiendl H. Immune reconstitution therapies: concepts for durable remission in multiple sclerosis. Nat Rev Neurol. 2020;16(1):56‐62. [DOI] [PubMed] [Google Scholar]
- 9. Investigators CT, Coles AJ, Compston DA, et al. Alemtuzumab vs. interferon beta‐1a in early multiple sclerosis. N Engl J Med. 2008;359(17):1786‐1801. [DOI] [PubMed] [Google Scholar]
- 10. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first‐line treatment for patients with relapsing‐remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819‐1828. [DOI] [PubMed] [Google Scholar]
- 11. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease‐modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829‐1839. [DOI] [PubMed] [Google Scholar]
- 12. Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE‐MS II 5‐year follow‐up: efficacy and safety findings. Neurology. 2017;89(11):1117‐1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE‐MS I 5‐year follow‐up: durable efficacy in the absence of continuous MS therapy. Neurology. 2017;89(11):1107‐1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giovannoni G, Cohen JA, Coles AJ, et al. Alemtuzumab improves preexisting disability in active relapsing‐remitting MS patients. Neurology. 2016;87(19):1985‐1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coles AJ, Achiron A, Traboulsee A, et al. Safety and efficacy with alemtuzumab over 13 years in relapsing‐remitting multiple sclerosis: final results from the open‐label TOPAZ study. Ther Adv Neurol Disord. 2023;16:17562864231194823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lopez Ruiz R, Sanchez Fernandez F, Ruiz de Arcos M, et al. Skin autoimmunity secondary to alemtuzumab in a tertiary care Spanish hospital. Neurol Clin Pract. 2022;12(1):29‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Varela L, Pappolla A, Heriz A, et al. Primary central nervous system vasculitis following alemtuzumab treatment for multiple sclerosis: a case report and literature review. Neurologist. 2023;28(4):270‐272. [DOI] [PubMed] [Google Scholar]
- 18. Costa GD, Comi G. A safety review of current monoclonal antibodies used to treat multiple sclerosis. Expert Opin Drug Saf. 2023;22(11):1011‐1024. [DOI] [PubMed] [Google Scholar]
- 19. Bass AD, Arroyo R, Boster AL, et al. Alemtuzumab outcomes by age: post hoc analysis from the randomized CARE‐MS studies over 8 years. Mult Scler Relat Disord. 2021;49:102717. [DOI] [PubMed] [Google Scholar]
- 20. Tuohy O, Costelloe L, Hill‐Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: long‐term safety and efficacy. J Neurol Neurosurg Psychiatry. 2015;86(2):208‐215. [DOI] [PubMed] [Google Scholar]
- 21. Alroughani R, AlMojel M, Al‐Hashel J, Ahmed SF. A real‐life study of alemtuzumab in persons with multiple sclerosis: Kuwait's experience. Mult Scler Relat Disord. 2023;74:104712. [DOI] [PubMed] [Google Scholar]
- 22. Bonitto JRG, Ayala OD, Botero LC. Real‐life evidence of treatment with alemtuzumab in patients diagnosed with relapsing‐remitting multiple sclerosis in Colombia. Mult Scler Relat Disord. 2022;61:103780. [DOI] [PubMed] [Google Scholar]
- 23. Bose G, Rush C, Atkins HL, Freedman MS. A real‐world single‐centre analysis of alemtuzumab and cladribine for multiple sclerosis. Mult Scler Relat Disord. 2021;52:102945. [DOI] [PubMed] [Google Scholar]
- 24. Brecl Jakob G, Barun B, Gomezelj S, et al. Effectiveness and safety of alemtuzumab in the treatment of active relapsing‐remitting multiple sclerosis: a multicenter, observational study. Neurol Sci. 2021;42:4591‐4597. [DOI] [PubMed] [Google Scholar]
- 25. Eichau S, Lopez Ruiz R, Ruiz de Arcos M, et al. Results of treatment with alemtuzumab in a Spanish cohort of patients with multiple sclerosis in the real world: the RealMS study. Front Neurol. 2023;14:1112193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez‐Real AM, Gonzalez I, Solar DM, et al. Alemtuzumab treatment in real clinical practice: Experience in a multicenter cohort. Mult Scler Relat Disord. 2023;75:104762. [DOI] [PubMed] [Google Scholar]
- 27. Rauma I, Mustonen T, Seppa JM, et al. Safety of alemtuzumab in a nationwide cohort of Finnish multiple sclerosis patients. J Neurol. 2021;269:824‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Russo CV, Sacca F, Frau J, et al. A real‐world study of alemtuzumab in a cohort of Italian patients. Eur J Neurol. 2022;29(1):257‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Theodorsdottir A, Debrabant B, Magyari M, et al. Alemtuzumab treatment in Denmark: a national study based on the Danish multiple sclerosis registry. Mult Scler. 2021;27:2254‐2266. [DOI] [PubMed] [Google Scholar]
- 30. Zmira O, Halpern AI, Abraham L, Achiron A. Efficacy and safety of alemtuzumab treatment in a real‐world cohort of patients with multiple sclerosis. Acta Neurol Belg. 2021;121(6):1513‐1518. [DOI] [PubMed] [Google Scholar]
- 31. Ziemssen T, Hoffmann F, Richter S, Engelmann U, White R. Alemtuzumab in a large Real‐life cohort: interim baseline data of the TREAT‐MS study. Front Neurol. 2021;12:620758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim H, Lee EJ, Kim SK, Kim KK, Lim YM. Efficacy and safety of alemtuzumab in Korean multiple sclerosis patients. Mult Scler Relat Disord. 2019;30:247‐251. [DOI] [PubMed] [Google Scholar]
