Abstract
Background:
To address the need for immunotherapy in patients with advanced primary hepatocellular carcinoma (HCC), combination with radiotherapy (RT) has emerged as a promising strategy. In preclinical studies, irradiated tumors released tumor antigens to synergistically increase the antitumor effect of immunotherapy. Hence, we investigated whether RT enhances the efficacy of anti-programmed death receptor-1 (PD-1) inhibitors in advanced HCC in real-world practice.
Methods:
Between August 2018 and June 2021, 172 patients with advanced primary HCC were enrolled in the tertiary center (Zhongshan Hospital of Fudan University); 95 were treated with a combination of RT and the inhibitor of PD-1 (RT-PD1 cohort), and 77 were administered anti-PD-1 therapy (PD1 cohort). The first cycle of PD-1 inhibitors was administered within 60 days or concurrently with RT. Propensity score matching for bias reduction was used to evaluate the clinical outcomes.
Results:
Among 71 propensity-matched pairs, median progression-free survival was 5.7 months in the RT-PD1 cohort vs. 2.9 months in the PD1 cohort (P <0.001). Median overall survival was 20.9 months in the RT-PD1 cohort vs. 11.2 months in the PD1 cohort (P = 0.018). Compared with patients in the PD1 cohort, patients in the RT-PD1 cohort had significantly higher objective response rates (40.8%, 29/71 vs. 19.7%, 14/71, P = 0.006) and disease control rates (62.0%, 44/71 vs. 31.0%, 22/71, P <0.001). The incidences of toxic effects were not significantly different between the two cohorts.
Conclusions:
RT plus anti-PD-1 therapy is well tolerated. RT enhances the efficacy of anti-PD-1 therapy in patients with advanced primary HCC by improving survival outcomes without increased toxic effects.
Keywords: Radiotherapy, Immune checkpoint inhibitor, Programmed cell death receptor-1, Hepatocellular carcinoma, Propensity score matching, Treatment outcome, Adverse effects
Introduction
The success of immune checkpoint inhibitors (ICIs) represented by programmed death receptor/ligand-1 (PD-1/PD-L1) antibodies has opened a new era of advanced hepatocellular carcinoma (HCC) treatment.[1,2,3,4] However, primary resistance of ICIs exposes patients to ineffective treatment,[5] possibly because certain tumor antigens are not detected. As the mechanisms of acquired resistance to ICIs remain unknown, only a subset of patients responds and presents long-term clinical benefits, leaving the need to investigate further options for non-responders.[6] Therefore, research efforts are being focused on various combined approaches to improve treatment outcomes. It is well documented that radiotherapy (RT) enhances the immune response in this setting.[7]
Radiotherapy can reshape the liver tumor microenvironment to prevent antigen-specific T cell loss.[8] In preclinical studies, irradiated tumors released more tumor antigens, presented antigens more effectively, and had higher T-cell infiltration. Combining RT with ICIs generated a further shrinkage of tumor in several solid tumor types compared with either of these treatments alone.[9,10,11,12,13] ICIs combined with local precision RT for advanced liver cancer may be a strategy to circumvent the primary or acquired resistance to ICIs, and further optimize the efficacy of therapy.[14] A previous study suggested that the priming effect of RT can rescue the immune response and resistance to ICIs in poor immunogenicity tumors.[15] Importantly, the administration of PD-1 inhibitors during RT reduces the aggregation of myeloid-derived suppressor cells and enhances the antitumor effects of CD8+ T cells that further help improve the efficacy of RT.[16] Our recently published study showed that PD-L1 expression in liver cancer cells increases after RT to enhance ICIs efficacy,[17] which reflected RT can enhance response to ICIs and synergistically augment the antitumor effect.[16,18,19]
Despite this, there is a scarcity of published prospective clinical data on combined RT and anti-PD-1 therapy in advanced HCC, except a few small series,[20,21,22] which were limited to the detection of potentially significant differences in response rates and outcomes when analyzed individually. In this study, we aimed to illustrate the efficacy and safety of RT plus anti-PD-1 therapy compared to anti-PD-1 monotherapy in advanced primary HCC with real-world evidence.
Methods
Patients
This retrospective study enrolled patients who received RT plus anti-PD-1 therapy (RT-PD1 cohort) or anti-PD-1 therapy alone (PD1 cohort) between August 2018 and June 2021 from Zhongshan Hospital of Fudan University. The Ethics Committee of Zhongshan Hospital Fudan University approved this retrospective study (No. B2021-828R2). The written informed consent of participants was waived due to the retrospective nature of the study.
Eligibility criteria for this study included patients aged 18 years or older who had received an initial diagnosis of advanced primary HCC. The diagnosis was established either through liver biopsy or by the presence of typical imaging features, characterized by hypervascularity in the arterial phase with subsequent washout observed in the portal venous or delayed phase. Treatment for these patients consisted of a combination of targeted therapy, encompassing vascular endothelial growth factor blockade and other anti-angiogenic drugs, administered concurrently with anti-PD-1 therapy. For patients infected with the hepatitis B virus (HBV), antiviral therapy was initiated before the commencement of anti-PD-1 therapy as a precautionary measure. Exclusion criteria encompassed individuals who had undergone a previous liver transplant, those with active autoimmune liver disease, individuals with diffuse lesions, those with severe ascites or hepatic encephalopathy, or individuals with concurrent malignant tumors. A total of 172 advanced primary HCC patients were finally enrolled. HCC staging was determined according to the Barcelona Clinic Liver Cancer (BCLC) classification.[4] The diagnosis of BCLC staging involved a detailed assessment of criteria, with BCLC-B stage assigned when multiple nodules were observed and BCLC-C stage assigned in the presence of observed extrahepatic metastasis or significant macrovascular invasion. Staging procedures relied on advanced imaging techniques, such as magnetic resonance imaging or computed tomography scans, to accurately evaluate the extent of the disease.
Propensity score matching (PSM) was performed using a nearest-neighbor algorithm with a caliper of width equal to 0.2 in a 1:1 ratio according to Eastern Cooperative Oncology Group (ECOG) score, sex, age (<50 years vs. ≥50 years), hepatitis B surface antigen (HBsAg) infection status, Child-Pugh score, extrahepatic metastasis, macroscopic vascular invasion, tumor size, tumor number, albumin–bilirubin (ALBI) grade, alpha-fetoprotein level, and targeted agents between the two groups to minimize bias caused by non-randomized selection of patients. After PSM, 71 matched pairs were identified.
Radiotherapy and immunotherapy
The RT-PD1 cohort was defined as the first cycle of PD-1 inhibitors administered within 60 days or concurrently with RT. For RT planning, according to guidelines of the Chinese Society of Clinical Oncology,[23] a vacuum immobilization bag was employed to ensure active respiratory control, thereby minimizing liver motion during treatment., including tomotherapy intensity-modulated radiotherapy (TOMO-IMRT) or stereotactic body radiotherapy (SBRT). TOMO-IMRT was delivered at a total dose of 20.0–60.0 Gy at 1.8–5.0 Gy per fraction in 53 cases (55.8%). SBRT was delivered at a total dose of 28.0–60.0 Gy at 1.8–10.0 Gy per fraction in 42 cases (44.2%). Modified individual doses were standardized using the regime of the RTOG guidelines,[24] taking into account tumor volume and the number of lesions. As the neoplasm volume and variety of lesions treated increased, lower doses were prescribed.
