Abstract
Background
Fibroblast growth factor receptor 2 (FGFR2) fusions and rearrangements are clinically actionable genomic alterations in cholangiocarcinoma (CCA). Pemigatinib is a selective, potent, oral inhibitor of FGFR1-3 and demonstrated efficacy in patients with previously treated, advanced/metastatic CCA with FGFR2 alterations in FIGHT-202 (NCT02924376). We report final outcomes from the extended follow-up period.
Patients and methods
The multicenter, open-label, single-arm, phase II FIGHT-202 study enrolled patients ≥18 years old with previously treated advanced/metastatic CCA with FGFR2 fusions or rearrangements (cohort A), other FGF/FGFR alterations (cohort B), or no FGF/FGFR alterations (cohort C). Patients received once-daily oral pemigatinib 13.5 mg in 21-day cycles (2 weeks on, 1 week off) until disease progression or unacceptable toxicity. The primary endpoint was objective response rate (ORR) in cohort A assessed as per RECIST v1.1 by an independent review committee; secondary endpoints included duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety.
Results
FIGHT-202 enrolled 147 patients (cohort A, 108; cohort B, 20; cohort C, 17; unconfirmed FGF/FGFR alterations, 2). By final analysis, 145 (98.6%) had discontinued treatment due to progressive disease (71.4%), withdrawal by patient (8.2%), or adverse events (AEs; 6.8%). Median follow-up was 45.4 months. The ORR in cohort A was 37.0% (95% confidence interval 27.9% to 46.9%); complete and partial responses were observed in 3 and 37 patients, respectively. Median DOR was 9.1 (6.0-14.5) months; median PFS and OS were 7.0 (6.1-10.5) months and 17.5 (14.4-22.9) months, respectively. The most common treatment-emergent AEs (TEAEs) were hyperphosphatemia (58.5%), alopecia (49.7%), and diarrhea (47.6%). Overall, 15 (10.2%) patients experienced TEAEs leading to pemigatinib discontinuation; intestinal obstruction and acute kidney injury (n = 2 each) occurred most frequently.
Conclusions
Pemigatinib demonstrated durable response and prolonged OS with manageable AEs in patients with previously treated, advanced/metastatic CCA with FGFR2 alterations in the extended follow-up period of FIGHT-202.
Key words: intrahepatic cholangiocarcinoma, precision medicine, next-generation sequencing, fibroblast growth factor receptor, pemigatinib, targeted therapy
Highlights
-
•
FIGHT-202 evaluated pemigatinib in patients with previously treated, advanced/metastatic CCA with FGFR2 rearrangements.
-
•
Median follow-up was 45.4 months; pemigatinib demonstrated an ORR of 37% and a median DOR of 9.1 months.
-
•
Median PFS and OS were 7.0 and 17.5 months, respectively.
-
•
AEs with pemigatinib treatment were manageable; during extended follow-up, no new safety signals were identified.
-
•
The importance of tumor molecular profiling and pemigatinib efficacy in CCA with FGFR2 fusions/rearrangements are described.
Introduction
Cholangiocarcinoma (CCA) accounts for ∼10%-25% of primary hepatic cancers and 3% of gastrointestinal tumors.1,2 In the United States, CCA incidence is increasing.3 Older patients, men, and people identifying as Asian/Pacific Islander generally have a higher CCA incidence.3 CCA is classified as intrahepatic (iCCA) or extrahepatic (perihilar or distal) based on location. Among CCA tumors, ∼10%-56% are iCCA.1,4,5 iCCA has high genomic heterogeneity, with 40%-50% of patients with CCA harboring one or more clinically actionable genomic alteration.6 Molecular profiling can identify patients most likely to benefit from targeted therapy based on clinically actionable genomic alterations and patterns of co-alterations.6, 7, 8 Fibroblast growth factor receptor 2 (FGFR2) fusions and rearrangements have been detected in 1%-13% of patients with iCCA.7,9, 10, 11 Compared to CCA without FGFR2 alterations, FGFR2 fusions are associated with longer overall survival (OS) from diagnosis.12 FGFRs regulate several cellular processes, including cell proliferation, survival, migration, and angiogenesis; dysregulation of these pathways drives tumorigenesis.13 Therefore, FGFR inhibitors are a rational targeted therapy to disrupt pathogenic FGFR signaling in CCA.14
Because of the asymptomatic nature of early-stage disease and nonspecific symptoms in later stages, CCA is often diagnosed in advanced stages when patients are ineligible for curative surgery.1,15 Approximately 65% of patients have unresectable disease, and up to half of them have lymph node metastases at time of diagnosis.16,17 Until recently, gemcitabine plus cisplatin chemotherapy was the first-line standard of care for treatment of unresectable or metastatic CCA.15,18 However, with the European Medicines Agency and United States Food and Drug Administration’s approval of durvalumab in combination with chemotherapy, and pembrolizumab in combination with chemotherapy for locally advanced or metastatic disease, chemoimmunotherapy is now widely accepted as the current standard of care.19, 20, 21, 22, 23 Modest response rates [∼20% objective response rate (ORR)] and a median survival of ∼11 months are typical with first-line chemotherapy.24,25 The addition of durvalumab to chemotherapy improves ORR to ∼27% and extended median OS to nearly 13 months.26 Improvement in OS has also been observed with the addition of pembrolizumab to gemcitabine and cisplatin, resulting in an OS of nearly 13 months.27
Despite this recent advance in therapy for unresectable or metastatic CCA,15 treatment options that exploit clinically actionable genomic alterations, including FGFR2 rearrangements, are needed. Pemigatinib is an oral, potent, selective FGFR1-3 inhibitor for treatment of adults with previously treated, unresectable, locally advanced or metastatic CCA with FGFR2 fusions or other rearrangements.28 In the primary analysis of FIGHT-202, a phase II study evaluating the safety and efficacy of pemigatinib in previously treated locally advanced or metastatic CCA, patients with FGFR2 fusions or rearrangements had an ORR of 35.5% at a median follow-up of 15.4 months.29 Here we report final efficacy and safety analyses from the extended follow-up period of the FIGHT-202 study (NCT02924376; EudraCT 2016-002422-36).
Patients and methods
Study design
FIGHT-202 was an open-label, single-arm, multicenter, phase II study conducted at 146 sites in the United States, Republic of Korea, UK, France, Italy, Thailand, Germany, Belgium, Israel, Spain, Japan, and Taiwan. The data cut-off date was 8 July 2021. FIGHT-202 consisted of three cohorts based on tumor FGF/FGFR alteration status: (A) FGFR2 rearrangements or fusions, (B) other FGF/FGFR alterations, or (C) no FGF/FGFR alterations (United States only). Enrollment and initial cohort assignment were permitted based on genomic testing results from a local laboratory. Final cohort assignment for statistical analyses was based on centrally confirmed next-generation sequencing results using the Foundation Medicine clinical trial assay (FoundationOne, Foundation Medicine, Cambridge, MA). FIGHT-202 was carried out as per the International Council for Harmonisation Guideline for Good Clinical Practice, Declaration of Helsinki, and local regulatory requirements. The study protocol was approved by the institutional review board of each site before patient enrollment. All patients provided written informed consent.