- 33. Hyun JW, Shin HJ, Jang H, Park NY, Kim SH, Kim HJ. Therapeutic outcome of alemtuzumab in Korean patients with multiple sclerosis: 2‐year follow‐up. J Clin Neurol. 2019;15(3):328‐333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Herman JA, Khalighinejad F, York K, et al. A real‐world cohort analysis of alemtuzumab outcomes in relapsing multiple sclerosis. Mult Scler Relat Disord. 2021;47:102619. [DOI] [PubMed] [Google Scholar]
- 35. Willis MD, Harding KE, Pickersgill TP, et al. Alemtuzumab for multiple sclerosis: long term follow‐up in a multi‐centre cohort. Mult Scler. 2016;22(9):1215‐1223. [DOI] [PubMed] [Google Scholar]
- 36. Huhn K, Bayas A, Doerck S, et al. Alemtuzumab as rescue therapy in a cohort of 50 relapsing‐remitting MS patients with breakthrough disease on fingolimod: a multi‐center observational study. J Neurol. 2018;265(7):1521‐1527. [DOI] [PubMed] [Google Scholar]
- 37. Prosperini L, Annovazzi P, Boffa L, et al. No evidence of disease activity (NEDA‐3) and disability improvement after alemtuzumab treatment for multiple sclerosis: a 36‐month real‐world study. J Neurol. 2018;265(12):2851‐2860. [DOI] [PubMed] [Google Scholar]
- 38. Frau J, Coghe G, Lorefice L, Fenu G, Musu L, Cocco E. Efficacy and safety of alemtuzumab in a real‐life cohort of patients with multiple sclerosis. J Neurol. 2019;266(6):1405‐1411. [DOI] [PubMed] [Google Scholar]
- 39. di Ioia M, Di Stefano V, Farina D, et al. Alemtuzumab treatment of multiple sclerosis in real‐world clinical practice: a report from a single Italian center. Mult Scler Relat Disord. 2020;38:101504. [DOI] [PubMed] [Google Scholar]
- 40. Kalincik T, Brown JWL, Robertson N, et al. Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing‐remitting multiple sclerosis: a cohort study. Lancet Neurol. 2017;16(4):271‐281. [DOI] [PubMed] [Google Scholar]
- 41. Ruck T, Bittner S, Wiendl H, Meuth SG. Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Int J Mol Sci. 2015;16(7):16414‐16439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Moser T, Schwenker K, Seiberl M, et al. Long‐term peripheral immune cell profiling reveals further targets of oral cladribine in MS. Ann Clin Transl Neurol. 2020;7(11):2199‐2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Killestein J, van Oosten B. Emerging safety issues in alemtuzumab‐treated MS patients. Mult Scler. 2019;25(9):1206‐1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Azevedo CJ, Kutz C, Dix A, Boster A, Sanossian N, Kaplan J. Intracerebral haemorrhage during alemtuzumab administration. Lancet Neurol. 2019;18(4):329‐331. [DOI] [PubMed] [Google Scholar]
- 45. Coles AJ, Jones JL, Vermersch P, et al. Autoimmunity and long‐term safety and efficacy of alemtuzumab for multiple sclerosis: benefit/risk following review of trial and post‐marketing data. Mult Scler. 2022;28(5):842‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Di Pauli F, Riedl K, Hegen H, et al. Alemtuzumab induced hemodynamic change in relapsing multiple sclerosis occurs independent of corticosteroid premedication ‐ a retrospective multicentre study. Mult Scler Relat Disord. 2022;63:103810. [DOI] [PubMed] [Google Scholar]
- 47. EMA . [website] 2020 [updated 16 January 2020 cited 2023 06.08.2023]. https://www.ema.europa.eu/en/medicines/human/referrals/lemtrada
- 48. Holmoy T, Fevang B, Olsen DB, Spigset O, Bo L. Adverse events with fatal outcome associated with alemtuzumab treatment in multiple sclerosis. BMC Res Notes. 2019;12(1):497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Devonshire V, Phillips R, Wass H, Da Roza G, Senior P. Monitoring and management of autoimmunity in multiple sclerosis patients treated with alemtuzumab: practical recommendations. J Neurol. 2018;265(11):2494‐2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rotondi M, Molteni M, Leporati P, Capelli V, Marino M, Chiovato L. Autoimmune thyroid diseases in patients treated with alemtuzumab for multiple sclerosis: an example of selective anti‐TSH‐receptor immune response. Front Endocrinol (Lausanne). 2017;8:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bsteh G, Riedl K, Krajnc N, et al. Has the pandemic changed treatment strategy in multiple sclerosis? Mult Scler Relat Disord. 2022;63:103912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sellner J, Rommer PS. Multiple sclerosis and SARS‐CoV‐2 vaccination: considerations for immune‐depleting therapies. Vaccines (Basel). 2021;9(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Simpson‐Yap S, De Brouwer E, Kalincik T, et al. Associations of disease‐modifying therapies with COVID‐19 severity in multiple sclerosis. Neurology. 2021;97(19):e1870‐e1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rabenstein M, Thomas OG, Carlin G, et al. The impact of hybrid immunity on immune responses after SARS‐CoV‐2 vaccination in persons with multiple sclerosis treated with disease‐modifying therapies. Eur J Neurol. 2023;30(12):3789‐3798. [DOI] [PubMed] [Google Scholar]
- 55. Beer S, Khan F, Kesselring J. Rehabilitation interventions in multiple sclerosis: an overview. J Neurol. 2012;259(9):1994‐2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1.
Supplemental Figure 2.
Supplemental Table 1.
Data Availability Statement
The data that support the findings of this study are available on reasonable request by qualified researchers from the corresponding author (JS).


