ABSTRACT
One significant complication of hepatitis B virus includes reactivation (HBVr) in the context of the use of immunosuppressive agents, such as corticosteroids and rituximab, among others. Limited data exist on the topic of HBVr risk in the context of tyrosine kinase inhibitors for which there is no strong guidance recommendation. We describe the clinical characteristics, diagnostic challenges, and the clinical course of a single patient with recurrent mantle cell lymphoma who developed HBVr after treatment with acalabrutinib, a Bruton tyrosine kinase inhibitor.
KEYWORDS: hepatitis B, hepatitis B reactivation, acalabrutinib, tyrosine kinase inhibitor, cholestatic hepatitis
INTRODUCTION
A significant complication of hepatitis B (HBV) includes reactivation (HBVr), which is defined as “loss of HBV immune control in HBV surface antigen (HBsAg)–positive, anti-HBc–positive or HBsAg–negative, anti-HBc–positive patients receiving immunosuppressive therapy for a concomitant medical condition; a rise in HBV DNA compared to baseline (or an absolute level of HBV DNA when a baseline is unavailable); and reverse seroconversion (seroreversion) from HBsAg negative to HBsAg positive for HBsAg-negative, anti-HBc–positive patients.”1 HBVr is often due to the use of immunosuppressive agents such as corticosteroids, rituximab, anthracyclines, cyclophosphamide, and vinca alkaloids.2,3 However, other agents have been implicated.
Limited data exist regarding HBVr risk in the context of tyrosine kinase inhibitors for which there is no strong guidance recommendation.4 Recently, HBVr was studied in the context of Bruton tyrosine kinase inhibitors, including acalabrutinib, and the HBVr risk was deemed to be moderate-level risk as approximately 10% of patients developed HBVr.5,6 We describe the clinical characteristics, diagnostic challenges, and the clinical course of a single patient who developed HBVr after treatment with acalabrutinib.
CASE REPORT
A 66-year-old man with history of obesity and type 2 diabetes mellitus was initially diagnosed with mantle cell lymphoma (MCL) after presenting with a neck mass and weight loss. He was screened and diagnosed with HBV exposure without active infection as HBV core antibody (HBcAb) was positive, HBV DNA was undetected, and HBsAg was negative. He was started on entecavir for HBVr prophylaxis and treated with rituximab, cyclophosphamide, vincristine sulfate, doxorubicin, methotrexate, and cytarabine for 6 months, achieving remission from MCL. Entecavir was continued for 12 months after chemotherapy was completed. Two years later, the patient was diagnosed with recurrent MCL. Serologic testing for hepatitis B reaffirmed his history of HBV exposure without active infection (without viremia). The patient underwent treatment with venetoclax and acalabrutinib for nearly 2 years without antiviral prophylaxis for HBVr because no strong evidence exists to guide recommendations for or against antiviral prophylaxis.
At the time of presentation to the hospital, the patient developed fever and was found to have an HBV DNA level of 421,000,000 IU; it was undetectable 3 years earlier. HBsAg had converted from nonreactive to reactive, and HBcAb immunoglobulin M (IgM) was also positive. Alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, bilirubin, and international normalized ratio were normal at that time. His last dose of rituximab had been nearly 5 years before this presentation. A new hepatitis B infection was not considered likely because the patient did not have any high-risk behaviors, had a previously reactive HBsAb, and had a history of HBV exposure.
Oral entecavir 0.5 mg/day was initiated to treat HBVr. Shortly afterward, liver enzymes rose and reached peak levels of alanine aminotransferase (ALT) 1160 U/L, aspartate aminotransferase (AST) 1057 U/L, and alkaline phosphatase (ALP) 410 U/L. Serologic workup for other acute liver disease, including hepatitis D, was unrevealing except for weakly positive antismooth muscle and antimitochondrial antibodies (Table 1). Abdomen magnetic resonance imaging/magnetic resonance cholangiopancreatography showed no biliary dilatation.
Table 1.