Images were contrasted on a 4D computed tomography (4D-CT) simulator through abdominal compression to evaluate the liver motion and determine internal target volume. Mega-Voltage CT was acquired before each course. The largest tumor was selected as the target lesion for radiotherapy, and up to three nodules were allowed under the condition that the tolerated dose was delivered to the liver. If multiple organ sites may be involved, it is up to the physician to decide what tumor localization to treat. The tumor should be at least 0.5 cm in diameter and no larger than 10.0 cm in diameter and radiotherapy treatment will be given before the start of PD-1. All the patients received anti-PD-1 therapy intravenously, according to a schedule of 240 mg every 2 weeks for nivolumab, 200 mg every 3 weeks for pembrolizumab, tislelizumab, and sintilimab, 240 mg every 3 weeks for toripalimab, or 200 mg every 3 weeks for camrelizumab. Additionally, continuous PD-1 inhibitors were administered unless there was disease progression or the occurrence of unacceptable toxicity. All patients included in the study did not undergo any additional treatment regimens following the completion of the provided treatment and before experiencing disease progression. The last follow-up time was recorded as the occurrence of death or loss to follow-up.
Response and safety assessment
Tumor response was assessed every 2–3 months by two independent radiologists according to RECIST (version 1.1).[25] The objective response rate (ORR) was defined as a sum of complete response (CR) and partial response (PR), and the disease control rate (DCR) was defined as the sum of CR, PR, and stable disease (SD). The best out-of-field (abscopal) rate was defined only in non-irradiated lesions.
Adverse events (AEs) were determined according to the Common Terminology Criteria for Adverse Events (CTCAE v5.0).[26] We assessed the development and severity of representative AEs at regular follow-up visits until 30 days after treatment cessation, including decreased appetite, fever, diarrhea, fatigue, nausea, myalgia, thrombocytopenia, leukopenia, hypothyroidism, hepatitis, myocarditis, pneumonitis, rash, and hypoadrenocorticism.
Statistical analysis
Categorical parameters are expressed as numbers and percentages and compared using χ2 or Fisher’s exact test. Progression-free survival (PFS) was measured from the start of RT to the date of progression or death due to any cause, or last follow-up. Overall survival (OS) was calculated as the interval between the initiation of RT until death due to any cause, or last follow-up. Log-rank tests were performed on Kaplan–Meier survival curves from treatment to PFS and OS. Prognostic factors were evaluated by univariate and multivariate Cox proportional hazard regression. The hazard ratios (HRs) and 95% confidence intervals (CIs) were presented. PSM and statistical analyses were performed using SPSS 26.0 (SPSS Inc., Chicago, IL, USA) and R software version 4.1.0 (R Core Team, Vienna, Austria). A P <0.05 was considered to be statistically significant.
Results
Baseline characteristics of RT-PD1 therapy and anti-PD-1 therapy
Of the 172 patients, 95 were in the RT-PD1 cohort and 77 in the PD1 cohort [Figure 1]. A total of 136 (79.1%) patients were infected with HBsAg, 122 (70.9%) received targeted therapy, and 151 (87.8%) had BCLC-C. There were 57 (33.1%) patients who had portal vein infiltration. In total, 57 individuals (33.1%) had macroscopic vascular invasion, including tumor thrombus in the main trunk of the portal vein (19 patients) and the inferior vena cava (13 patients); 121 (70.3%) had extrahepatic metastasis, with metastatic sites in the lung (74 cases), lymph nodes (18 cases), bone (41 cases), pleural (3 cases), and adrenal (11 cases). Eighteen patients (10.5%) received first-line therapy, and 154 patients (89.5%) received second-line or later therapy [Table 1]. Of the anti-PD-1 antibodies, camrelizumab, nivolumab, toripalimab, pembrolizumab, tislelizumab, and sintilimab were administered in 35, 20, 32, 19, 16, and 50 patients, respectively. The median duration of anti-PD-1 treatment in the RT-PD1 cohort was 5.8 months (range, 0.7–25.0), vs. 3.6 months (range, 0.7–15.7) in the PD1 cohort. The median number of anti-PD-1 immunotherapy cycles in the RT-PD1 cohort was 6 (range, 1–23), vs. 4 (range, 1–21) in the PD1 cohort.
Figure 1.
Flow diagram of patients with advance HCC who underwent either RT plus anti-PD-1 therapy or anti-PD-1 therapy. HCC: Hepatocellular carcinoma; PD-1: Programmed death receptor-1; RFA: Radiofrequency ablation; RT: Radiotherapy.
Table 1.
Patients’ baseline demographic characteristics before and after PSM.
| Variables | Before PSM | After PSM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 172) | RT + PD1 (n = 95) | PD1 (n = 77) | Statistic values | P value | Overall (n = 142) | RT + PD1 (n = 71) | PD1 (n = 71) | Statistic values | P value | |
| Sex | 2.076* | 0.150 | 2.095* | 0.148 | ||||||
| Female | 24 (14.0) | 10 (10.5) | 14 (18.2) | 20 (14.1) | 7 (9.9) | 13 (18.3) | ||||
| Male | 148 (86.0) | 85 (89.5) | 63 (81.8) | 122 (85.9) | 64 (90.1) | 58 (81.7) | ||||
| Age (years) | 2.752* | 0.097 | 0.300* | 0.584 | ||||||
| <50 | 56 (32.6) | 36 (37.9) | 20 (26.0) | 43 (30.3) | 23 (32.4) | 20 (28.2) | ||||
| ≥50 | 116 (67.4) | 59 (62.1) | 57 (74.0) | 99 (69.7) | 48 (67.6) | 51 (71.8) | ||||
| HBsAg | 0.111* | 0.739 | 0.041* | 0.839 | ||||||
| Negative | 36 (20.9) | 19 (20.0) | 17 (22.1) | 31 (21.8) | 16 (22.5) | 15 (21.1) | ||||
| Positive | 136 (79.1) | 76 (80.0) | 60 (77.9) | 111 (78.2) | 55 (77.5) | 56 (78.9) | ||||