Patients
Eligibility requirements have been published previously.29 Briefly, eligible patients were ≥18 years old, had advanced/metastatic or surgically unresectable CCA with radiographically measurable disease as per RECIST v1.1, disease progression after one or more line of prior systemic therapy, documented FGF/FGFR gene alteration, life expectancy ≥12 weeks, and Eastern Cooperative Oncology Group (ECOG) performance status ≤2. Patients with inadequate hepatic or renal function, history or current evidence of ectopic mineralization or calcification, or current evidence of clinically significant corneal or retinal disorder were ineligible.
Treatment
All patients self-administered pemigatinib over 21-day cycles (2 weeks on/1 week off) at a starting oral dose of 13.5 mg once daily until documented radiologic disease progression, unacceptable toxicity, consent withdrawal, or physician decision.
Endpoints and assessments
The primary endpoint was ORR in patients with FGFR2 fusions or rearrangements (cohort A) as determined by an independent review committee (IRC). ORR was defined as the percentage of patients with complete (CR) or partial responses (PR) as per RECIST v1.1. Disease was assessed by computed tomography or magnetic resonance imaging every 6 weeks through week 12, and every 9 weeks thereafter for all cohorts; patients who discontinued study treatment for reasons other than disease progression were assessed every 9 weeks during follow-up.
Secondary endpoints were ORR in patients with FGF/FGFR alterations other than FGFR2 fusions or rearrangements (cohort B) and ORR in patients without FGF/FGFR alterations (cohort C). Additional secondary endpoints assessed in all cohorts were progression-free survival [PFS; time from first dose to progressive disease (PD) or death], duration of response (DOR; time from the date of CR or PR until PD), disease control rate (DCR; CR + PR + stable disease), and OS (time from first dose to death due to any cause) for all cohorts.
Safety and tolerability were based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 and were assessed at screening, during treatment, at the end of treatment, and during follow-up.
Genomic analysis of baseline tumor samples was carried out as previously described.6
Statistical analyses
The efficacy-assessable population included all patients with centrally confirmed FGF/FGFR alteration status who received one or more dose of pemigatinib. The primary analysis of ORR was carried out in cohort A based on IRC-assessed tumor responses. The Clopper–Pearson method was used to estimate the 95% confidence interval (CI) for ORR. Analyses of ORR and 95% CI estimation in cohorts A and B combined, cohort B, and cohort C, as well as DCR analyses, were carried out in the same way as the analysis of ORR for cohort A. The Kaplan–Meier method was used to assess PFS, DOR, and OS. Exploratory analysis of ORR, PFS, and OS in subgroups based on demographic and baseline clinical characteristics was carried out for cohort A. The safety-assessable population included all enrolled patients who received one or more dose of pemigatinib; safety data were summarized descriptively. Statistical analysis of the effect of co-alterations on OS was carried out using the log-likelihood ratio test and Kaplan–Meier method as described previously.6
Results
Patients
At final data cut-off, 147 patients were enrolled, including 108 in cohort A (FGFR2 fusions or rearrangements), 20 in cohort B (other FGF/FGFR alterations), and 17 in cohort C (no FGF/FGFR alterations). Two patients had undetermined FGF/FGFR status as per central review and were excluded from efficacy evaluations. A detailed analysis of FGFR2 rearrangements (cohort A)29 and other genomic alterations (cohorts B and C) has been previously published.6 Fifteen and 93 patients assigned to cohort A had FGFR2 rearrangements and fusions (a subset of rearrangements in which the fusion partner is predicted to be translated in-frame with FGFR2),6 respectively; 56 unique fusion partner genes were identified. The most common fusion partner was BICC1 (n = 32, 29.6%). In cohort B, the most common FGF/FGFR alteration was FRS2 amplification (n = 9, 45.0%), followed by FGF3, FGF4, FGF19 amplification (n = 5, 25.0%), and FGFR2 C382R point mutation (n = 4, 20.0%). In cohort C, the most frequently detected genomic alterations were in CDKN2A (n = 7, 41.2%), KRAS (n = 7, 41.2%), and IDH1 (n = 5, 29.4%).
Median (range) age was 59.0 (26-78) years; 101 patients (68.7%) were <65 years old (Table 1). Most patients were women (57.8%), white (70.7%), and enrolled in North America (60.5%). Cohort A included higher percentages of women and patients aged <65 years old compared with cohorts B and C. Most patients (n = 132, 89.8%) had iCCA; of these, 107 (99.1%) were in cohort A (Table 1). The most common sites of extrahepatic metastases were the lymph nodes (54.4%) and lung (53.1%). At final data cut-off, 145 patients (98.6%) overall had discontinued treatment (Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2024.103488). The most common reason for pemigatinib discontinuation across cohorts was PD (71.4%), followed by withdrawal by patient (8.2%) and adverse events (AEs; 6.8%). In cohort A, 106 patients (98.1%) discontinued pemigatinib, with PD (71.3%) being the most common primary reason. Median (range) duration of exposure to pemigatinib was 5.9 (0.2-51.1) months overall and was approximately five times longer in cohort A [7.2 (0.2-51.1) months] versus cohorts B [1.4 (0.2-12.9) months] and C [1.2 (0.2-4.7) months]. Treatment information after pemigatinib discontinuation was available for 58 patients (39.5%); of these, 56.9% received one additional line, 19.0% received two lines, and 24.1% received three or more lines of therapy. The most common treatments immediately following pemigatinib discontinuation were chemotherapy (56.9%), futibatinib (17.2%), and immune checkpoint inhibitors (10.3%).
Table 1.