Liver serology workup
| Variable | Reference range, adults | Result |
| Hepatitis A IgM antibody | Negative | Negative |
| Hepatitis B surface antigen | Nonreactive | Positive |
| Hepatitis B surface antibody | Nonreactive | Nonreactive |
| Hepatitis B total core antibody | Nonreactive | Positive |
| Hepatitis B IgM core antibody | Nonreactive | Positive |
| Hepatitis B DNA (IU/mL) | Undetected | 421,000,000 |
| Hepatitis C antibody | Nonreactive | Nonreactive |
| Hepatitis C RNA (IU/mL) | Undetected | Undetected |
| Hepatitis D antibody | Negative | Negative |
| Hepatitis E IgM antibody | Negative | Negative |
| Hepatitis E IgG antibody | Negative | Negative |
| Hepatitis E RNA (IU/mL) | Undetected | Undetected |
| HIV-1 antigen | Nonreactive | Nonreactive |
| HIV-1 antibody | Nonreactive | Nonreactive |
| HIV-2 antibody | Nonreactive | Nonreactive |
| CMV IgM | Negative | Negative |
| EBV viral capsid antigen IgM antibody | Negative | Negative |
| EBV DNA (copies/mL) | Not detected | Not detected |
| HSV-1 DNA (copies/mL) | Not detected | Not detected |
| HSV-2 DNA (copies/mL) | Not detected | Not detected |
| HHV-6 DNA (copies/mL) | Not detected | Not detected |
| Adenovirus DNA (copies/mL) | Not detected | Not detected |
| Anti-nuclear antibody titer | Negative | Negative |
| Anti-smooth muscle antibody | Negative | Positive (1:40 titer) |
| Anti-mitochondrial antibody | Normal: 0, borderline 0.1–0.3 | 0.1 |
| Anti-liver kidney microsomal type 1 antibody | Negative | Negative |
| IgG (mg/dL) | 610–1,616 | 706 |
| IgM (mg/dL) | 35–242 | 483 |
| IgA (mg/dL) | 85–499 | 210 |
| Ceruloplasmin (mg/dL) | 19.0–31.0 | 35.5 |
| Alpha-1 antitrypsin (mg/dL) | 100–190 | 209 |
| Ferritin (ng/mL) | 30–400 | 5,636 |
| HFE gene mutation analysis | — | Negative for C282Y and H63D |
| Thyroid-stimulating hormone (TSH) (mcunit/mL) | 0.27–4.2 | 2.01 |
| Antitissue transglutaminase IgA antibody (U/mL) | <4.0 | 1.8 |
| Antitissue transglutaminase IgG antibody (U/mL) | <6.0, weakly positive 6–9 | 8.4 |
CMV, cytomegalovirus; EBV, Epstein–Barr virus; HHV, human herpesvirus; HSV, herpes simplex virus; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M.
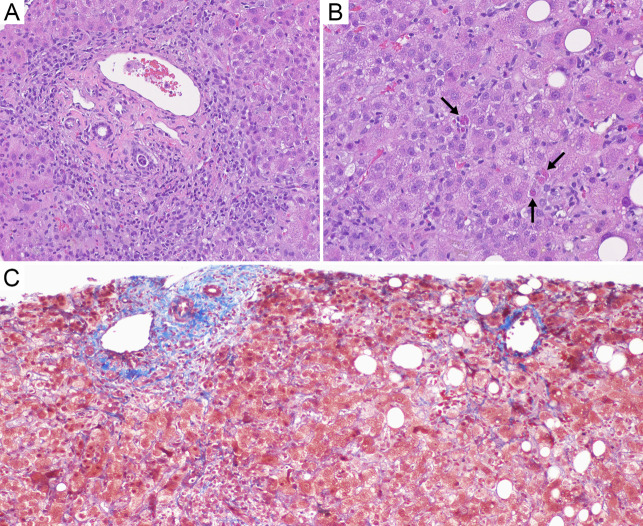
As his liver enzymes improved, the total bilirubin increased to a peak of 10.6 mg/dL with a peak direct component of 8.4 mg/dL. However, throughout this episode, his international normalised ratio (INR) remained normal with no evidence of liver failure. The delayed rise in bilirubin was concerning for hepatic dysfunction, specifically drug-induced liver injury related to a concurrent autoimmune-like process, given the weakly positive anti-smooth muscle antibody and anti-mitochondrial antibody; however, the immunoglobulin G (IgG) and IgM levels were normal (Table 1). Diagnostic liver biopsy revealed panacinar hepatitis with mild steatosis, no lymphoma involvement, and no advanced fibrosis (Figure 1). Given the clinical context, results were most consistent with HBVr because of acalabrutinib treatment rather than idiopathic autoimmune hepatitis (AIH) or drug-induced AIH.
Figure 1.
(A–C) Liver biopsy. (A) Hematoxylin and eosin (H&E) stain: Portal tract is expanded by mixed inflammatory infiltrates with marked inflammatory interface activity. (B) H&E stain: Lobular hepatitis is characterized by sinusoidal lymphohistiocytic inflammation and multiple acidophil bodies (arrows). (C) Trichrome stain highlights the lack of pre-existing bridging fibrosis or cirrhosis, with a portal tract at the left and a central vein at the right.