| Child-Pugh grade | 2.722* | 0.099 | 1.143* | 0.285 | ||||||
| A | 137 (79.7) | 80 (84.2) | 57 (74.0) | 115 (81.0) | 60 (84.5) | 55 (77.5) | ||||
| B | 35 (20.3) | 15 (15.8) | 20 (26.0) | 27 (19.0) | 11 (15.5) | 16 (22.5) | ||||
| ECOG performance status | 2.427* | 0.119 | 0.067* | 0.796 | ||||||
| 0 | 26 (15.1) | 18 (18.9) | 8 (10.4) | 17 (12.0) | 9 (12.7) | 8 (11.3) | ||||
| 1 | 146 (84.9) | 77 (81.1) | 69 (89.6) | 125 (88.0) | 62 (87.3) | 63 (88.7) | ||||
| BCLC | 6.400* | 0.011 | 2.117* | 0.146 | ||||||
| B | 21 (12.2) | 17 (17.9) | 4 (5.2) | 13 (9.2) | 9 (12.7) | 4 (5.6) | ||||
| C | 151 (87.8) | 78 (82.1) | 73 (94.8) | 129 (90.8) | 62 (87.3) | 67 (94.4) | ||||
| NLR | 3.0 (2.1–5.1) | 3.0 (1.9–5.5) | 3.0 (2.1–5.0) | 2986.5† | 0.956 | 3.1 (2.0–5.2) | 3.1 (1.9–5.5) | 3.0 (2.1–5.0) | 2106.0† | 0.978 |
| ALBI grade | 11.160* | 0.004 | 4.239* | 0.120 | ||||||
| 1 | 102 (59.3) | 67 (70.5) | 35 (45.5) | 82 (57.7) | 47 (66.2) | 35 (49.3) | ||||
| 2 | 68 (39.5) | 27 (28.4) | 41 (53.2) | 58 (40.8) | 23 (32.4) | 35 (49.3) | ||||
| 3 | 2 (1.2) | 1 (1.1) | 1 (1.3) | 2 (1.4) | 1 (1.4) | 1 (1.4) | ||||
| Extrahepatic metastasis | 0.078* | 0.780 | 0.575* | 0.448 | ||||||
| Without | 51 (29.7) | 29 (30.5) | 22 (28.6) | 38 (26.8) | 17 (23.9) | 21 (29.6) | ||||
| With | 121 (70.3) | 66 (69.5) | 55 (71.4) | 104 (73.2) | 54 (76.1) | 50 (70.4) | ||||
| Macroscopic vascular invasion | 5.942* | 0.015 | 3.147* | 0.076 | ||||||
| Without | 115 (66.9) | 71 (74.7) | 44 (57.1) | 94 (66.2) | 52 (73.2) | 42 (59.2) | ||||
| With | 57 (33.1) | 24 (25.3) | 33 (42.9) | 48 (33.8) | 19 (26.8) | 29 (40.8) | ||||
| Tumor size (mm) | 5.271* | 0.022 | 1.812* | 0.178 | ||||||
| <50 | 97 (56.4) | 61 (64.2) | 36 (46.8) | 76 (53.5) | 42 (59.2) | 34 (47.9) | ||||
| ≥50 | 75 (43.6) | 34 (35.8) | 41 (53.2) | 66 (46.5) | 29 (40.8) | 37 (52.1) | ||||
| Tumor number | 3.215* | 0.073 | 2.817* | 0.093 | ||||||
| <4 | 89 (51.7) | 55 (57.9) | 34 (44.2) | 70 (49.3) | 40 (56.3) | 30 (42.3) | ||||
| ≥4 | 83 (48.3) | 40 (42.1) | 43 (55.8) | 72 (50.7) | 31 (43.7) | 41 (57.7) | ||||
| AFP (ng/mL) | 2.724* | 0.099 | 0.255* | 0.613 | ||||||
| <400 | 99 (57.6) | 60 (63.2) | 39 (50.6) | 77 (54.2) | 40 (56.3) | 37 (52.1) | ||||
| ≥400 | 73 (42.4) | 35 (36.8) | 38 (49.4) | 65 (45.8) | 31 (43.7) | 34 (47.9) | ||||
| Targeted therapy | 2.191* | 0.139 | 0.341* | 0.559 | ||||||
| Without | 50 (29.1) | 32 (33.7) | 18 (23.4) | 35 (24.6) | 19 (26.8) | 16 (22.5) | ||||
| With | 122 (70.9) | 63 (66.3) | 59 (76.6) | 107 (75.4) | 52 (73.2) | 55 (77.5) | ||||
| Previous therapy | 6.819* | 0.146 | 6.821* | 0.146 | ||||||
| No | 18 (10.5) | 12 (12.6) | 6 (7.8) | 12 (16.9) | 8 (11.3) | 4 (2.8) | ||||
| Resection | 63 (36.6) | 40 (42.1) | 23 (29.9) | 52 (73.2) | 31 (43.7) | 21 (14.8) | ||||
| Ablation | 30 (17.4) | 12 (12.6) | 18 (23.4) | 19 (26.8) | 10 (14.1) | 9 (6.3) | ||||
| TACE | 139 (80.8) | 70 (73.7) | 69 (89.6) | 112 (157.7) | 46 (64.8) | 66 (46.5) | ||||
| Systemic chemotherapy | 9 (5.2) | 4 (4.2) | 5 (6.5) | 9 (12.7) | 4 (5.6) | 5 (3.5) | ||||
| Anti-PD-1 antibodies | 1.965* | 0.854 | 1.025* | 0.961 | ||||||
| Camrelizumab | 35 (20.3) | 18 (18.9) | 17 (22.1) | 31 (43.7) | 16 (22.5) | 15 (10.6) | ||||
| Nivolumab | 20 (11.6) | 11 (11.6) | 9 (11.7) | 16 (22.5) | 7 (9.9) | 9 (6.3) | ||||
| Toripalimab | 32 (18.6) | 15 (15.8) | 17 (22.1) | 28 (39.4) | 13 (18.3) | 15 (10.6) | ||||
| Pembrolizumab | 19 (11.0) | 11 (11.6) | 8 (10.4) | 16 (22.5) | 8 (11.3) | 8 (5.6) | ||||
| Tislelizumab | 16 (9.3) | 10 (10.5) | 6 (7.8) | 15 (21.1) | 9 (12.7) | 6 (4.2) | ||||
| Sintilimab | 50 (29.1) | 30 (31.6) | 20 (26) | 36 (50.7) | 18 (25.4) | 18 (12.7) | ||||
Data are presented as median (Q1-Q3) or n (%). *χ2 value; †U value. AFP: Alpha fetoprotein; ALBI: Albumin–bilirubin; BCLC: Barcelona Clinic Liver Cancer; ECOG: Eastern Cooperative Oncology Group; HBsAg: Hepatitis B surface antigen; NLR: Neutrophil-to-lymphocyte ratio; PD1: Anti-programmed death receptor-1 inhibitor; PD-1: Programmed death receptor-1; PSM: Propensity score matching; RT: Radiotherapy; TACE: Transcatheter arterial chemoembolization.
The median time between immunotherapy and radiation was 7 days (range 0–60 days). The tumor sites selected for RT were primarily liver lesions. The median radiation dose delivered was 3.0 Gy (1.8–10.0). The patients who received radiotherapy approach were SBRT (n = 42) and TOMO-IMRT (n = 53). Fifty-one patients received palliative RT and 44 patients received RT for local control. The details of the radiotherapy treatment regimens are displayed in Supplementary Table 1, http://links.lww.com/CM9/B995. After performing PSM, we obtained one-to-one matching cohorts (71 patients per group) for the RT-PD1 cohort vs. PD1 cohort [Table 1]. The baseline variables between the matched cohorts were not found significant differences.
Comparison of tumor responses between the groups
According to the imaging assessment, the ORR was 42.1% (40/95) and 20.7% (16/77) in the RT-PD1 and PD1 cohorts, respectively (P = 0.003). The DCR was also significantly higher in the RT-PD1 cohort than in the PD1 cohort (68.4%, 65/95 vs. 32.5%, 25/77; P <0.001). The time to achieve the best response was similar in both cohorts (3.4 months vs. 2.8 months, P = 0.065; Table 2). After performing PSM, compared with patients in the PD1 cohort, patients in the RT-PD1 cohort had a significantly higher ORR (29/71, 40.8% vs. 14/71, 19.7%, P = 0.006; Table 2) and DCR (44/71, 62.0% vs. 22/71, 31.0%, P <0.001; Table 2). The time to achieve best response was similar in both cohorts after PSM (3.4 months vs. 2.8 months, P = 0.113; Table 2).
Table 2.
