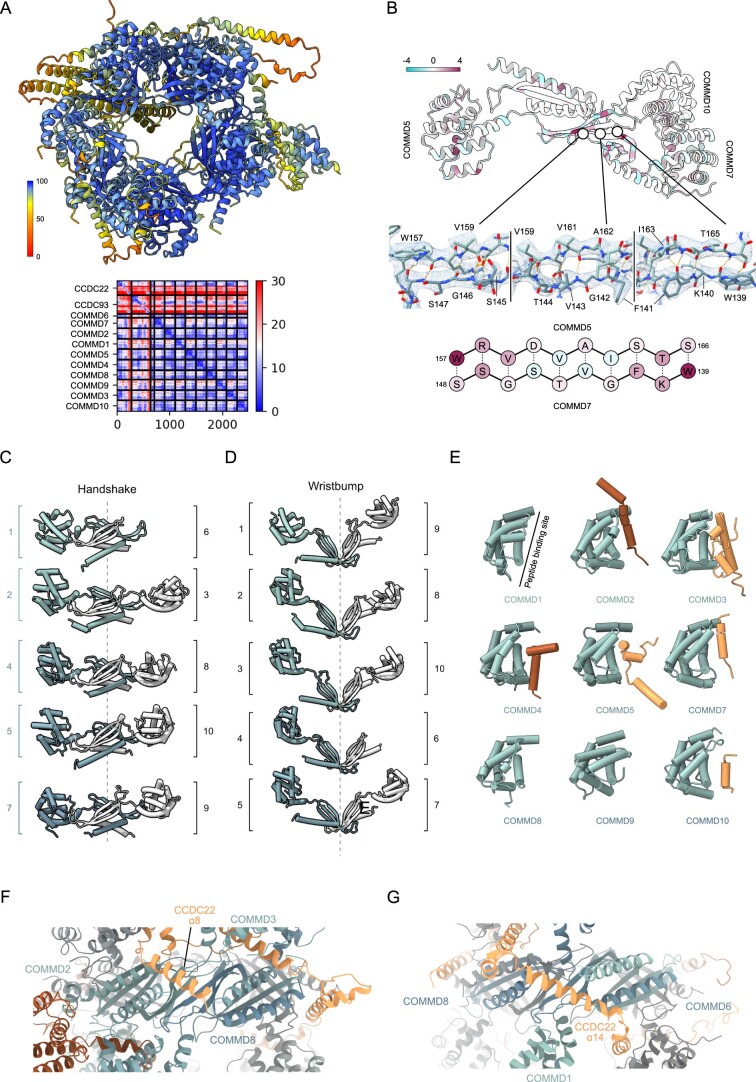
Extended Data Fig. 3. Molecular models of Commander complex top half, related to Fig. 3.
a, AF2 prediction and the predicted alignment error (PAE) plot of the top half of the Commander complex, constituting the full sequences of COMMDs and residues 120–392 of CCDC22 and residues 21–377 of CCDC93. b, Top: Example wristbump interface between COMMD5 and COMMD7. Middle: three closeups of the model in cryo-EM density, highlighting the residues involved in the wristbump interaction interface between COMMD5 and COMMD7. Bottom: schematic representation of the example wristbump interface. Coloring is by sequence conservation within the human COMMD proteins in Top and Bottom subpanels. c-d, Structural models of all c, handshake and d, wristbump interactions. e, Models of NTDs of COMMD proteins (except COMMD6) depicted alongside parts of CCDC93 or CCDC22 that interact with them at the peptide binding site. f-g, Detail of f CCDC22 α8 or g CCDC22 α14 binding site on the COMMD-ring.