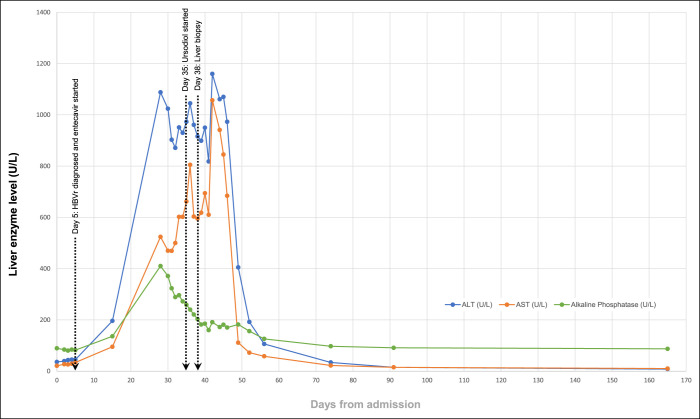
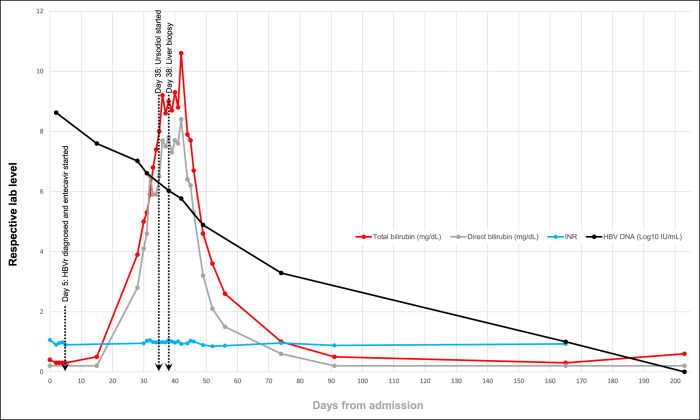
Progressive hyperbilirubinemia was attributed to a delayed phenomenon in the biochemical laboratory test results. Ten weeks after entecavir initiation, ALT, AST, ALP, and bilirubin normalized (Figures 2 and 3). HBV DNA level decreased steadily on entecavir therapy, and after approximately 6 months, it became undetectable (Figure 3), and HBsAg became nonreactive. Given continued normal mental status and INR levels (Figure 3), there was no evidence of acute liver failure.
Figure 2.
Trends in results of liver biochemical testing (serum alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase levels), displayed in days relative to admission on day 0 (lines connect available data points).
Figure 3.
Trends in results of liver biochemical testing (total bilirubin, direct bilirubin, and INR) and HBV DNA (log10 scale) displayed in days relative to admission on day 0 (lines connect available data points). HBV, hepatitis B virus; INR, international normalised ratio.
Indefinite treatment with entecavir was eventually recommended. MCL treatment with venetoclax and acalabrutinib was discontinued because of HBVr from acalabrutinib, and the patient is currently on surveillance.
DISCUSSION
HBVr may manifest as silent reactivation, overt hepatitis with liver dysfunction, or acute liver failure.7 Although our patient did not have the encephalopathy and impaired synthetic function (INR of 1.5 or higher) indicative of acute liver failure,8 he did have acute liver injury, as evidenced by his severely elevated liver chemistries with histological evidence of hepatitis.
Various immunosuppressive regimens are deemed high risk (ie, greater than 10% risk as per American Gastroenterological Association [AGA] guidelines) vs moderate risk (ie, 1%-10% risk as per AGA guidelines) of HBVr.7–9 Although patients with positive HBsAg are at highest risk of HBVr, patients with past exposure to HBV without active infection, defined as negative HBsAg and HBV DNA but positive HBcAb, are at risk of HBVr as well.2,10 There is a paucity of data regarding HBVr prophylaxis for those receiving low- or moderate-risk therapy. Current practice recommendations from the American Association for the Study of Liver Disease and AGA are to monitor HBV DNA, HBsAg, and ALT and start prophylaxis/treatment if indicated (i.e., on-demand therapy) where prescribing an antiviral is optional.7,11
Bruton tyrosine kinase inhibitors are perceived as moderate risk of HBVr, but current data are insufficient to recommend universal HBVr prophylaxis on these treatments, although close monitoring for HBVr is warranted.4,5,12,13 Nucleoside inhibitors tenofovir and entecavir are first-line therapy for both HBVr prophylaxis and treatment and have been shown to be superior to lamivudine.7
Although the exact incidence or risk of venetoclax and acalabrutinib causing HBVr is not known, there have been rare cases of HBVr with concurrent acalabrutinib use with other immunosuppressive agents.14 Venetoclax's adverse effects of lymphopenia and neutropenia might hypothetically predispose to HBVr, but there are no clinical reports regarding this.15
During the diagnostic evaluation for this patient, the rise in the bilirubin despite decrease in the HBV DNA level led to concern about whether another liver injury process was potentially causing progressive synthetic dysfunction. In light of improving liver enzymes, improving HBV DNA levels with entecavir treatment, in conjunction with liver biopsy results, drug-induced liver injury (DILI) by acalabrutinib was less likely. There are no data reporting a drug-induced AIH related to acalabrutinib.14 The diagnosis remained compatible with HBVr.
