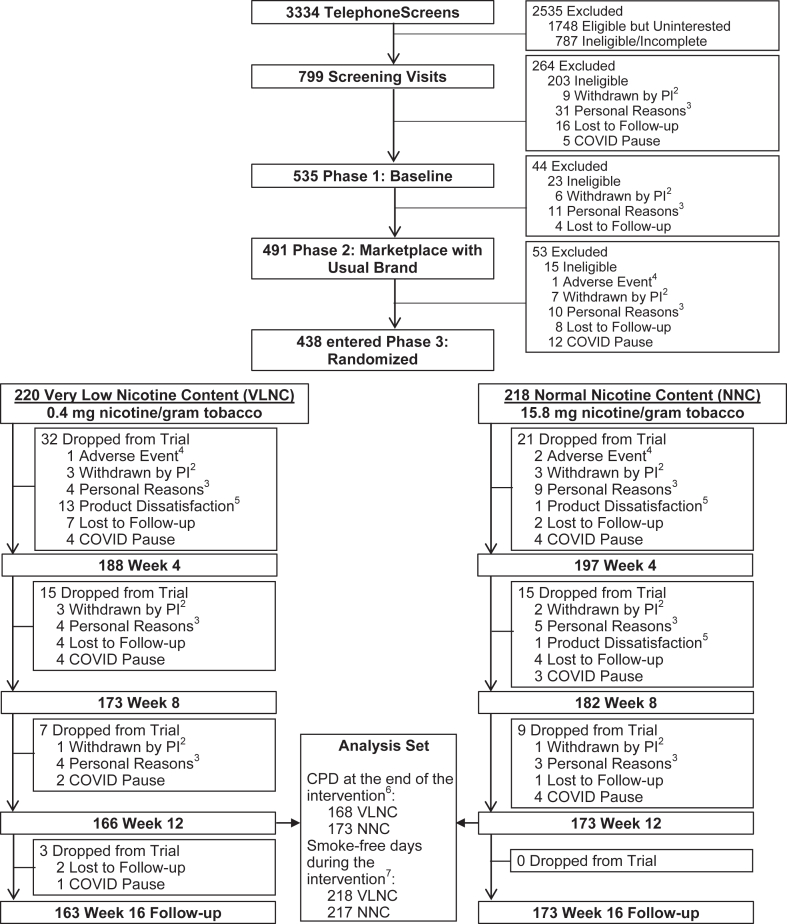
Fig. 1.
Consort Diagram1. Footnote: 1Study sites included University of Minnesota (lead; Institutional Review Board [IRB] number 00000937), University of California, San Francisco (IRB number 17–22748), Duke University (IRB number Pro00086465), Brown University, University of Pennsylvania, and Wake Forest University (IRB number 00061623, includes Brown University and University of Pennsylvania). 2Withdrawn by Principal Investigator (PI): Included unstable physical/mental health, pregnancy, low carbon monoxide, ineligible Cigarettes per Day (CPD), no internet access, non-adherence to protocol. 3Personal Reasons: Included life complications, study burden, moved out of area, lost interest, time constraints, unwilling to use non-menthol cigarettes at University of California, San Francisco site (menthol cigarettes banned in San Francisco), incarceration. 4Adverse Event: Reasons for self-withdrawal due to adverse events included difficulty tolerating withdrawal symptoms or a new illness or injury that required the individual's time and/or focus. 5Product dissatisfaction: Unwilling to smoke the study cigarette or use other Experimental Marketplace products. 6The different sample size in the CPD outcomes at the end of intervention (i.e., mean CPD over 7 days before Week 12) than the number of participants at Week 12 was due to the fact that some participants dropped out between visits but had useable Interactive Voice Response (IVR) data; all 438 participants were included in the analysis of CPD outcomes by using imputation methods. 7The sample size of smoke-free days during the intervention was based on the number of paticipants who responded to the IVR for ≥1 day during the intervention; 435 out of 438 participants were included in the analysis of the smoke-free days outcome; no imputation.