FIGURE 8.
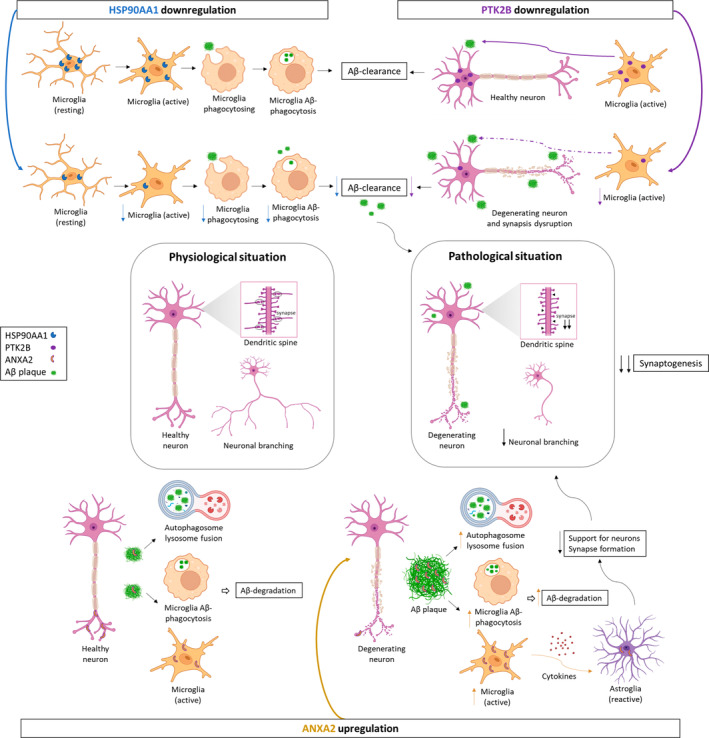
Potential role of HSP90AA1, PTK2B, and ANXA2 in synaptic decline through microglial cells in AD. HSP90AA1 promotes Aβ clearance through activation of microglial phagocytosis in AD. Its downregulation decreases microglial activation, phagocytosis, and consequently Aβ clearance at synapses. PTK2B, expressed in neurons, promotes normal macrophage polarization and migration to inflamed areas in synaptic structures. However, PTK2B is downregulated, and consequently, microglial cell activation and migration to Aβ deposits are reduced, contributing to neurodegeneration and synapse disruption. ANXA2, present in neurons as well, could contain Aβ pathology through autophagosome‐lysosome fusion and the activation of microglial cells. Therefore, its upregulation could increase autophagosome‐lysosome fusion and Aβ‐mediated microglial activation and subsequently promote Aβ degradation. On the other hand, active microglia secrete proinflammatory cytokines that can damage neurons either directly or indirectly by activating neurotoxic astrocytes. Additionally, ANXA2 is expressed in reactive astrocytes, which lose many normal astrocytic functions, including neuron support and synapse formation. As a result, it leads to the formation of fewer and weaker synapses and a decline in synaptogenesis. Therefore, synaptic homeostasis is disrupted with a marked decrease in the growth and branching of dendrites and axons, and neurodegeneration occurs in the EC of AD patients. Figure created with BioRender.com. Aβ, amyloid‐β; AD, Alzheimer's disease; EC, entorhinal cortex.