Patient demographics and baseline clinical characteristics (safety-assessable population)
| Parameter | FGFR2 fusions or rearrangements (n = 108) | Other FGF/FGFR alterations (n = 20) | No FGF/FGFR alterations (n = 17) | Total (N = 147)a |
|---|---|---|---|---|
| Age, median (range), years | 55.5 (26-77) | 63.0 (45-78) | 65.0 (49-78) | 59.0 (26-78) |
| <65, n (%) | 83 (76.9) | 10 (50.0) | 6 (35.3) | 101 (68.7) |
| 65-<75, n (%) | 20 (18.5) | 7 (35.0) | 8 (47.1) | 35 (23.8) |
| ≥75, n (%) | 5 (4.6) | 3 (15.0) | 3 (17.6) | 11 (7.5) |
| Sex, n (%) | ||||
| Female | 66 (61.1) | 11 (55.0) | 7 (41.2) | 85 (57.8) |
| Male | 42 (38.9) | 9 (45.0) | 10 (58.8) | 62 (42.2) |
| Region, n (%) | ||||
| North America | 64 (59.3) | 6 (30.0) | 17 (100.0) | 89 (60.5) |
| Western Europe | 32 (29.6) | 3 (15.0) | 0 | 35 (23.8) |
| Rest of worldb | 12 (11.1) | 11 (55.0) | 0 | 23 (15.6) |
| Race, n (%) | ||||
| White | 79 (73.1) | 9 (45.0) | 14 (82.4) | 104 (70.7) |
| Asian | 12 (11.1) | 11 (55.0) | 0 | 23 (15.6) |
| Black/African American | 7 (6.5) | 0 | 1 (5.9) | 8 (5.4) |
| American Indian/Alaska native | 0 | 0 | 1 (5.9) | 1 (0.7) |
| Other/missing | 10 (9.3) | 0 | 1 (5.9) | 11 (7.5) |
| Time since initial diagnosis, median (range), years | 1.3 (0.2-11.1) | 0.7 (0.2-2.5) | 1.0 (0.3-4.3) | 1.1 (0.2-11.1) |
| ECOG performance status, n (%) | ||||
| 0 | 46 (42.6) | 7 (35.0) | 6 (35.3) | 60 (40.8) |
| 1 | 57 (52.8) | 10 (50.0) | 8 (47.1) | 76 (51.7) |
| 2 | 5 (4.6) | 3 (15.0) | 3 (17.6) | 11 (7.5) |
| Metastatic disease,cn (%) | ||||
| Yes | 89 (82.4) | 20 (100.0) | 16 (94.1) | 126 (85.7) |
| No | 16 (14.8) | 0 | 1 (5.9) | 18 (12.2) |
| Missing or not evaluable | 3 (2.8) | 0 | 0 | 3 (2.0) |
| Prior systemic therapies, n (%) | ||||
| 1 | 65 (60.2) | 12 (60.0) | 11 (64.7) | 89 (60.5) |
| 2 | 30 (27.8) | 7 (35.0) | 2 (11.8) | 39 (26.5) |
| ≥3 | 13 (12.0) | 1 (5.0) | 4 (23.5) | 19 (12.9) |
| Prior cancer surgery, n (%) | 38 (35.2) | 6 (30.0) | 4 (23.5) | 48 (32.7) |
| Prior radiation, n (%) | 29 (26.9) | 3 (15.0) | 5 (29.4) | 37 (25.2) |
| CCA location, n (%) | ||||
| Intrahepatic | 107 (99.1) | 13 (65.0) | 10 (58.8) | 132 (89.8) |
| Extrahepatic | 1 (0.9) | 4 (20.0) | 7 (41.2) | 12 (8.2) |
| Other | 0 | 3 (15.0) | 0 | 3 (2.0) |
| History of hepatitis, n (%) | ||||
| Hepatitis B | 4 (3.7) | 1 (5.0) | 0 | 5 (3.4) |
| Hepatitis C | 1 (0.9) | 1 (5.0) | 0 | 2 (1.4) |
| Sites of disease at baseline, n (%)d | ||||
| Liver | 102 (94.4) | 17 (85.0) | 17 (100.0) | 138 (93.9) |
| Lymph nodes | 58 (53.7) | 11 (55.0) | 10 (58.8) | 80 (54.4) |
| Lung | 59 (54.6) | 9 (45.0) | 10 (58.8) | 78 (53.1) |
| Bone | 21 (19.4) | 4 (20.0) | 2 (11.8) | 27 (18.4) |
| Ascites | 8 (7.4) | 5 (25.0) | 2 (11.8) | 15 (10.2) |
| Pancreas | 7 (6.5) | 1 (5.0) | 2 (11.8) | 11 (7.5) |
CCA, cholangiocarcinoma; ECOG, Eastern Cooperative Oncology Group; FGF, fibroblast growth factor; FGFR, FGF receptor.
Total number includes two patients who did not have confirmed FGF/FGFR status by central laboratory testing and were not assigned to any cohort.
Rest of the world includes Israel, Japan, Republic of Korea, Taiwan, and Thailand.
Patients with nonmetastatic disease have no evidence of extrahepatic metastasis. Patients with metastatic disease may have had intrahepatic and extrahepatic metastases.
Specific sites reported in >5% of patients overall are shown.
Response to treatment
Overall, median (range) follow-up for the efficacy-assessable population was 45.4 (19.9-53.7) months.
Cohort A
Median (range) follow-up for efficacy-assessable patients in cohort A was 42.9 (19.9-52.2) months. ORR (95% CI) based on IRC-assessed confirmed tumor responses was 37.0% (27.9% to 46.9%); three patients (2.8%) achieved CR, and 37 (34.3%) had PR (Table 2). Among 93 patients with FGFR2 fusions, ORR (95% CI) was 36.6% (26.8% to 47.2%), including two patients (2.2%) with CR and 32 (34.4%) with PR. ORR (95% CI) among 15 patients with FGFR2 rearrangements was 40.0% (16.3% to 67.7%), including 1 patient (6.7%) with CR and five (33.3%) with PR. In cohort A, outcomes were generally similar across baseline demographic and clinical characteristic subgroups; ORR was numerically higher in patients with ECOG status of 0 versus 1 or 2 (50.0% versus 27.4%) and nonmetastatic (excludes patients with extrahepatic metastases) versus metastatic disease (includes patients with intrahepatic and extrahepatic metastases; 50.0% versus 34.8%), whereas the number of prior therapies did not affect ORR (36.9%, 36.7%, and 38.5%, respectively, for 1, 2, or ≥3 prior therapies; Supplementary Figure S2, available at https://doi.org/10.1016/j.esmoop.2024.103488).
Table 2.
Efficacy outcomes (efficacy-assessable population)
| Parameter | FGFR2 fusions or rearrangements (n = 108) | Other FGF/FGFR alterations (n = 20) | No FGF/FGFR alterations (n = 17) |
|---|---|---|---|
| Duration of follow-up, median (range), months | 42.9 (19.9-52.2) | 47.5 (43.7-51.1) | 51.9 (49.5-53.7) |
| ORR, n (%) | 40 (37.0) | 0 | 0 |
| 95% CI | 27.9-46.9 | 0-16.8 | 0-19.5 |
| Best overall response, n (%) | |||
| CR | 3 (2.8) | 0 | 0 |
| PR | 37 (34.3) | 0 | 0 |
| SD | 49 (45.4) | 8 (40.0) | 3 (17.6) |
| Progressive disease | 16 (14.8) | 7 (35.0) | 11 (64.7) |
| Not evaluable | 3 (2.8) | 5 (25.0) | 3 (17.6) |
| Time to response, median (range), months | 2.7 (0.7-16.6) | — | — |
| DOR | |||
| Events, n (%) | 30 (75.0) | 0 | 0 |
| Censored, n (%) | 10 (25.0) | 0 | 0 |
| Median (95% CI), months | 9.1 (6.0-14.5) | — | — |
| ≥12 months, n (%)a | 12 (30.0) | — | — |
| Kaplan–Meier estimate (95% CI) | |||
| 6 months | 67.8 (50.4-80.3) | — | — |
| 12 months | 41.2 (24.8-56.8) | — | — |
| DCR, n (%) | 89 (82.4) | 8 (40.0) | 3 (17.6) |
| 95% CI | 73.9-89.1 | 19.1-63.9 | 3.8-43.4 |
| PFS | |||
| Events, n (%) | 85 (78.7) | 17 (85.0) | 15 (88.2) |
| Censored, n (%) | 23 (21.3) | 3 (15.0) | 2 (11.8) |
| Median (95% CI), months | 7.0 (6.1-10.5) | 2.1 (1.2-4.9) | 1.5 (1.4-1.8) |
| Kaplan–Meier estimate (95% CI) | |||
| 6 months | 61.1 (51.0-69.8) | 25.3 (8.1-47.1) | 6.8 (0.4-26.3) |
| 12 months | 32.3 (22.9-42.1) | 0 (NE-NE) | 0 (NE-NE) |
| OS | |||
| Deaths, n (%) | 76 (70.4) | 18 (90.0) | 15 (88.2) |
| Censored, n (%) | 32 (29.6) | 2 (10.0) | 2 (11.8) |
| Median (95% CI), months | 17.5 (14.4-22.9) | 6.7 (2.1-10.6) | 4.0 (2.0-4.6) |
| Kaplan–Meier estimate (95% CI) | |||
| 6 months | 88.7 (81.0-93.4) | 50.8 (26.6-70.7) | 26.7 (8.3-49.6) |
| 12 months | 67.6 (57.7-75.6) | 22.6 (7.0-43.4) | 13.3 (2.2-34.6) |
CR, complete response; DCR, disease control rate; DOR, duration of response; FGF, fibroblast growth factor; FGFR, FGF receptor; NE, not evaluable; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; PR, partial response; SD, stable disease.