Comparison of efficacy of RT-PD1 group with PD1 group based on tumor response before and after PSM.
| Variables | Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall (n = 172) | RT + PD1 (n = 95) | PD1 (n = 77) | P value | Overall (n = 142) | RT + PD1 (n = 71) | PD1 (n = 71) | P value | |
| Tumor response, n (%) | <0.001 | 0.003 | ||||||
| CR | 5 (2.9) | 4 (4.2) | 1 (1.3) | 3 (2.1) | 2 (2.8) | 1 (1.4) | ||
| PR | 51 (29.7) | 36 (37.9) | 15 (19.5) | 40 (28.2) | 27 (38.0) | 13 (18.3) | ||
| SD | 34 (19.8) | 25 (26.3) | 9 (11.7) | 23 (16.2) | 15 (21.1) | 8 (11.3) | ||
| Progressive disease | 82 (47.7) | 30 (31.6) | 52 (67.5) | 76 (53.5) | 27 (38) | 49 (69) | ||
| Response rate (%) | ||||||||
| ORR | 32.6 | 42.1 | 20.7 | 0.003 | 30.3 | 40.8 | 19.7 | 0.006 |
| DCR | 52.3 | 68.4 | 32.5 | <0.001 | 46.5 | 62.0 | 31.0 | <0.001 |
| Median time to achieve best response (months) | 3.1 | 2.8 | 3.4 | 0.065 | 3.0 | 2.8 | 3.4 | 0.113 |
CR: Complete response; DCR: Disease control rate; ORR: Objective response rate; PD1: Anti-programmed death receptor-1 inhibitor; PR: Partial response; PSM: Propensity score matching; RT: Radiotherapy; SD: Stable disease.
Comparison of survival outcomes between the groups
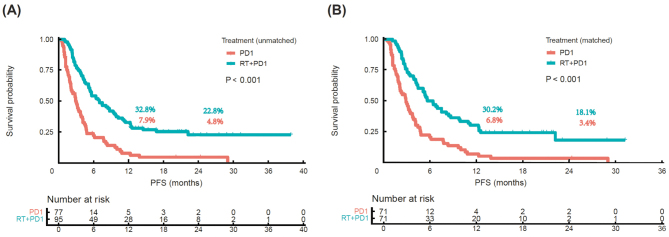
Median PFS in the RT-PD1 cohort was higher than that in the PD1 cohort (7.0 months vs. 3.0 months; P <0.001, Figure 2A). The 1-year PFS rates were 32.8% and 7.9%, and the 2-year PFS rates were 22.8% and 4.8%, respectively. Median PFS was significantly different between the RT-PD1 cohort and PD1 cohort after performing PSM (5.7 months vs. 2.9 months; P <0.001, Figure 2B). The 1-year PFS rates were 30.2% and 6.8%, and the 2-year PFS rates were 18.1% and 3.4% after PSM, respectively.
Figure 2.
Kaplan–Meier survival curves for PFS between RT-PD1 and PD1 cohorts before and after PSM. (A) Unmatched and (B) matched cohorts. P values are calculated using log-rank test. PD1: Anti-programmed death receptor-1 inhibitor; PFS: Progression-free survival; PSM: Propensity score matching; RT: Radiotherapy.
The multivariate analysis using Cox regression model indicated that RT-PD1 treatment (HR: 0.474; 95% CI: 0.316–0.711; P <0.001) and age (HR: 1.639; 95% CI: 1.076–2.495; P = 0.021) were independent prognostic factors for PFS in the unmatched cohorts [Table 3]. The multivariate analysis also showed that RT-PD1 treatment (HR: 0.446; 95% CI: 0.301–0.661; P <0.001) and age (HR: 1.873; 95% CI: 1.202–2.919; P = 0.006) were independent prognostic factor for PFS in the matched cohorts [Table 3].
Table 3.
Prognostic factors affecting PFS of patients using univariable and multivariable analyses before and after PSM.
| Variables | Before PSM | After PSM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | |
| Sex | ||||||||||||
| Female | 1 (ref) | 1 (ref) | ||||||||||
| Male | 1.062 | 0.654–1.726 | 0.807 | 1.103 | 0.647–1.881 | 0.717 | ||||||
| Age | ||||||||||||
| <50 years | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| ≥50 years | 1.542 | 1.084–2.192 | 0.016 | 1.639 | 1.076–2.495 | 0.021 | 1.815 | 1.224–2.693 | 0.003 | 1.873 | 1.202–2.919 | 0.006 |
| HBsAg | ||||||||||||
| Negative | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| Positive | 1.66 | 1.059–2.603 | 0.027 | 1.085 | 0.660–1.784 | 0.749 | 2.043 | 1.245–3.352 | 0.005 | 1.251 | 0.734–2.132 | 0.410 |
| Child-Pugh grade | ||||||||||||
| A | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| B | 2.003 | 1.323–3.032 | 0.001 | 1.492 | 0.849–2.623 | 0.165 | 1.992 | 1.253–3.166 | 0.004 | 1.448 | 0.841–2.492 | 0.182 |
| ECOG performance status | ||||||||||||
| 0 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| 1 | 3.027 | 1.690–5.424 | <0.001 | 1.42 | 0.631–3.198 | 0.397 | 2.216 | 1.151–4.267 | 0.017 | 1.644 | 0.808–3.344 | 0.170 |
| ALBI | ||||||||||||
| 1 | 1 (ref) | 0.001 | 1 (ref) | 1 (ref) | 0.001 | 1 (ref) | 0.353 | |||||
| 2 | 0.55 | 0.134–2.257 | 0.407 | 0.960 | 0.603–1.529 | 0.864 | 1.958 | 1.345–2.851 | <0.001 | 1.183 | 0.773–1.811 | 0.438 |
| 3 | 1.078 | 0.262–4.429 | 0.917 | 1.002 | 0.209–4.810 | 0.998 | 2.794 | 0.678–11.509 | 0.155 | 2.873 | 0.591–13.974 | 0.191 |
| NLR | 1.026 | 1.000–1.052 | 0.046 | 1.007 | 0.976–1.040 | 0.642 | 1.022 | 0.995–1.049 | 0.114 | |||
| Extrahepatic metastasis | ||||||||||||
| Without | 1 (ref) | 1 (ref) | 1 (ref) | |||||||||
| With | 1.628 | 1.111–2.385 | 0.012 | 1.094 | 0.614–1.949 | 0.759 | 1.432 | 0.942–2.176 | 0.093 | |||
| Macroscopic vascular invasion | ||||||||||||
| Without | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| With | 1.823 | 1.280–2.597 | <0.001 | 1.148 | 0.719–1.835 | 0.563 | 1.685 | 1.150–2.469 | 0.007 | 1.249 | 0.815–1.912 | 0.307 |
| Tumor size | ||||||||||||
| <50 mm | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| ≥50 mm | 1.512 | 1.076–2.124 | 0.017 | 1.129 | 0.762–1.674 | 0.545 | 1.525 | 1.057–2.200 | 0.024 | 1.165 | 0.779–1.741 | 0.457 |
| Tumor number | ||||||||||||
| <4 | 1 (ref) | 1 (ref) | 1 (ref) | |||||||||
| ≥4 | 2.390 | 1.692–3.376 | <0.001 | 1.194 | 0.805–1.771 | 0.378 | 1.116 | 0.765–1.627 | 0.570 | |||
| AFP | ||||||||||||
| <400 ng/mL | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| ≥400 ng/mL | 1.768 | 1.260–2.483 | 0.001 | 1.224 | 0.812–1.848 | 0.335 | 1.624 | 1.126–2.341 | 0.009 | 1.144 | 0.759–1.725 | 0.521 |
| Treatment | ||||||||||||
| PD1 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| RT + PD1 | 0.395 | 0.280–0.556 | <0.001 | 0.474 | 0.316–0.711 | <0.001 | 0.418 | 0.288–0.606 | <0.001 | 0.446 | 0.301–0.661 | <0.001 |
| Targeted therapy | ||||||||||||
| Without | 1 (ref) | 1 (ref) | ||||||||||
| With | 1.393 | 0.952–2.039 | 0.088 | 1.196 | 0.781–1.832 | 0.409 | ||||||
AFP: Alpha fetoprotein; ALBI: Albumin–bilirubin; CI: Confidence interval; ECOG: Eastern Cooperative Oncology Group; HR: Hazard ratio; HBsAg: Hepatitis B surface antigen; NLR: Neutrophil-to-lymphocyte ratio; PD1: Anti-programmed death receptor-1 inhibitor; PFS: Progression-free survival; PSM: Propensity score matching; RT: Radiotherapy.
