Figure 6:
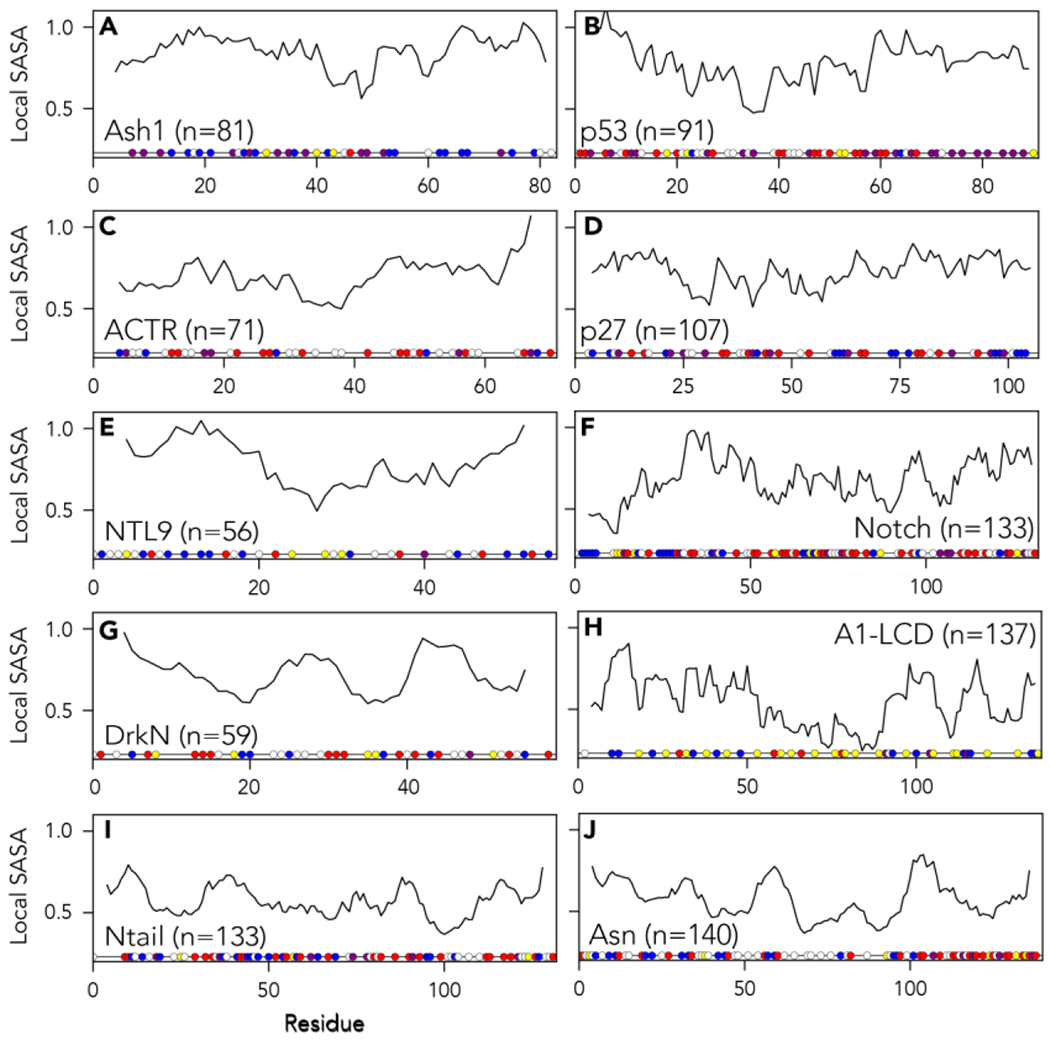
Normalized local solvent-accessible surface area (SASA) using an eight-residue sliding window and a 10 Å probe size. Normalization is done using excluded volume (EV) reference simulations to account for side-chain-dependent differences in solvent accessibility. Amino acid residues are colored as in Fig 3. Distinct patterns of accessibility are observed across different proteins, indicating long- and short-range intramolecular interactions can influence the accessibility of local binding sites.
