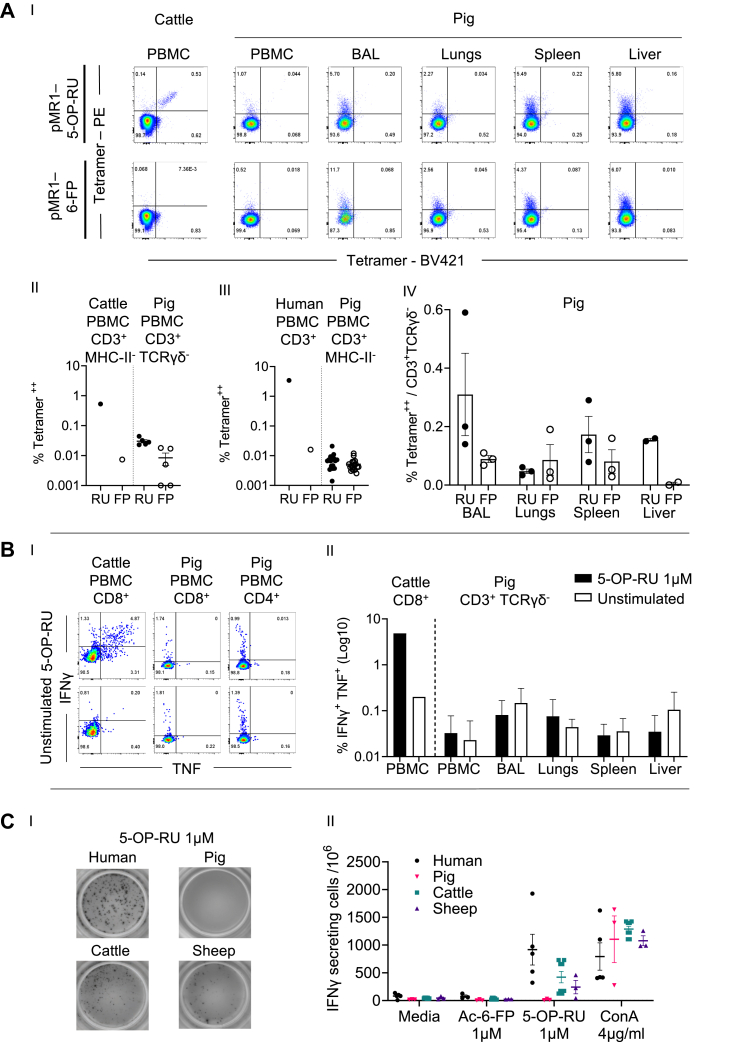
Figure 5.
Detailed characterization of MAIT cells in pigs and sheep.A, (I) representative flow cytometry plots of cells stained with pig MR1–5-OP-RU (pMR1–5-OP-RU) or MR1–6-FP (pMR1–6-FP) tetramers conjugated to PE or BV421 among CD3+ γδTCR- pig PBMC, BAL, lungs spleen, and liver (and for comparison among CD3+ cattle PBMC), (II) the proportion of cells double positive for pig MR1–5-OP-RU (RU) or MR1–6-FP (FP) tetramers conjugated to PE or BV421 among CD3+ γδTCR- T cells in pig PBMC (n = 5) (and for comparison among CD3+ MHC-II- cattle PBMC (n = 1)), (III) the proportion of cells double positive for pig MR1–5-OP-RU (RU) or MR1–6-FP (FP) tetramers conjugated to PE or BV421 among CD3+ MHC-II- pig PBMC (n = 18) (and for comparison among CD3+ human PBMC (n = 1)), and (IV) the proportion of cells double positive for pig MR1–5-OP-RU (RU) or MR1–6-FP (FP) tetramers conjugated to PE or BV421 among CD3+ γδTCR− T cells in pig (n = 2 or 3) BAL, lungs, spleen, and liver lymphocytes. B, representative flow cytometry plots (I) and quantification of the frequency (II) of the IFNγ and TNF intracellular cytokine response in cattle CD8+ PBMC (n = 1) and CD3+ γδTCR- pig (n = 3) PBMC, BAL, lungs, liver, and spleen lymphocytes, following stimulation with 1 μM 5-OP-RU for 6 h. Mean ± SEM are shown. C, representative images (I) and quantification (II) of secreted IFNγ assessed by ELISpot following coincubation of human (n = 3), pig (n = 3), cattle (n = 4), and sheep (n = 3) PBMC with medium, 1 μM Ac-6-FP, 1 μM 5-OP-RU, or 4 μg/ml ConA for 18 h. Mean SCF/106 cells ± SEM are shown. Ac-6-FP, acetyl-6-FP; 5-OP-RU, 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil; 6-FP, 6-formylpterin; MAIT, mucosal-associated invariant T; MHC, major histocompatibility complex; MR1, MHC-I related protein 1; TCR, T cell receptor; PBMC, peripheral blood mononuclear cell.