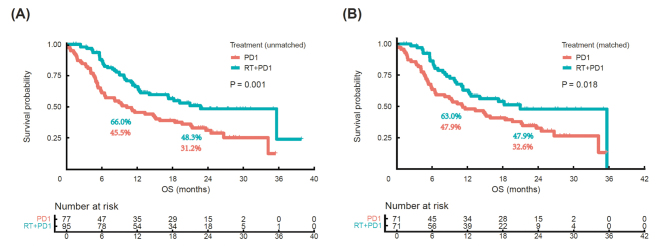
The follow-up period ended on November 22, 2022. The median follow-up duration was 23.3 months (95% CI: 21.5–25.1) in the two cohorts. During the follow-up period, 97 (56.1%) of the 172 patients died (42 [44.2%] and 55 [71.4%] patients in the RT-PD1 and PD1 cohorts, respectively). The median OS was significantly longer in the RT-PD1 cohort than that in the PD1 cohort (22.7 months vs. 10.2 months; P = 0.001; Figure 3A). The 1-year OS rates were 66.0% and 45.5%, and the 2-year OS rates were 48.3% and 31.2%, respectively. Median OS was also significantly different between the RT-PD1 and PD1 cohorts after PSM (20.9 and 11.2 months; P = 0.018; Figure 3B). The 1-year OS rates were 63.0% and 47.9%, and the 2-year OS rates were 47.9% and 32.6% after performing PSM, respectively.
Figure 3.
Kaplan–Meier survival curves for OS between RT-PD1 and PD1 cohorts before and after PSM. (A) Unmatched and (B) matched cohorts. P values are calculated using log-rank test. OS: Overall survival; PD1: Anti-programmed death receptor-1 inhibitor; PSM: Propensity score matching; RT: Radiotherapy.
The multivariate analysis suggested that RT-PD1 treatment (HR: 0.607; 95% CI: 0.379–0.971; P = 0.037), age (HR: 2.016; 95% CI: 1.239–3.281; P = 0.005), and Child-Pugh score (HR: 2.784; 95% CI: 1.499–5.172; P = 0.001) were independent prognostic factors for OS in the unmatched cohorts [Table 4]. Moreover, RT-PD1 treatment (HR: 0.595; 95% CI: 0.359–0.983; P = 0.043), age (HR: 2.385; 95% CI: 1.379–4.123; P 0.002), and Child-Pugh score (HR: 2.402; 95% CI: 1.259–4.580; P = 0.008) were also independent prognostic factors for OS in the matched cohorts [Table 4].
Table 4.
Prognostic factors affecting OS of patients using univariable and multivariable analyses before and after PSM.
| Variable | Before matching | After matching | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariable | Multivariable | Univariable | Multivariable | |||||||||
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Sex | ||||||||||||
| Female | 1 (ref) | 1 (ref) | ||||||||||
| Male | 1.120 | 0.635–1.976 | 0.696 | 1.180 | 0.638–2.181 | 0.598 | ||||||
| Age | ||||||||||||
| <50 years | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| ≥50 years | 1.855 | 1.23–2.797 | 0.003 | 2.016 | 1.239–3.281 | 0.005 | 2.193 | 1.397–3.444 | 0.001 | 2.385 | 1.379–4.123 | 0.002 |
| HBsAg | ||||||||||||
| Negative | 1 (ref) | 1 (ref) | 1 (ref) | |||||||||
| Positive | 1.525 | 0.902–2.579 | 0.116 | 2.093 | 1.132–3.873 | 0.019 | 1.533 | 0.781–3.012 | 0.215 | |||
| Child-Pugh grade | ||||||||||||
| A | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| B | 3.308 | 2.133–5.13 | <0.001 | 2.784 | 1.499–5.172 | 0.001 | 2.876 | 1.763–4.694 | 0.001 | 2.402 | 1.259–4.580 | 0.008 |
| ECOG performance status | ||||||||||||
| 0 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| 1 | 4.019 | 1.753–9.216 | 0.001 | 2.01 | 0.823–4.91 | 0.126 | 2.738 | 1.106–6.783 | 0.029 | 1.676 | 0.614–4.572 | 0.313 |
| ALBI | ||||||||||||
| 1 | 1 (ref) | 0.001 | 1 (ref) | 1 (ref) | 0.020 | 1 (ref) | 0.353 | |||||
| 2 | 2.115 | 1.412–3.168 | <0.001 | 0.906 | 0.521–1.579 | 0.729 | 1.581 | 1.016–2.461 | 0.042 | 0.984 | 0.585–1.657 | 0.953 |
| 3 | 3.901 | 0.935–16.272 | 0.062 | 1.584 | 0.314–8.002 | 0.578 | 5.086 | 1.211–21.356 | 0.026 | 3.152 | 0.573–17.335 | 0.187 |
| NLR | 1.032 | 1.008–1.056 | 0.009 | 1.008 | 0.982–1.036 | 0.541 | 1.028 | 1.003–1.054 | 0.028 | 1.008 | 0.981–1.036 | 0.577 |
| Extrahepatic metastasis | ||||||||||||
| Without | 1 (ref) | 1 (ref) | ||||||||||
| With | 1.474 | 0.934–2.327 | 0.096 | 1.281 | 0.772–2.128 | 0.338 | ||||||
| Macroscopic vascular invasion | ||||||||||||
| Without | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| With | 2.099 | 1.394–3.162 | <0.001 | 1.338 | 0.808–2.217 | 0.257 | 1.810 | 1.159–2.827 | 0.009 | 1.400 | 0.800–2.450 | 0.238 |
| Tumor size | ||||||||||||
| <50 mm | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| ≥50 mm | 1.942 | 1.297–2.906 | 0.001 | 1.354 | 0.860–2.130 | 0.190 | 1.907 | 1.224–2.969 | 0.004 | 1.386 | 0.832–2.311 | 0.210 |
| Tumor number | ||||||||||||
| <4 | 1 (ref) | 1 (ref) | 1 (ref) | |||||||||
| ≥4 | 3.175 | 2.092–4.819 | <0.001 | 1.439 | 0.912–2.269 | 0.118 | 0.692 | 0.434–1.105 | 0.123 | |||
| AFP | ||||||||||||
| <400 ng/mL | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| ≥400 ng/mL | 1.888 | 1.259–2.831 | 0.002 | 1.036 | 0.646–1.662 | 0.882 | 1.731 | 1.113–2.692 | 0.015 | 1.010 | 0.587–1.740 | 0.970 |
| Treatment | ||||||||||||
| PD1 | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||||||
| RT + PD1 | 0.517 | 0.345–0.776 | 0.001 | 0.607 | 0.379–0.971 | 0.037 | 0.585 | 0.373–0.917 | 0.019 | 0.595 | 0.359–0.983 | 0.043 |
| Targeted therapy | ||||||||||||
| Without | 1 (ref) | 1 (ref) | ||||||||||
| With | 1.186 | 0.754–1.866 | 0.459 | 1.054 | 0.635–1.751 | 0.838 | ||||||
AFP: Alpha fetoprotein; ALBI: Albumin–bilirubin; CI: Confidence interval; ECOG, Eastern Cooperative Oncology Group; HR: Hazard ratio; HBsAg: Hepatitis B surface antigen; NLR: Neutrophil-to-lymphocyte ratio; OS: Overall survival; PD1: Anti-programmed death receptor-1 inhibitor; PSM: Propensity score matching; RT: Radiotherapy.