Calculated as the percentage of patients with DOR ≥12 months among all patients with CR or PR (n = 40).
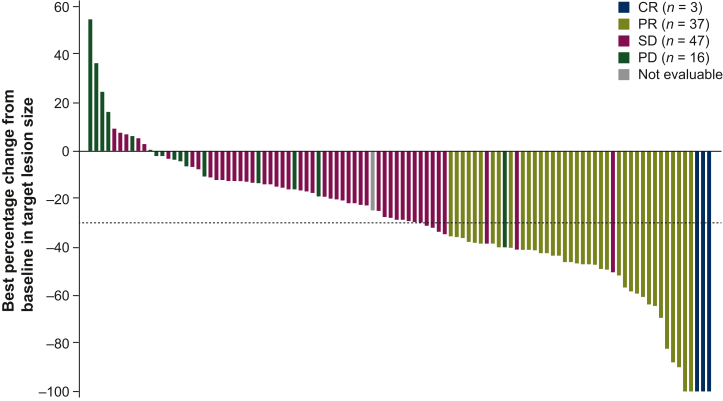
Median (range) time to response in cohort A was 2.7 (0.7-16.6) months, with a median (95% CI) DOR of 9.1 (6.0-14.5) months (Supplementary Figure S3, available at https://doi.org/10.1016/j.esmoop.2024.103488). Among patients with CR or PR, 12 (30.0%) had DOR ≥12 months. Most patients with DOR ≥12 months had only one line of prior therapy (n = 8, 66.7%); four (33.3%) patients had disease in the liver only. Four patients with DOR ≥12 months had BAP1 co-alterations and none had TP53 or PBRM1 co-alterations. DCR (95% CI) in cohort A was 82.4% (73.9% to 89.1%). Among the 104 patients with postbaseline target lesion measurements, 93 had reduction in sum of target lesion diameters, and 48 patients had reductions of >30%. Median (range) best percentage change from baseline in sum of target lesion diameters was −28.4% (−100% to +55%; Figure 1).
Figure 1.
Best percentage change from baseline in target lesion size based on IRC assessment among efficacy-assessable patients in cohort A (FGFR2 rearrangements or fusions). The dashed line indicates criterion for PR (≥30% decrease in sum of target lesion diameters). CR, complete response; FGFR, fibroblast growth factor receptor; IRC, independent review committee; PD, progressive disease; PR, partial response; SD, stable disease.
Cohort B
Median (range) follow-up for efficacy-assessable patients in cohort B was 47.5 (43.7-51.1) months. No objective responses were observed (Table 2). Median (range) best percentage change from baseline in sum of target lesion diameters was 0% (−41% to +91%; Supplementary Figure S4A, available at https://doi.org/10.1016/j.esmoop.2024.103488).
Cohort C
Median (range) follow-up for efficacy-assessable patients in cohort C was 51.9 (49.5-53.7) months. No objective responses were observed (Table 2). Median (range) best percentage change from baseline in sum of target lesion diameters was 6.2% (−33% to +74%; Supplementary Figure S4B, available at https://doi.org/10.1016/j.esmoop.2024.103488).
Progression-free survival and overall survival
Median (95% CI) PFS based on IRC assessment in cohort A was 7.0 (6.1-10.5) months; Kaplan–Meier estimate of PFS at 12 months was 32.3% (Table 2; Figure 2A). Analysis of PFS among patient subgroups in cohort A revealed that outcomes were generally similar irrespective of patient demographic and baseline clinical characteristics; PFS was numerically shorter among those with metastatic versus nonmetastatic disease (6.9 versus 17.5 months, respectively) and similar between patients with 1, 2, or ≥3 lines of prior therapy (7.0, 8.9, and 6.8 months, respectively; Supplementary Figure S5, available at https://doi.org/10.1016/j.esmoop.2024.103488). PFS for patients with FGFR2 C382R point mutations (n = 4) in cohort B was 1.1, 4.0, 6.9, and 9.0 months, respectively. In cohorts B and C overall, median PFS (2.1 and 1.5 months, respectively) and Kaplan–Meier estimates of PFS at evaluable time points were significantly lower than in cohort A.
Figure 2.
Kaplan–Meier estimates of (A) PFS based on IRC assessment, (B) OS for all cohorts, and (C) OS in cohort A stratified by response (efficacy-assessable population). IRC, independent review committee; NE, not estimable; OS, overall survival; PFS, progression-free survival.
At data cut-off, 32 patients (29.6%) in cohort A were alive and censored for survival (Table 2; Figure 2B). Median (95% CI) OS was 17.5 (14.4-22.9) months; Kaplan–Meier estimate of 12-month survival was 67.6%. Median (95% CI) OS was notably longer among responders [46.1 (21.5-not estimable) months] versus non-responders [13.7 (9.6-16.2) months; Figure 2C]. The analysis of OS among patient subgroups in cohort A revealed that outcomes were generally similar across patient demographic and baseline clinical characteristic subgroups (Supplementary Figure S6, available at https://doi.org/10.1016/j.esmoop.2024.103488). Median OS was numerically shorter among patients with ECOG performance status of 1 or 2 versus 0 (14.7 versus 27.7 months, respectively) and for those with versus without metastatic disease (16.2 versus 42.4 months). Patients with co-alterations in TP53 [hazard ratio (HR) (95% CI) 3.33 (1.48-7.52), P = 0.002] and PBRM1 [HR (95% CI) 2.46 (1.24-4.87), P = 0.007] had significantly worse OS compared with patients without the co-alterations (Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103488). Two patients each in cohorts B and C (10.0% and 11.8%, respectively) were alive and censored for survival. Median (95% CI) OS was 6.7 (2.1-10.6) months for cohort B and 4.0 (2.0-4.6) months for cohort C; Kaplan–Meier estimates of OS were significantly lower than in cohort A.
Safety
Overall, all patients experienced one or more treatment-emergent AE (TEAE), and 101 (68.7%) had at least one grade ≥3 TEAE. The most common TEAEs of any grade were hyperphosphatemia (58.5%), alopecia (49.7%), and diarrhea (47.6%). Hypophosphatemia (14.3%), stomatitis (6.8%), and arthralgia (6.1%) were the most common grade ≥3 TEAEs (Supplementary Table S2, available at https://doi.org/10.1016/j.esmoop.2024.103488). Clinically notable TEAEs occurred in 81.6% of patients and included hyperphosphatemia (60.5%), nail toxicity (44.9%), hypophosphatemia (23.8%), and serous retinal detachment (4.8%). Kaplan–Meier estimates of median (95% CI) time to onset of the first occurrence of any hyperphosphatemia or nail toxicity TEAE were 0.49 (0.26-0.69) months and 5.98 (4.80-7.92) months, respectively, and were non-estimable for the first occurrence of hypophosphatemia and serous retinal detachment due to the low incidence of these events. None of these TEAEs led to pemigatinib discontinuation.
