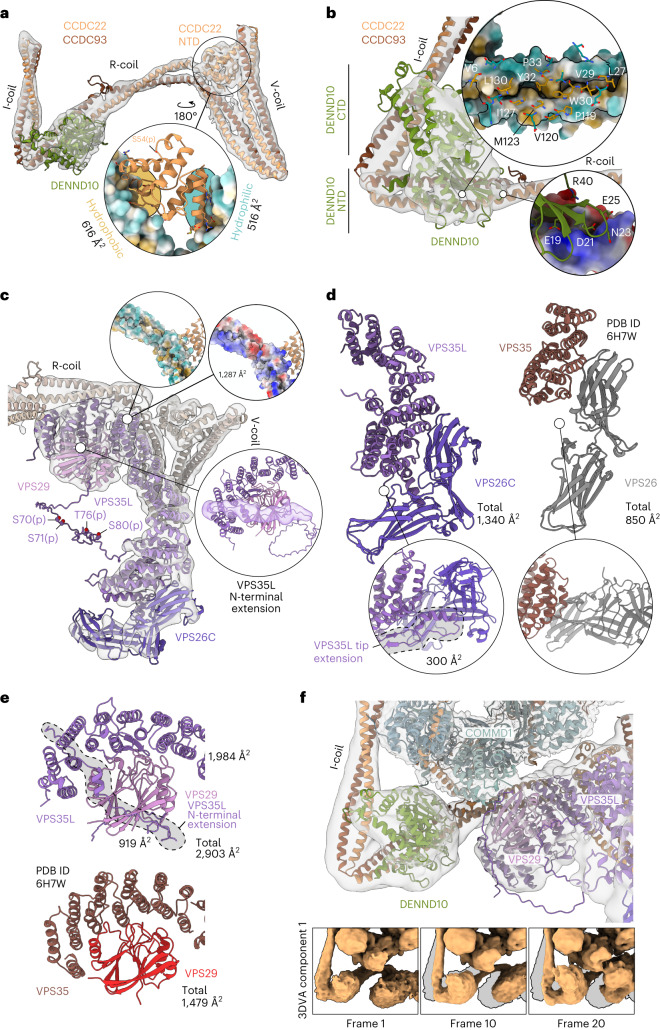
Fig. 4. Structures and interactions of the effector subunits.
a, CCDC22/93 coiled-coil regions, NTD of CCDC22 and DENND10 in density. The inset shows the binding site of CCDC22 NN–CH between the C-terminal V-coil characterized by hydrophobic and hydrophilic interfaces. Ser54 phosphorylation site identified in this study is indicated. b, DENND10 interface with R-coil of CCDC22/93. The major interface consists of a hydrophobic core along the groove of the R-coil and a charged patch on the C-terminal side of R-coil. c, The structure of the Retriever subcomplex in the context of Commander. Four experimentally identified phosphorylation sites (Ser70, Ser71, Thr76 and Ser80) are indicated in the disordered intervening N-terminal region of the model. The insets show the Retriever interfaces with CCDC22/93 through the C-terminal part of VPS35L, mainly via charged interactions. The N-terminal extension of VPS35L is shown in density where contributions from VPS29 and VPS35L C-terminal parts have been masked and low-pass filtered. d, Interfaces between the mobile NTD of COMMD1 with both DENND10 and VPS29/VPS35L. The insets show the first, middle and last frames of component 1 from 3D variability analysis (3DVA), reconstructed from particles using the intermediates job type. e, Interaction between VPS35L and VPS29 (top) compared to corresponding interaction in fungal Retromer arch (PDB 6H7W). The N-terminal extension of VPS35L accounts for nearly 33% of the buried interface. f, Interaction between VPS35L and VPS26C compared to the fungal VPS35–VPS26 interface. The insets show that VPS35L contains an extended helix that provides an expanded binding interface to VPS26C. Side chains in a and b are included only for visualization purposes.