An exploratory analysis revealed that when patients were stratified by RT modality, a comparison of patients treated with SBRT-PD1 vs. those treated with TOMO-IMRT-PD1 reveals a significant difference in survival (median PFS, 10.2 months vs. 4.4 months, P = 0.001; median OS, 35.6 months vs. 12.1 months, P <0.001, Supplementary Figure 1, http://links.lww.com/CM9/B995). Moreover, higher OS and PFS were noticed in the patients treated with fractions size higher than 5 Gy [Supplementary Figure 2, http://links.lww.com/CM9/B995]. The median PFS and OS were higher in the time interval between anti-PD-1 therapy and RT within 7 days than after 7 days (median PFS, 9.4 months vs. 4.5 months, P = 0.019; median OS, 35.6 months vs. 12.2 months, P = 0.019, Supplementary Figure 3, http://links.lww.com/CM9/B995). Importantly, patients receiving intrahepatic radiotherapy demonstrated a marked improvement in both OS and PFS compared to those receiving extrahepatic lesion radiotherapy (median PFS, 9.4 months vs. 5.4 months, P = 0.015; median OS, not reached vs. 12.1 months, P = 0.001, Supplementary Figure 4, http://links.lww.com/CM9/B995). While there was no statistically significant difference in the ORR, the DCR exhibited a significant improvement with intrahepatic radiotherapy compared to extrahepatic radiotherapy [Supplementary Table 2, http://links.lww.com/CM9/B995].
Effects of RT-PD1 treatment without increasing adverse effects
The distribution of AEs before and after PSM is summarized in Table 5. Treatment-related AEs were occurred in 46 (59.7%) and 59 (62.1%) patients in the PD1 and RT-PD1 cohorts, respectively. Grade 3 to 4 AEs occurred in 7 (9.1%) patients in the PD1 cohort and 9 (9.5%) in the RT-PD1 cohort. The most frequent treatment-related AEs of all grades were fatigue, decreased appetite, rash, and nausea.
Table 5.
Treatment-related AEs Summary before and after PSM.
| AE | Before PSM | After PSM | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RT + PD1 (n = 95) | PD1 (n = 77) | P value | RT + PD1 (n = 71) | PD1 (n = 71) | P value | |||||||
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | Any grade | Grade 3–4 | |
| Decreased appetite | 18 (18.9) | 0 (0) | 18 (23.4) | 0 (0) | 0.478 | – | 16 (22.5) | 0 (0) | 14 (19.7) | 0 (0) | 0.299 | – |
| Fever | 12 (12.6) | 1 (1.1) | 10 (13) | 0 (0) | 0.945 | 0.572 | 9 (12.7) | 1 (1.4) | 8 (11.3) | 0 (0) | 0.475 | 0.572 |
| Diarrhea | 8 (8.4) | 0 (0) | 6 (7.8) | 0 (0) | 0.881 | – | 4 (5.6) | 0 (0) | 2 (2.8) | 0 (0) | 0.496 | – |
| Fatigue | 26 (27.4) | 0 (0) | 21 (27.3) | 0 (0) | 0.989 | – | 18 (25.4) | 0 (0) | 16 (22.5) | 0 (0) | 0.285 | – |
| Nausea | 15 (15.8) | 0 (0) | 14 (18.2) | 0 (0) | 0.677 | – | 11 (15.5) | 0 (0) | 12 (16.9) | 0 (0) | 0.751 | – |
| Myalgia | 7 (7.4) | 0 (0) | 3 (3.9) | 0 (0) | 0.522 | – | 2 (2.8) | 0 (0) | 3 (4.2) | 0 (0) | 0.811 | – |
| Thrombocytopenia | 7 (7.4) | 1 (1.1) | 5 (6.5) | 2 (2.6) | 0.823 | 0.854 | 4 (5.6) | 1 (1.4) | 5 (7) | 0 (0) | 0.746 | 0.572 |
| Leukopenia | 4 (4.2) | 0 (0) | 3 (3.9) | 0 (0) | 0.776 | – | 2 (2.8) | 0 (0) | 0 (0) | 0 (0) | 0.199 | – |
| Hepatitis | 7 (7.4) | 2 (2.1) | 5 (6.5) | 2 (2.6) | 0.823 | 0.832 | 2 (2.8) | 2 (2.8) | 5 (7) | 2 (2.8) | 0.623 | 0.832 |
| Hypothyroidism | 7 (7.4) | 0 (0) | 7 (9.1) | 0 (0) | 0.681 | – | 5 (7) | 0 (0) | 2 (2.8) | 0 (0) | 0.289 | – |
| Myocarditis | 8 (8.4) | 2 (2.1) | 6 (7.8) | 2 (2.6) | 0.880 | 0.832 | 5 (7) | 2 (2.8) | 2 (2.8) | 2 (2.8) | 0.289 | 0.832 |
| Pneumonitis | 12 (12.6) | 0 (0) | 9 (11.7) | 0 (0) | 0.851 | – | 9 (12.7) | 0 (0) | 7 (9.9) | 0 (0) | 0.332 | – |
| Rash | 17 (17.9) | 4 (4.2) | 14 (18.2) | 4 (5.2) | 0.961 | 0.953 | 10 (14.1) | 2 (2.8) | 7 (9.9) | 3 (4.2) | 0.220 | 0.811 |
| Hypoadrenocorticism | 2 (2.1) | 1 (1.1) | 2 (2.6) | 0 (0) | 0.831 | 0.572 | 1 (1.4) | 1 (1.4) | 2 (2.8) | 0 (0) | 0.854 | 0.572 |
Data are expressed as n (%). AE: Adverse event; PD1: Anti-programmed death receptor-1 inhibitor; PSM: Propensity score matching; RT: Radiotherapy.
Considering the pre-propensity-matched RT-PD1 and PD1 cohorts as a whole, the incidence and types of AEs were similar, most of them were grade 1 to 2 in severity, and the AE profiles were similar between the two groups. The findings before PSM were approximately the same as the results for overall population analysis after PSM.
Discussion
To the best of our knowledge, this study represents one of the most extensive assessments of retrospective obtained data to identify a synergistic effect of RT on response to anti-PD-1 therapy in patients with advanced primary HCC. We obtained primary evidence on the superiority of RT-PD1 over anti-PD-1 therapy in terms of OS, PFS, and tumor response in the treatment of HCC without an increase in toxic effects caused by both RT and related immunotherapies. The credibility of these results was provided by PSM analysis that simulated randomization of a prospective study and reduced potential biases associated with confounding variables in study design.