Treatment-related TEAEs occurred in 91.8% of patients, and 32.7% experienced grade ≥3 treatment-related TEAEs (Table 3). Overall, the most common treatment-related TEAEs were hyperphosphatemia (53.7%), alopecia (46.3%), and diarrhea (36.1%); the most common grade ≥3 treatment-related TEAEs were hypophosphatemia (8.8%), stomatitis (6.1%), and arthralgia and palmar–plantar erythrodysesthesia syndrome (4.1% each). Serious AEs (SAEs) occurred in 46.3% of patients overall; the most common SAEs included abdominal pain (4.8%), pyrexia (4.8%), and cholangitis (4.1%). Six patients (4.1%) experienced fatal TEAEs, including failure to thrive (n = 2), as well as biliary obstruction, cholangitis, sepsis, and pleural effusion (n = 1 each); none were deemed related to treatment.
Table 3.
Treatment-related treatment-emergent adverse events (safety-assessable population)
| Events |
FGFR2 fusions or rearrangements (n = 108) |
Other FGF/FGFR alterations (n = 20) |
No FGF/FGFR alterations (n = 17) |
Total (N = 147)a |
||||
|---|---|---|---|---|---|---|---|---|
| Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | Any grade | Grade ≥3 | |
| Any treatment-related TEAE, n (%)b | 102 (94.4) | 40 (37.0) | 17 (85.0) | 6 (30.0) | 14 (82.4) | 1 (5.9) | 135 (91.8) | 48 (32.7) |
| Hyperphosphatemia | 55 (50.9) | 0 | 11 (55.0) | 0 | 12 (70.6) | 0 | 79 (53.7) | 0 |
| Alopecia | 61 (56.5) | 0 | 3 (15.0) | 0 | 2 (11.8) | 0 | 68 (46.3) | 0 |
| Diarrhea | 44 (40.7) | 4 (3.7) | 5 (25.0) | 0 | 4 (23.5) | 1 (5.9) | 53 (36.1) | 5 (3.4) |
| Stomatitis | 43 (39.8) | 9 (8.3) | 4 (20.0) | 0 | 3 (17.6) | 0 | 51 (34.7) | 9 (6.1) |
| Dysgeusia | 42 (38.9) | 0 | 3 (15.0) | 0 | 3 (17.6) | 0 | 50 (34.0) | 0 |
| Fatigue | 38 (35.2) | 2 (1.9) | 4 (20.0) | 0 | 6 (35.3) | 0 | 48 (32.7) | 2 (1.4) |
| Dry mouth | 38 (35.2) | 0 | 2 (10.0) | 0 | 1 (5.9) | 0 | 43 (29.3) | 0 |
| Nausea | 32 (29.6) | 2 (1.9) | 2 (10.0) | 0 | 3 (17.6) | 0 | 38 (25.9) | 2 (1.4) |
| Decreased appetite | 25 (23.1) | 0 | 5 (25.0) | 1 (5.0) | 4 (23.5) | 0 | 35 (23.8) | 1 (0.7) |
| Dry eye | 33 (30.6) | 0 | 0 | 0 | 0 | 0 | 34 (23.1) | 1 (0.7) |
| Dry skin | 24 (22.2) | 1 (0.9) | 0 | 0 | 0 | 0 | 26 (17.7) | 1 (0.7) |
| Arthralgia | 21 (19.4) | 5 (4.6) | 2 (10.0) | 1 (5.0) | 0 | 0 | 23 (15.6) | 6 (4.1) |
| Palmar–plantar erythrodysesthesia syndrome | 22 (20.4) | 6 (5.6) | 1 (5.0) | 0 | 0 | 0 | 23 (15.6) | 6 (4.1) |
| Constipation | 21 (19.4) | 0 | 1 (5.0) | 0 | 0 | 0 | 22 (15.0) | 0 |
| Hypophosphatemia | 17 (15.7) | 11 (10.2) | 2 (10.0) | 2 (10.0) | 0 | 0 | 19 (12.9) | 13 (8.8) |
| Vomiting | 15 (13.9) | 1 (0.9) | 1 (5.0) | 0 | 1 (5.9) | 0 | 17 (11.6) | 1 (0.7) |
| Pain in extremity | 15 (13.9) | 0 | 0 | 0 | 0 | 0 | 15 (10.2) | 0 |
| Weight decreased | 11 (10.2) | 1 (0.9) | 3 (15.0) | 0 | 0 | 0 | 14 (9.5) | 1 (0.7) |
| Hyponatremia | 3 (2.8) | 1 (0.9) | 3 (15.0) | 3 (15.0) | 2 (11.8) | 0 | 8 (5.4) | 4 (2.7) |
FGF, fibroblast growth factor; FGFR, FGF receptor; TEAE, treatment-emergent adverse event.
Total number includes two patients who did not have confirmed FGF/FGFR status by central laboratory testing and were not assigned to any cohort.
All any-grade TEAEs occurring in ≥10% and grade ≥3 TEAEs occurring in ≥2% of the total population are shown.
Overall, TEAEs led to pemigatinib dose interruptions, dose reductions, and discontinuations in 42.2%, 13.6%, and 10.2% of patients, respectively. The most frequent TEAEs leading to dose interruptions were stomatitis (8.2%), palmar–plantar erythrodysesthesia syndrome (6.1%), and arthralgia (4.8%). TEAEs leading to dose reductions in more than two patients were arthralgia, palmar–plantar erythrodysesthesia syndrome, and stomatitis [n = 5 (3.4%) each]. TEAEs leading to pemigatinib discontinuations in more than one patient were intestinal obstruction and acute kidney injury (n = 2 each).
Discussion
In the final analysis of FIGHT-202, continued benefit of pemigatinib in patients with previously treated advanced or metastatic CCA with FGFR2 rearrangements or fusions was observed over an extended follow-up period, including a 37% ORR, a median DOR of 9.1 months, and a median PFS and OS of 7.0 and 17.5 months, respectively. No patients with other or without FGF/FGFR alterations responded to pemigatinib. No new safety concerns were identified; the most common treatment-related TEAE was hyperphosphatemia (54%; all cases were grade 1 or 2).
CCA is typically unresectable at diagnosis,16 and mortality rates, primarily driven by iCCA, are increasing.30,31 Standard-of-care first-line treatment for unresectable or metastatic CCA is gemcitabine and cisplatin plus durvalumab in many countries, whereas gemcitabine plus cisplatin chemotherapy remains the standard of care where durvalumab has not yet been approved.15,18,32 However, many patients do not respond to treatment, and second-line therapies provide only limited benefit.33 A meta-analysis of retrospective and phase II studies reporting second-line chemotherapy for bile duct cancers showed a mean response rate of 7.7% and a mean OS of only 7.2 months.34 A post hoc analysis of FIGHT-202 assessing PFS in patients by prior systemic therapy showed that median PFS in patients with FGFR2 fusions or rearrangements treated with second-line pemigatinib was 7.0 months.35 Patients with prior second-line therapy (chemotherapy, 93%) had a median PFS of 4.2 months, possibly suggesting that second-line targeted therapy may improve outcomes in patients with FGFR2 fusions or rearrangements over chemotherapy.35 In contrast with the historically poor responses to second-line treatments for CCA, we demonstrated the continued clinical benefit of pemigatinib in previously treated CCA with FGFR2 fusions or rearrangements over an extended follow-up period.
