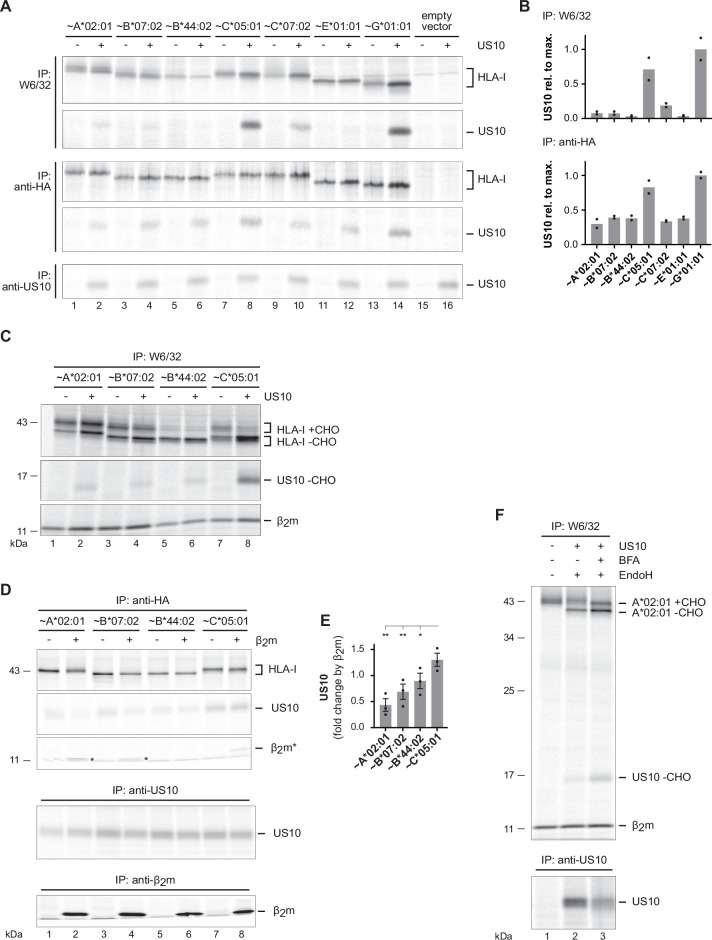
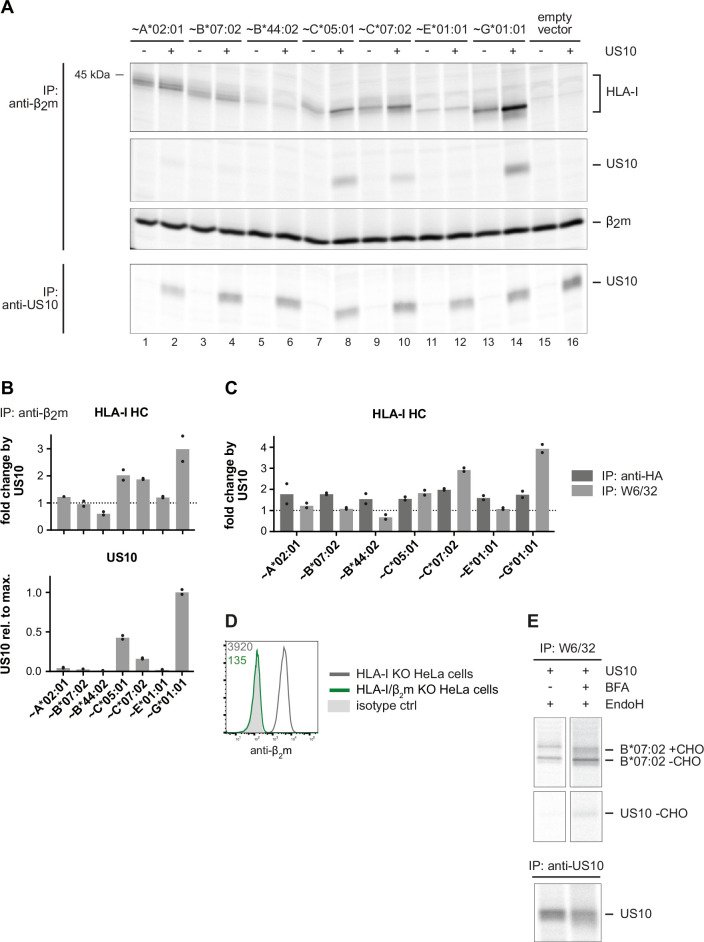
Figure 4. US10 binding to β2m/HC heterodimers correlates with human leucocyte antigen class I (HLA-I) endoplasmic reticulum (ER) retention.
(A) HLA-I KO HeLa cells were transiently co-transfected with indicated HA-HLA-I-expressing plasmids comprising a mutated gRNA binding site together with a US10- or a control-pIRES-EGFP plasmid. To improve assembly of HLA-E, UL40 (comprising an HLA-E ligand) was expressed with HLA-E. Cells were metabolically labeled for 2 hr and immunoprecipitations were performed as in Figure 1C; antibodies were applied as indicated on the left (Figure 4—source data 1 and 2). (B) Relative signal strengths from single bands of US10 in the W6/32 and (upper panel) anti-HA immunoprecipitation (lower panel) samples are shown. Dots represent individual values and bars mean values thereof from two independent experiments (biological replicates). The ratio (US10/control) of single bands of HLA-I HCs in the anti-HA and W6/32 immunoprecipitation samples is shown. Dots represent individual values and bars mean values thereof from two independent experiments (biological replicates). (C) HLA-I KO HeLa cells were transfected and treated as in (A). Immunoprecipitation was performed with W6/32 and subsequently an EndoH digest was performed (Figure 4—source data 3). (D) HLA-I/β2m, double KO HeLa cells were transiently transfected with US10, HA-tagged HLA-I, and β2m as indicated. At 20 hr post-transfection, cells were metabolically labeled for 2 hr and immunoprecipitation was performed as indicated (Figure 4—source data 4). One of three independent experiments is shown. (E) The intensity of the US10 bands co-immunoprecipitated with anti-HA was quantified, and the ratios of the samples with and without β2m were determined from three independent experiments (biological replicates). Significance was calculated using one-way paired ANOVA followed by Dunnett’s multiple comparison test. (F) HLA-I KO HeLa cells were transfected with HA-HLA-A*02:01 and US10 or a control plasmid. At 20 hr post-transfection, cells were treated with brefeldin A (BFA) during metabolic labeling for 2 hr. Subsequently, an immunoprecipitation using anti-HA was performed. Indicated samples were subjected to EndoH digestion prior to SDS-PAGE separation (Figure 4—source data 5). One of two independent experiments is shown in (A), (C), and (F).