RT synergistically enhances the antitumor effect of immunotherapy via multiple potential mechanisms, such as increasing the visibility of tumor antigens, attracting leukocytes into the tumor tissue, and modulating the tumor microenvironment.[12,16,27,28] Thus, combining immunotherapy and radiotherapy is being considered as a possible way of improving clinical benefits in patients who are not responding or have become resistant to immunotherapy. Recent clinical studies suggested that combining pembrolizumab and radiotherapy improved the responses and outcomes of metastatic non-small-cell lung cancer.[29] However, combination strategy is still under preclinical and clinical development for patients with liver cancer.[35] We demonstrated that combining RT with PD-L1 blockade results in a better response in preclinical liver tumors.[17] An important concept in this regard is the abscopal effect, which is reflected to generate a systemic antitumor immune response at unirradiated out-of-field lesions.[30] It is thought that RT increases systemic antigen release from tumor tissue, enhancing antigen recognition by antigen-presenting cells, thereby subsequently presenting antigens to T cells as a result (CD8+ cytotoxic T lymphocytes in particular). Activation of the above cells triggers both local and systemic antitumor immune responses. In addition, sublethal doses of RT have been demonstrated to attract T cells to tumors by modulating their microenvironment, while low-dose RT decreases the immunosuppressive cell signaling induced by high doses.[32,33,34]
The use of RT for local control is imperative as the out-of-field lesion can be better controlled with improved systemic therapy.[30] The present analysis mainly included irradiated intrahepatic lesions and indicated the abscopal effect was induced at a much higher frequency with the inclusion of RT. Of the patients with metastatic disease, >50% (44 out of 66 patients evaluated) treated with RT-PD1 in the present study developed the best out-of-field control response [Supplementary Table 3, http://links.lww.com/CM9/B995]. We also looked at the out-of-field control response rate to each RT modality. The out-of-field control response rates were 73.9% for SBRT and 48.8% for TOMO-IMRT (P = 0.05). Consistent with previous reports,[35,36] patients treated with SBRT-PD1 had better survival outcomes compared with patients treated with TOMO-IMRT-PD1. These results indicate that combined anti-PD-1 with SBRT is more likely to elicit an abscopal immune effect of non-target radiotherapy, while improved clinical outcomes with lower tumor burden.
Furthermore, the levels of tumor-specific T cells increased during and after radiotherapy, as described in a previous study.[37] The interval of ICIs after RT is the focus of investigation in this field. Therefore, we also included patients who received anti-PD-1 therapy shortly after RT (<60 days) to minimize the delay of systemic treatment that may have increased the susceptibility of their immune systems to T-cell penetration and activation. Previous studies have shown a survival advantage for patients with brain metastasis of melanoma who received SBRT with synchronized ICIs compared with non-synchronized ICIs.[38] The PACIFIC trial also revealed that the administration of durvalumab within 14 days after simultaneous chemoradiotherapy significantly improved the OS benefit in multivariate analysis.[39] Similarly, our study found that the median PFS and OS were higher in the time interval between RT and anti-PD-1 therapy within 7 days than after 7 days. Based on the above results, it is suggested that concurrent therapy is achieved robust anti-tumor and a significant survival benefit than non-synchronized therapy.
The optimal radiation dose and fractionation regimens for stimulating a systemic antitumor immune response remain unclear. Intriguingly, our subgroup analyses indicated that RT-PD1 with the largest fraction size ≥5 Gy significantly prolonged OS and PFS compared with the largest fraction size <5 Gy [Supplementary Figure 2, http://links.lww.com/CM9/B995]. Two patients showed a CR assessed by the RECIST after receiving ≥5 Gy RT in 6 fractions with anti-PD-1 therapy. Moreover, the responses were durable–both patients remained disease-free for 13.0 and 4.3 months and were still alive at 24.2 months and 29 months of follow-up, respectively. Our findings are consistent with those of preclinical studies, and many lines of evidence in vivo suggest that the immune-modulating effects of hypofractionated RT are more pronounced than single-dose RT, leading to a better systemic response.[34,40,41,42] However, we could not further perform PSM by the radiation dose and fractionation due to the low sample size. This observation remains striking and warrants further clinical investigation.
In the present study, the most frequent AEs associated with RT plus anti-PD-1 therapy were fatigue, decreased appetite, rash, fever, and nausea. A majority of the patients in the present study experienced grade 1–2 immune-mediated AEs. The above-mentioned AEs were reversible and manageable; and, the incidences of treatment-related toxic effects had no significant difference in both cohorts. Safety measures showed a good profile consistent with findings of previous studies on RT plus anti-PD-1 therapy.[12,43,44,45,46,47]
Our analysis is limited by its retrospective nature, though PSM was used to mitigate potential biases associated with the study design. Second, there was heterogeneity in the study population that included patients who received radiotherapy at different times during the disease course. Third, the standard of dose fractionation regimen, target volume, and role of PD-L1 remained to be explored.
In conclusion, the study demonstrates that RT-PD1 therapy may improve PFS by activating systemic antitumor immune responses. Collectively supporting the combination therapy is associated with superior OS outcomes. Coupled with the absence of increased toxic effects, it further accentuates the potential clinical significance of the RT-PD1 therapy approach. A prospective randomized trial is currently underway to validate these results (ClinicalTrials.gov identifier: NCT03857815).
Acknowledgments
This abstract has been selected for an Oral presentation in 2022 American Society for Radiation Oncology Annual Meeting.[48]
Funding
This study was supported a grant from the National Natural Science Foundation of China (No. 82073479).
Conflicts of interest
None.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Supplementary Material
Footnotes
Shujung Hsu and Yencheng Chao contributed equally to this work.
How to cite this article: Hsu SJ, Chao YC, Hu Y, Zhang Y, Hong WF, Chen YX, Chen RX, Zeng ZC, Du SS. Radiotherapy enhances efficacy of PD-1 inhibitors in advanced hepatocellular carcinoma: A propensity-matched real-world study. Chin Med J 2024;137:1332–1342. doi: 10.1097/CM9.0000000000003124
References
- 1.Yau T Hsu C Kim TY Choo SP Kang YK Hou MM, et al. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J Hepatol 2019;71:543–552. doi: 10.1016/j.jhep.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 2.El-Khoueiry AB Sangro B Yau T Crocenzi TS Kudo M Hsu C, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu AX Finn RS Edeline J Cattan S Ogasawara S Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–952. doi: 10.1016/S1470-2045(18)30351-6. [DOI] [PubMed] [Google Scholar]
- 4.Reig M Forner A Rimola J Ferrer-Fàbrega J Burrel M Garcia-Criado Á, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: An EASL position paper. J Hepatol 2021;75:960–974. doi: 10.1016/j.jhep.2021.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Federico P Petrillo A Giordano P Bosso D Fabbrocini A Ottaviano M, et al. Immune checkpoint inhibitors in hepatocellular carcinoma: Current status and novel perspectives. Cancers (Basel) 2020;12:3025. doi: 10.3390/cancers12103025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Z, Peng Y, Peng X, Xiao D, Shi Y, Tao Y. Effects of radiation therapy on tumor microenvironment: An updated review. Chin Med J 2023;136:2802–2811. doi: 10.1097/CM9.0000000000002535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu J Green MD Li S Sun Y Journey SN Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dovedi SJ Adlard AL Lipowska-Bhalla G McKenna C Jones S Cheadle EJ, et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res 2014;74:5458–5468. doi: 10.1158/0008-5472.CAN-14-1258. [DOI] [PubMed] [Google Scholar]