The success of the FGFR inhibitors pemigatinib, erdafitinib, futibatinib, and RLY-4008 in patients with previously treated CCA tumors with FGFR2 fusions or rearrangements29,36, 37, 38, 39, 40 represents a paradigm shift toward personalized medicine.41 The European Society for Medical Oncology (ESMO) and United States National Comprehensive Cancer Network (NCCN) treatment guidelines currently suggest biomarker-guided treatments based not only on FGFR2 alterations but also microsatellite instability-high/mismatch repair-deficient, ERBB2-positive/mutated, NTRK fusion-positive, and BRAF- and IDH1-mutated tumors.15,18 NCCN also suggests biomarker-guided treatments for tumor mutation burden-high and RET fusion-positive tumors,15 and ESMO suggests treatment for patients with BRCA1/2 or PALB2 mutations.18 Targeted therapies are currently recommended as second-line treatments; however, extending these findings into the first-line setting may improve clinical outcomes in patients with CCA with specific genomic alterations. For example, the ongoing randomized phase III FIGHT-302 clinical study (NCT03656536)42 is evaluating first-line pemigatinib versus chemotherapy in patients with CCA with FGFR2 rearrangements.
The clinical utility of FGFR inhibition in CCA is hampered by primary and secondary resistance to FGFR inhibitors. Understanding mechanisms of tumor resistance is therefore critical. In FIGHT-202, patients with co-occurring alterations in one or more tumor suppressor gene had significantly shorter PFS than those without tumor suppressor gene alterations; ORR was not significantly different.6 Consistent with previous PFS and ORR findings, TP53 and PBRM1 co-alterations were also associated with significantly shorter OS in this final analysis of FIGHT-202.6 Patients with co-alterations in BAP1 also tended to have numerically shorter OS, consistent with the worse PFS previously reported in patients with this co-alteration.6 In FIGHT-202, all patients with reductions in tumor size followed by PD (n = 8) had developed one or more mutation in the kinase domain of FGFR2 predicted to promote kinase activation or impair pemigatinib binding.6 Larger-scale molecular profiling may enhance understanding of alterations that prevent or reverse FGFR inhibition in CCA.
FGFR inhibitors that irreversibly bind to FGFR2 (e.g. futibatinib, RLY-4008) may have greater antitumor activity in CCA with FGFR2 resistance mutations compared with ATP-competitive inhibitors (e.g. pemigatinib).14 However, whether the covalent binding mechanism translates to clinically meaningful longer PFS is unknown. Ongoing phase I/II studies of RLY-4008 will provide more data to address this question.40 Preliminary work suggests that sequential treatment of patients with acquired ‘on target’ resistance to ATP-competitive inhibitors with irreversible FGFR2 inhibitors is possible in a subgroup of patients; this concept should be evaluated in future studies.
Although targeting the tumor microenvironment (TME) with immune checkpoint inhibitors has proven to be an effective therapeutic strategy in many solid tumors, these drugs, with the exception of durvalumab26 and pembrolizumab,27 have been used with limited success in CCA.1 Recent TME-based transcriptomic analyses demonstrated that approximately two-thirds of iCCA tumors exhibit an immunologically cold ‘non-inflamed’ TME.43 The largest subtype of non-inflamed tumors was significantly enriched in FGFR2 fusions as well as BAP1 and IDH1/2 mutations.43 A TOPAZ-1 post hoc analysis found that patients with biliary tract cancer with clinically actionable genomic alterations, including FGFR2 rearrangements, had an OS benefit from first-line durvalumab plus gemcitabine and cisplatin versus chemotherapy alone.44 The number of patients with FGFR2 rearrangements was very small; therefore, whether adding durvalumab to standard chemotherapy benefits patients with FGFR2 alterations to the same extent as patients without FGFR2 alterations remains an open question. FGFR inhibition coupled with TME-targeted therapies, such as those that deplete cells contributing to immunosuppressive TME phenotypes, may improve clinical outcomes in specific patient populations.43 Further characterization of the TME in FGFR2-altered CCA may be warranted to understand the impact of dysregulated FGFR2 signaling on the TME and to identify patients who might benefit most from combination therapies.
Limitations of FIGHT-202 have been discussed previously.29 The study design included no active comparator treatment arm. Small numbers in patient subgroups also limit interpretation of efficacy based on demographic and disease characteristic factors.
Conclusions
This final analysis of FIGHT-202 demonstrated continued durable response, prolonged OS, and manageable AEs in patients with previously treated advanced or metastatic CCA with FGFR2 fusions or rearrangements, further supporting regulatory approvals of pemigatinib based on this single-arm, phase II study.28,45 These results highlight the need for early molecular testing in CCA. The phase III FIGHT-302 study will further elucidate the role of FGFR inhibitors in biomarker-selected CCA. Routine comprehensive genomic profiling is needed to discover novel actionable FGFR2 alterations and identify patients who might benefit from FGFR inhibition.
Acknowledgements
Writing assistance was provided by Erin McClure, PhD, an employee of ICON (Blue Bell, PA, USA), and was funded by Incyte (Wilmington, DE, USA).
Funding
This work was supported by Incyte (no grant number).