In conclusion, our case highlights the risk of HBVr in patients previously exposed to HBV without active infection and receive lower-risk immunosuppressants. A delayed rise in bilirubin in patients with HBVr does not necessarily correlate with further hepatic injury, but there can be a potential lag time between improvement of the HBV viral load and the biochemical response.
DISCLOSURES
Author contributions: AS Shaikh, SC Abraham, and HC Zhang both made substantial contributions to acquire and interpret the data of the work and draft the manuscript. AS Shaikh, LS Wang, HA Torres, and HC Zhang all made substantial contributions to conceive and design the work as well as revise the manuscript. All authors gave approval of the final version to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. AS Shaikh is the article guarantor.
Financial disclosure: Dr Torres is or has been the principal investigator for research grants from the National Cancer Institute, Gilead Sciences, and Merck & Co., Inc., with all funds paid to MD Anderson Cancer Center. Dr Torres is or has been a paid scientific advisor for AbbVie, Inc., Gilead Sciences, Janssen Pharmaceuticals, Inc., Merck & Co., Inc., and Dynavax Technologies; MD Anderson Cancer Center is managing the terms of these arrangements in accordance with its conflict-of-interest policies. All other authors do not have any actual or potential financial conflicts or disclosures.
Previous presentation: Presented at ACG's 2023 Annual Scientific Meeting on October 23, 2023, in Vancouver, Canada.
Informed patient consent was obtained for this case report.
Contributor Information
Susan C. Abraham, Email: suabraham@mdanderson.org.
Lan S. Wang, Email: LWang25@mdanderson.org.
Harrys A. Torres, Email: HTorres@mdanderson.org.
Hao Chi Zhang, Email: HZhang20@mdanderson.org.
REFERENCES
- 1.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67(4):1560–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim HY, Kim W. Chemotherapy-related reactivation of hepatitis B infection: Updates in 2013. World J Gastroenterol 2014;20(40):14581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang JP, Feld JJ, Hammond SP, et al. Hepatitis B virus screening and management for patients with cancer prior to therapy: ASCO provisional clinical opinion update. J Clin Oncol 2020;38(31):3698–715. [DOI] [PubMed] [Google Scholar]
- 4.Mustafayev K, Torres H. Hepatitis B virus and hepatitis C virus reactivation in cancer patients receiving novel anticancer therapies. Clin Microbiol Infect 2022;28(10):1321–7. [DOI] [PubMed] [Google Scholar]
- 5.Chiu CY, Ahmed S, Thomas SK, et al. Hepatitis B virus reactivation in patients receiving Bruton tyrosine kinase inhibitors. Clin Lymphoma Myeloma Leuk 2023;23(8):610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Azzam A, Khaled H, Ashry BO, et al. HBV reactivation in patients receiving Bruton tyrosine kinase inhibitors (BTKIs): A systematic review and meta-analysis. Curr Infect Dis Rep 2024;26:1–13. [Google Scholar]
- 7.Myint A, Tong MJ, Beaven SW. Reactivation of hepatitis B virus: A review of clinical guidelines. Clin Liver Dis (Hoboken) 2020;15(4):162–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah NJ, Royer A, John S. Acute liver failure. In: StatPearls. Treasure Island (FL): StatPearls Publishing; https://www.ncbi.nlm.nih.gov/books/NBK482374 (2024). [PubMed] [Google Scholar]
- 9.Papatheodoridis GV, Lekakis V, Voulgaris T, et al. Hepatitis B virus reactivation associated with new classes of immunosuppressants and immunomodulators: A systematic review, meta-analysis, and expert opinion. J Hepatol 2022;77(6):1670–89. [DOI] [PubMed] [Google Scholar]
- 10.Bozza C, Cinausero M, Iacono D, Puglisi F. Hepatitis B and cancer: A practical guide for the oncologist. Crit Rev Oncol Hematol 2016;98:137–46. [DOI] [PubMed] [Google Scholar]
- 11.Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: Current concepts, management strategies, and future directions. Gastroenterology 2017;152(6):1297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ni Y, Gao L, Lu Y, et al. Risk of HBV reactivation in relapsed or refractory diffuse large B-cell lymphoma patients receiving Bruton tyrosine kinase inhibitors therapy. Front Immunol 2022;13:982346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mak JWY, Law AWH, Law KWT, Ho R, Cheung CKM, Law MF. Prevention and management of hepatitis B virus reactivation in patients with hematological malignancies in the targeted therapy era. World J Gastroenterol 2023;29(33):4942–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Acalabrutinib. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; https://www.ncbi.nlm.nih.gov/books/NBK548486/ (2012). [PubMed] [Google Scholar]
- 15.LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Venetoclax. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; https://www.ncbi.nlm.nih.gov/books/NBK548460/ (2012). [PubMed] [Google Scholar]