- 10.Twyman-Saint Victor C Rech AJ Maity A Rengan R Pauken KE Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zeng J See AP Phallen J Jackson CM Belcaid Z Ruzevick J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys 2013;86:343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong X Li X Jiang T Xie H Zhu Z Zhou F, et al. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J Thorac Oncol 2017;12:1085–1097. doi: 10.1016/j.jtho.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Yin Y Wang J Yi J Zhang K Yin Z Jin S, et al. AZD1775 and anti-PD-1 antibody synergistically sensitize hepatoma to radiotherapy. Chin Med J 2024;137:222–231. doi: 10.1097/CM9.0000000000002988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Billan S, Kaidar-Person O, Gil Z. Treatment after progression in the era of immunotherapy. Lancet Oncol 2020;21:e463–e476. doi: 10.1016/S1470-2045(20)30328-4. [DOI] [PubMed] [Google Scholar]
- 15.Yuan Z Fromm A Ahmed KA Grass GD Yang GQ Oliver DE, et al. Radiotherapy rescue of a nivolumab-refractory immune response in a patient with PD-L1-negative metastatic squamous cell carcinoma of the lung. J Thorac Oncol 2017;12:e135–e136. doi: 10.1016/j.jtho.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 16.Deng L Liang H Burnette B Beckett M Darga T Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest 2014;124:687–695. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du SS Chen GW Yang P Chen YX Hu Y Zhao QQ, et al. Radiation therapy promotes hepatocellular carcinoma immune cloaking via PD-L1 upregulation induced by cGAS-STING activation. Int J Radiat Oncol Biol Phys 2022;112:1243–1255. doi: 10.1016/j.ijrobp.2021.12.162. [DOI] [PubMed] [Google Scholar]
- 18.Kim KJ, Kim JH, Lee SJ, Lee EJ, Shin EC, Seong J. Radiation improves antitumor effect of immune checkpoint inhibitor in murine hepatocellular carcinoma model. Oncotarget 2017;8:41242–41255. doi: 10.18632/oncotarget.17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Young KH Baird JR Savage T Cottam B Friedman D Bambina S, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One 2016;11:e157164. doi: 10.1371/journal.pone.0157164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith WH Law AS Hulkower M McGee HM Lehrer EJ Schwartz M, et al. The effect of radiation therapy on the objective response and outcomes with nivolumab for hepatocellular carcinoma. Acta Oncol 2020;59:940–943. doi: 10.1080/0284186X.2020.1769860. [DOI] [PubMed] [Google Scholar]
- 21.Chiang CL, Chan A, Chiu K, Kong FS. Combined stereotactic body radiotherapy and checkpoint inhibition in unresectable hepatocellular carcinoma: A potential synergistic treatment strategy. Front Oncol 2019;9:1157. doi: 10.3389/fonc.2019.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan C Ruohoniemi D Shanbhogue KP Wei J Welling TH Gu P, et al. Safety of combined yttrium-90 radioembolization and immune checkpoint inhibitor immunotherapy for hepatocellular carcinoma. J Vascular Interventional Radiol 2020;31:25–34. doi: 10.1016/j.jvir.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 23.Wang FH Zhang XT Li YF Tang L Qu XJ Ying JE, et al. The Chinese Society of Clinical Oncology (CSCO): Clinical guidelines for the diagnosis and treatment of gastric cancer, 2021. Cancer Commun (Lond) 2021;41:747–795. doi: 10.1002/cac2.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Timmerman R. A Story of hypofractionation and the table on the wall. Int J Radiat Oncol Biol Phys 2022;112:4–21. doi: 10.1016/j.ijrobp.2021.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz LH Litière S de Vries E Ford R Gwyther S Mandrekar S, et al. RECIST 1.1-update and clarification: From the RECIST committee. Eur J Cancer 2016;62:132–137. doi: 10.1016/j.ejca.2016.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairchild AT, Tanksley JP, Tenenbaum JD, Palta M, Hong JC. Interrater reliability in toxicity identification: Limitations of current standards. Int J Radiat Oncol Biol Phys 2020;107:996–1000. doi: 10.1016/j.ijrobp.2020.04.040. [DOI] [PubMed] [Google Scholar]
- 27.Formenti SC Rudqvist NP Golden E Cooper B Wennerberg E Lhuillier C, et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med 2018;24:1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Demaria S Ng B Devitt ML Babb JS Kawashima N Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 29.Theelen WSME Chen D Verma V Hobbs BP Peulen HMU Aerts JGJV, et al. Pembrolizumab with or without radiotherapy for metastatic non-small-cell lung cancer: A pooled analysis of two randomised trials. Lancet Respir Med 2021;9:467–475. doi: 10.1016/S2213-2600(20)30391-X. [DOI] [PubMed] [Google Scholar]
- 30.Menon H Chen D Ramapriyan R Verma V Barsoumian HB Cushman TR, et al. Influence of low-dose radiation on abscopal responses in patients receiving high-dose radiation and immunotherapy. J Immunother Cancer. 2019;7:237. doi: 10.1186/s40425-019-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klug F Prakash H Huber PE Seibel T Bender N Halama N, et al. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 32.Carozza JA Böhnert V Nguyen KC Skariah G Shaw KE Brown JA, et al. Extracellular cGAMP is a cancer cell-produced immunotransmitter involved in radiation-induced anti-cancer immunity. Nat Cancer 2020;1:184–196. doi: 10.1038/s43018-020-0028-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menon H Ramapriyan R Cushman TR Verma V Kim HH Schoenhals JE, et al. Role of radiation therapy in modulation of the tumor stroma and microenvironment. Front Immunol 2019;10:193. doi: 10.3389/fimmu.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanpouille-Box C Alard A Aryankalayil MJ Sarfraz Y Diamond JM Schneider RJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuo Y Yoshida K Nishimura H Ejima Y Miyawaki D Uezono H, et al. Efficacy of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein tumor thrombosis/inferior vena cava tumor thrombosis: Evaluation by comparison with conventional three-dimensional conformal radiotherapy. J Radiat Res 2016;57:512–523. doi: 10.1093/jrr/rrw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang JF Lo CH Lee MS Lin CS Dai YH Shen PC, et al. Stereotactic ablative radiotherapy versus conventionally fractionated radiotherapy in the treatment of hepatocellular carcinoma with portal vein invasion: A retrospective analysis. Radiat Oncol 2019;14:180. doi: 10.1186/s13014-019-1382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaue D Comin-Anduix B Ribas A Zhang L Goodglick L Sayre JW, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res 2008;14:4883–4890. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian JM, Yu JB, Kluger HM, Chiang VL. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016;122:3051–3058. doi: 10.1002/cncr.30138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gray JE Villegas A Daniel D Vicente D Murakami S Hui R, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage III NSCLC-update from PACIFIC. J Thorac Oncol 2020;15:288–293. doi: 10.1016/j.jtho.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dewan MZ Galloway AE Kawashima N Dewyngaert JK Babb JS Formenti SC, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379–5388. doi: 10.1158/1078-0432.CCR-09-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys 2012;83:1306–1310. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demaria S, Formenti SC. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front Oncol 2012;2:153. doi: 10.3389/fonc.2012.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaverdian N Lisberg AE Bornazyan K Veruttipong D Goldman JW Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barker CA Postow MA Khan SA Beal K Parhar PK Yamada Y, et al. Concurrent radiotherapy and ipilimumab immunotherapy for patients with melanoma. Cancer Immunol Res 2013;1:92–98. doi: 10.1158/2326-6066.CIR-13-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seung SK Curti BD Crittenden M Walker E Coffey T Siebert JC, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2 – Tumor and immunological responses. Sci Transl Med 2012;4:137ra74. doi: 10.1126/scitranslmed.3003649. [DOI] [PubMed] [Google Scholar]
- 46.Hubbeling HG Schapira EF Horick NK Goodwin KEH Lin JJ Oh KS, et al. Safety of combined PD-1 pathway inhibition and intracranial radiation therapy in non-small cell lung cancer. J Thorac Oncol 2018;13:550–558. doi: 10.1016/j.jtho.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 47.Luke JJ Lemons JM Karrison TG Pitroda SP Melotek JM Zha Y, et al. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol 2018;36:1611–1618. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsu SJ Chen Y Yang P Hu Y Chen R Zeng Z, et al. Radiotherapy enhance the immune checkpoint inhibitors efficacy in advanced liver cancer. Int J Radiat Oncol Biol Phys 2022;114:S105. doi: 10.1016/j.ijrobp.2022.07.532. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.