Disclosure
AV received personal fees from Amgen, AstraZeneca, Bayer, BeiGene, BMS, Celgene, Delcath, Eisai, Eli Lilly and Company, Hengrui, Incyte Corporation, Ipsen, Medac Pharma, Merck, Pieris, QED Therapeutics, Roche, Sanofi, Servier, and Shire. VS received institutional grants from Agios, BMS, Celgene, Clovis Oncology, Debiopharm, FibroGen, Incyte, MedImmune, Merck, National Cancer Institute, and Rafael Pharmaceuticals; received institutional grants and personal fees from Halozyme Therapeutics, Incyte, and Ipsen; and received personal fees from KLUS Pharma, NewLink Genetics, and QED Therapeutics. AH received grants from Incyte; received personal fees from Amgen, Bayer, Debiopharm, Eisai, Eli Lilly and Company, Incyte, Servier, and Spectrum Pharmaceuticals; received other fees from the Drug Development Department (DITEP); and received grants from AstraZeneca, BMS, Boehringer Ingelheim, Janssen Cilag, Merck, Novartis, Pfizer, Roche, and Sanofi. GMV received personal fees from Alexion, Amgen, Astellas, Bayer Healthcare, Celgene, Exelixis, Genentech, Incyte, and Novartis; and received research funds paid directly to institution from Astellas, Boston Scientific, Celgene, Eli Lilly and Company, EMD Serono, E.R. Squibb and Sons, Incyte, and Merck. DM received grants from Incyte; received grants and personal fees from Celgene, Evotec, Incyte, iOnctura, and Shire; and received personal fees from Baxter and Eli Lilly and Company. RMAR received grants from Incyte; received grants outside of the institution from Actinium Pharmaceuticals and Seattle Genetics; and received personal fees from Sirtex Medical. ASP received personal fees from AAA Pharmaceutical, Amgen, Eisai, Incyte, and Ipsen; received grants and personal fees from BMS and Taiho Pharmaceutical Group; received grants from Exelixis and Incyte; and received other fees from Actinium Pharmaceuticals, Alexion, Aptose Biosciences, Immunomedics, and Seattle Genetics. MJB received grants from Adaptimmune, Agios, ARIAD, Basilea, Bioline, Boston BioMed, Celgene, Dicerna Pharmaceuticals, EMD Merck Serono, Halozyme Therapeutics, Incyte, Ionis Pharmaceuticals, MedImmune, Mirna Therapeutics, Novartis, Puma Biotherapeutics, QED Therapeutics, Redhill Pharmaceuticals, Senhwa Biosciences, SillaJen, Sun BioPharma, Taiho Pharmaceutical Group, and Toray; received personal fees from ADC Therapeutics, AstraZeneca, Exelixis, G1 Therapeutics, Immunovative Therapies, Inspyr Therapeutics, Lynx Group, Merck, and Western Oncolytics; and received other fees from AVEO Oncology, Intercept Pharmaceuticals, OncBioMune Pharmaceuticals, and Pieris. AGM received grants from BMS. DYO received personal fees from Aslan Pharmaceuticals, Bayer, Genentech/Roche, Halozyme Therapeutics, Merck Serono, Novartis, Taiho Pharmaceutical Group, and Zymeworks; received grants from Array, Eli Lilly and Company, Green Cross, and Novartis; and received grants and personal fees from AstraZeneca. ED received research grants paid directly to the institution and honoraria from Pfizer; received research grants paid directly to the institution and consulting fees from Incyte; received consulting fees from Basilea, G1 Therapeutics, Helsinn, QED, and Taiho; and received research grants paid directly to the institution from AstraZeneca, Eli Lilly, Gilead, Ipsen, Lutris, MedImmune, NGM Biopharmaceuticals, Relay, and Zymeworks. DVC received grants from Incyte; and received personal fees from Astellas, BMS, Eli Lilly and Company, Five Prime, Foundation Medicine, Genentech/Roche, Gritstone Oncology, Guardant Health, Merck, Taiho Pharmaceutical Group, and Tempus. EVC received personal fees from Astellas, AstraZeneca, and Incyte; received grants and personal fees from Bayer, BMS, Celgene, Eli Lilly and Company, Merck KGaA, Merck Sharp & Dohme, Novartis, Roche, and Servier; and received grants from Amgen, Boehringer Ingelheim, and Ipsen. CFL, HZ, and MLV are employees and shareholders of Incyte. GKAA received research funding from Arcus, AstraZeneca, BioNtech, BMS, Celgene, Genentech/Roche, Helsinn, Puma, QED, Servier, Silenseed, Yiviva, and has a consulting or advisory role in Alnylam, AstraZeneca, Autem, Beigene, Berry Genomics, Boehringer Ingelheim, Celgene, Cend, CytomX, Eisai, Eli Lilly, Exelixis, Flatiron, Genentech/Roche, Helio, Helsinn, Incyte, Ipsen, Merck, Newbridge, Novartis, QED, Rafael, Servier, Silenseed, Sobi, Vector, and Yiviva. DG has declared no conflicts of interest.
Data sharing
Incyte (Wilmington, DE, USA) is committed to data sharing that advances science and medicine while protecting patient privacy. Qualified external scientific researchers may request anonymized datasets owned by Incyte for the purpose of conducting legitimate scientific research. Researchers may request anonymized datasets from any interventional study (except phase I studies) for which the product and indication have been approved on or after 1 January 2020 in at least one major market (e.g. USA, EU, JPN). Data will be available for request after the primary publication or 2 years after the study has ended. Information on Incyte’s clinical trial data sharing policy and instructions for submitting clinical trial data requests are available at: https://www.incyte.com/Portals/0/Assets/Compliance%20and%20Transparency/clinical-trial-data-sharing.pdf?ver=2020-05-21-132838-960.
Supplementary data
References
- 1.Banales J.M., Marin J.J.G., Lamarca A., et al. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020;17(9):557–588. doi: 10.1038/s41575-020-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tyson G.L., El-Serag H.B. Risk factors for cholangiocarcinoma. Hepatology. 2011;54(1):173–184. doi: 10.1002/hep.24351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Javle M., Lee S., Azad N.S., et al. Temporal changes in cholangiocarcinoma incidence and mortality in the United States from 2001 to 2017. Oncologist. 2022;27(10):874–883. doi: 10.1093/oncolo/oyac150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hang H., Jeong S., Sha M., et al. Cholangiocarcinoma: anatomical location-dependent clinical, prognostic, and genetic disparities. Ann Transl Med. 2019;7(23):744. doi: 10.21037/atm.2019.12.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izquierdo-Sanchez L., Lamarca A., La Casta A., et al. Cholangiocarcinoma landscape in Europe: diagnostic, prognostic and therapeutic insights from the ENSCCA registry. J Hepatol. 2022;76(5):1109–1121. doi: 10.1016/j.jhep.2021.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Silverman I.M., Hollebecque A., Friboulet L., et al. Clinicogenomic analysis of FGFR2-rearranged cholangiocarcinoma identifies correlates of response and mechanisms of resistance to pemigatinib. Cancer Discov. 2021;11(2):326–339. doi: 10.1158/2159-8290.CD-20-0766. [DOI] [PubMed] [Google Scholar]
- 7.Lowery M.A., Ptashkin R., Jordan E., et al. Comprehensive molecular profiling of intrahepatic and extrahepatic cholangiocarcinomas: potential targets for intervention. Clin Cancer Res. 2018;24(17):4154–4161. doi: 10.1158/1078-0432.CCR-18-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kendre G., Murugesan K., Brummer T., et al. Charting co-mutation patterns associated with actionable drivers in intrahepatic cholangiocarcinoma. J Hepatol. 2023;78(3):614–626. doi: 10.1016/j.jhep.2022.11.030. [DOI] [PubMed] [Google Scholar]
- 9.Jusakul A., Cutcutache I., Yong C.H., et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov. 2017;7(10):1116–1135. doi: 10.1158/2159-8290.CD-17-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kongpetch S., Jusakul A., Lim J.Q., et al. Lack of targetable FGFR2 fusions in endemic fluke-associated cholangiocarcinoma. JCO Glob Oncol. 2020;6:628–638. doi: 10.1200/GO.20.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murugesan K., Necchi A., Burn T.C., et al. Pan-tumor landscape of fibroblast growth factor receptor 1-4 genomic alterations. ESMO Open. 2022;7(6) doi: 10.1016/j.esmoop.2022.100641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abou-Alfa G.K., Bibeau K., Schultz N., et al. Effect of FGFR2 alterations on overall and progression-free survival in patients receiving systemic therapy for intrahepatic cholangiocarcinoma. Target Oncol. 2022;17(5):517–527. doi: 10.1007/s11523-022-00906-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chioni A.M., Grose R.P. Biological significance and targeting of the FGFR axis in cancer. Cancers (Basel) 2021;13(22):5681. doi: 10.3390/cancers13225681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogel A., Segatto O., Stenzinger A., Saborowski A. FGFR2 inhibition in cholangiocarcinoma. Annu Rev Med. 2023;74:293–306. doi: 10.1146/annurev-med-042921-024707. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Hepatobiliary Cancers. https://www.nccn.org/login Version 2.2022. Available at.
