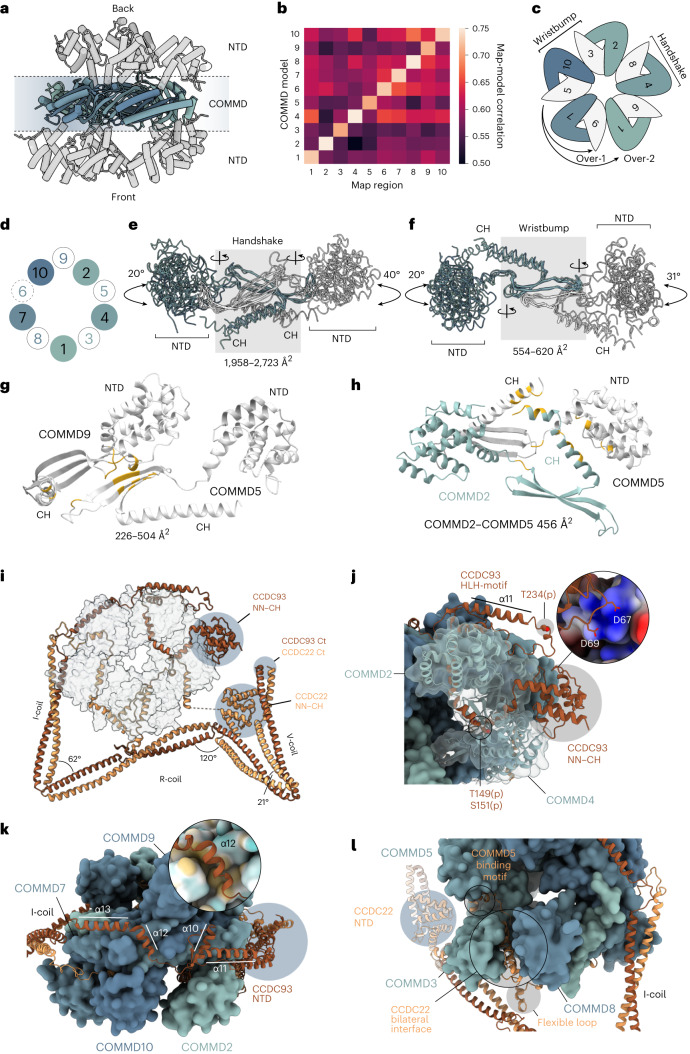
Fig. 3. Structure of the COMMD-ring.
a, The structure of the COMMD-ring is shown as a ribbon representation from the side. COMMD domains and NTDs are indicated. b, Heatmap depicting model-to-map cross-correlation coefficients for COMMD domains placed in each COMMD map region after real space refinement. c, Schematic diagram of COMMD-ring subunit organization. COMMDs with NTDs toward the viewer are indicated in blue-green shades, while COMMDs with NTDs away from the viewer are indicated in white. Handshake, wristbump, over-1 and over-2 interactions are indicated. d, Schematic representation of the NTD organization within the COMMD-ring. Numbers indicate COMMD proteins. Colors as in c. COMMD6 is indicated in dashed lines as it lacks an NTD. e,f, Superposed COMMD chains depicting the handshake (e) and wristbump (f) interactions between two chains with range of interaction surface areas for each COMMD pair. Angles of NTD rotation along the indicated hinge axes are shown. Colors as in c. g, Model of over-1 interaction between COMMDs 2 and 5. Interacting residues in gold, with buried surface area ranges indicated. h, Model of over-2 interaction between COMMDs 5 and 2, colors as in g. i, Overview of CCDCs intertwined within the COMMD-ring. j–l, Major CCDC interaction sites with COMMD chains. j, CCDC93 NN–CH domain binds the side of COMMD4 NTD and encircles the COMMD2 NTD in a headlock with HLH motif contacting the NN–CH domain. Experimentally identified phosphorylation sites are indicated. k, Top view of the COMMD-ring, showing the arrangement of CCDC93 α10–α13. The inset shows the hydrophobic binding pocket of α12 on COMMD7. Coloring indicates hydrophobic (yellow) and hydrophilic (cyan) surfaces. l, CCDC22 forms a bilateral interaction interface between COMMD3 and COMMD8. The loop protruding out of the density map between α13 and α14 of CCDC22 as well as the NN–CH domain of CCDC22 are indicated. NTD, N-terminal domain; CH, C-terminal helix; Ct, C-terminus.