- 16.Khan S.A., Davidson B.R., Goldin R.D., et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012;61(12):1657–1669. doi: 10.1136/gutjnl-2011-301748. [DOI] [PubMed] [Google Scholar]
- 17.DeOliveira M.L., Cunningham S.C., Cameron J.L., et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel A., Bridgewater J., Edeline J., et al. Biliary tract cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(2):127–140. doi: 10.1016/j.annonc.2022.10.506. [DOI] [PubMed] [Google Scholar]
- 19.IMFINZI® (durvalumab) Wilmington, DE: AstraZeneca; 2022. Full Prescribing Information. [Google Scholar]
- 20.KEYTRUDA® (pembrolizumab) Merck; Rahway, NJ, USA: 2023. Full Prescribing Information. [Google Scholar]
- 21.National Comprehensive Cancer Network (NCCN) Biliary Tract Cancers. April 9, 2024. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Version 1.2024. [Google Scholar]
- 22.IMFINZI® (durvalumab) Summary of Product Characteristics. Södertälje, Sweden: AstaZeneca AB. 2023 [Google Scholar]
- 23.KEYTRUDA® (pembrolizumab) Summary of Product Characteristics. Haarlem, The Netherlands: Merck Sharp & Dohme B.V. 2020 [Google Scholar]
- 24.Valle J., Wasan H., Palmer D.H., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362(14):1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 25.Okusaka T., Nakachi K., Fukutomi A., et al. Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer. 2010;103(4):469–474. doi: 10.1038/sj.bjc.6605779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh D.-Y., Ruth He A., Qin S., et al. Durvalumab plus gemcitabine and cisplatin in advanced biliary tract cancer. NEJM Evid. 2022;1(8) doi: 10.1056/EVIDoa2200015. [DOI] [PubMed] [Google Scholar]
- 27.Kelley R.K., Ueno M., Yoo C., et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023;401(10391):1853–1865. doi: 10.1016/S0140-6736(23)00727-4. [DOI] [PubMed] [Google Scholar]
- 28.PEMAZYRE® (pemigatinib) Incyte Corporation; Wilmington, DE, USA: 2022. Full Prescribing Information. [Google Scholar]
- 29.Abou-Alfa G.K., Sahai V., Hollebecque A., et al. Pemigatinib for previously treated, locally advanced or metastatic cholangiocarcinoma: a multicentre, open-label, phase 2 study. Lancet Oncol. 2020;21(5):671–684. doi: 10.1016/S1470-2045(20)30109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bertuccio P., Malvezzi M., Carioli G., et al. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71(1):104–114. doi: 10.1016/j.jhep.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Yao K.J., Jabbour S., Parekh N., Lin Y., Moss R.A. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics database. BMC Gastroenterol. 2016;16(1):117. doi: 10.1186/s12876-016-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AstraZeneca Imfinzi plus chemotherapy recommended for approval in the EU by CHMP as first immunotherapy regimen for advanced biliary tract cancer. https://www.astrazeneca.com/media-centre/press-releases/2022/imfinzi-plus-chemotherapy-recommended-for-approval-in-the-eu-by-chmp-as-first-immunotherapy-regimen-for-advanced-biliary-tract-cancer.html Available at.
- 33.Tsimafeyeu I., Temper M. Cholangiocarcinoma: an emerging target for molecular therapy. Gastrointest Tumors. 2021;8(4):153–158. doi: 10.1159/000517258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamarca A., Hubner R.A., Ryder W.D., Valle J.W. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25(12):2328–2338. doi: 10.1093/annonc/mdu162. [DOI] [PubMed] [Google Scholar]
- 35.Bibeau K., Feliz L., Lihou C.F., Ren H., Abou-Alfa G.K. Progression-free survival in patients with cholangiocarcinoma with or without FGF/FGFR alterations: a FIGHT-202 post hoc analysis of prior systemic therapy response. JCO Precis Oncol. 2022;6 doi: 10.1200/PO.21.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Javle M., Roychowdhury S., Kelley R.K., et al. Infigratinib (BGJ398) in previously treated patients with advanced or metastatic cholangiocarcinoma with FGFR2 fusions or rearrangements: mature results from a multicentre, open-label, single-arm, phase 2 study. Lancet Gastroenterol Hepatol. 2021;6(10):803–815. doi: 10.1016/S2468-1253(21)00196-5. [DOI] [PubMed] [Google Scholar]
- 37.Feng Y.-H., Su W.-C., Oh D.-Y., et al. Updated analysis with longer follow up of a phase 2a study evaluating erdafitinib in Asian patients (pts) with advanced cholangiocarcinoma (CCA) and fibroblast growth factor receptor (FGFR) alterations. J Clin Oncol. 2022;40(suppl 4):430. [Google Scholar]
- 38.Loriot Y., Schuler M.H., Iyer G., et al. Tumor agnostic efficacy and safety of erdafitinib in patients (pts) with advanced solid tumors with prespecified fibroblast growth factor receptor alterations (FGFRalt) in RAGNAR: interim analysis (IA) results. J Clin Oncol. 2022;40(suppl 16):3007. [Google Scholar]
- 39.Goyal L., Meric-Bernstam F., Hollebecque A., et al. Futibatinib for FGFR2-rearranged intrahepatic cholangiocarcinoma. N Engl J Med. 2023;388(3):228–239. doi: 10.1056/NEJMoa2206834. [DOI] [PubMed] [Google Scholar]
- 40.Borad M.J., Schram A.M., Kim R.D., et al. Updated dose escalation results for ReFocus, a first-in-human study of highly selective FGFR2 inhibitor RLY-4008 in cholangiocarcinoma and other solid tumors. J Clin Oncol. 2023;41(suppl 16):4009. [Google Scholar]
- 41.Malenica I., Donadon M., Lleo A. Molecular and immunological characterization of biliary tract cancers: a paradigm shift towards a personalized medicine. Cancers (Basel) 2020;12(8):2190. doi: 10.3390/cancers12082190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bekaii-Saab T.S., Valle J.W., Van Cutsem E., et al. FIGHT-302: first-line pemigatinib vs gemcitabine plus cisplatin for advanced cholangiocarcinoma with FGFR2 rearrangements. Future Oncol. 2020;16(30):2385–2399. doi: 10.2217/fon-2020-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin-Serrano M.A., Kepecs B., Torres-Martin M., et al. Novel microenvironment-based classification of intrahepatic cholangiocarcinoma with therapeutic implications. Gut. 2023;72(4):736–748. doi: 10.1136/gutjnl-2021-326514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Valle J.W., Qin S., Antonuzzo L., et al. 68O Impact of mutation status on efficacy outcomes in TOPAZ-1: a phase III study of durvalumab (D) or placebo (PBO) plus gemcitabine and cisplatin (plus GC) in advanced biliary tract cancer (BTC) Ann Oncol. 2022;33:S1457. [Google Scholar]
- 45.PEMAZYRE (pemigatinib) 2022. Summary of Product Characteristics. Amsterdam, The Netherlands: Incyte Biosciences Distribution. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.