Abstract
Purpose
Stroke-associated pneumonia (SAP) usually complicates stroke and is linked to adverse prognoses. Triglycerides, total cholesterol, and body weight index (TCBI) is a new and simple calculated nutrition index. This study seeks to investigate the association between TCBI and SAP incidence, along with its predictive value.
Patients and Methods
Nine hundred and sixty-two patients with acute ischemic stroke were divided into SAP group and Non-SAP group. The TCBI was divided into three layers: T1, TCBI < 948.33; T2, TCBI 948.33–1647.15; T3, TCBI > 1647.15. Binary Logistic regression analysis was used to determine the relationship between TCBI levels and the incidence of SAP. Furthermore, restricted cubic splines (RCS) analysis was utilized to evaluate the influence of TCBI on the risk of SAP.
Results
TCBI in the SAP group was markedly lower compared to that in the Non-SAP group (P < 0.001). The Logistic regression model revealed that, using T3 layer as the reference, T1 layer had the highest risk for SAP prevalence (OR = 2.962, 95% CI: 1.600–5.485, P = 0.001), with confounding factors being controlled. The RCS model found that TCBI had a linear relationship with SAP (P for nonlinear = 0.490, P for overall = 0.004). Moreover, incorporating TCBI into the A2DS2 (Age, atrial fibrillation, dysphagia, sex, and severity) model substantially enhanced the initial model’s predictive accuracy.
Conclusion
Low TCBI was associated with a higher risk of SAP. In clinical practice, TCBI has shown predictive value for SAP, contributing to early intervention and treatment of SAP.
Keywords: acute ischemic stroke, stroke-associated pneumonia, nutritional status, TCBI
Introduction
Stroke stands as a primary global cause of mortality and chronic disability, with clinical research indicating that the majority of stroke-related fatalities are not directly due to the stroke itself, but rather its complications. Stroke-associated pneumonia (SAP) constitutes a frequent complication among acute ischemic stroke (AIS) patients, with an incidence rate ranging from 7% to 38%.1,2 SAP detrimentally impacts patient neurological recovery, significantly alters prognosis, and is a major mortality factor in stroke cases.3,4 Research has associated factors such as age, dysphagia, severity of stroke, inflammatory response, and stroke-induced immunosuppressive syndrome with an increased risk of SAP.5,6 The early detection of potential patients holds clinical significance for implementing surveillance and prevention measures. However, relying on a single biomarker presents limited predictive capability regarding disease outcome. Several risk stratification scoring systems, including the Friedant pneumonia prediction score, A2DS2 (Age, atrial fibrillation, dysphagia, sex, and severity) score, Kwon pneumonia score, and Preventive Antibiotics in Stroke Study pneumonia Rule, etc.,7–9 have been developed to forecast the incidence of SAP events. The A2DS2 score, determined by factors including age, dysphagia, gender, atrial fibrillation, and stroke severity, is the prevalent risk assessment tool in clinical settings, corroborated across various external cohorts.10–12
Studies have shown a correlation between nutritional status and stroke-related adverse outcomes and complications.13,14 Patients exhibiting malnutrition at baseline exhibited a higher propensity for developing SAP.15 Therefore, insights into malnutrition assessment could facilitate the early detection of AIS patients with a high risk of SAP. TCBI (triglycerides, total cholesterol, and body weight index) is a new simple calculated nutritional index,16 encompassing serum triglycerides (TG), total cholesterol (TC), and body weight (BW). Previous research has demonstrated that TCBI serves as both a nutritional and prognostic marker in patients suffering from coronary artery disease, acute decompensated heart failure, and hemodynamic instability within intensive care settings.16–18 In the Chinese population with H-type hypertension, there exists a negative correlation between TCBI and stroke incidence.19 In patients with AIS, TCBI has been found to be associated with adverse outcomes.20
Currently, research on the connection between TCBI and SAP is still lacking. Therefore, this study hypothesizes that a lower TCBI is associated with an increased incidence of SAP events in patients with AIS. If so, we will evaluate whether TCBI is valuable in predicting SAP incidence and improving the predictive effectiveness of traditional A2DS2 model.
Materials and Methods
Study Design and Population
Patients with AIS who were admitted to the Department of Neurology at the Affiliated Huai’an Hospital of Xuzhou Medical University between March 2021 and May 2022 were prospectively enrolled in the study. This study was conducted in strict accordance with the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Affiliated Huai’an Hospital of Xuzhou Medical University (Approval number: HEYLL202137). Written informed consent was obtained from each participant.
The inclusion criteria were as follows: (1) Confirmed by computed tomography or magnetic resonance imaging to meet the diagnostic criteria for AIS; (2) The time from onset to admission is less than 7 days; (3) Age ≥ 18 years old.
The exclusion criteria were as follows: (1) Transient ischemic attack; (2) Active infection or prophylactic use of antibiotics within 2 weeks prior to admission; (3) Combined with a history of central nervous system diseases, such as brain trauma, cerebral hemorrhage or hydrocephalus; (4) The presence of swallowing dysfunction before stroke; (5) Patients with severe liver and kidney dysfunction (Child-Pugh C or serum creatinine >177 umol/L); (6) Patients with diseases of the blood system, cancer or receiving immunosuppressive therapy; (7) Clinical data are incomplete.
Data Collection
General data of stroke patients were collected, including demographic characteristics: gender, age, height and weight. Previous medical histories include: hypertension, diabetes, coronary heart disease, atrial fibrillation, smoking, drinking and a history of stroke. Stroke-related data: National Institutes of Health Stroke Scale (NIHSS) at admission and stroke subtypes (based on TOAST classification). Treatment received during hospitalization: endovascular treatment, intravenous thrombolysis therapy and drug use. Laboratory data include: hemoglobin, albumin, total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). Swallowing function data collection: The swallowing function of patients was evaluated by modified water swallowing test within 24 hours after admission. A2DS2 scoring model score collection: This is a model for predicting clinical risk of SAP based on factors such as age, dysphagia, male, atrial fibrillation, and stroke severity (NIHSS) on a scale of 0 to 10. The A2DS2 score has been validated in multiple external cohorts.10–12
Diagnosis of SAP
According to the revised standards of the Centers for Disease Control and Prevention, patients diagnosed with SAP are those who experience lower respiratory tract infections within the first seven days following an acute stroke.21
Assessment of TCBI
Fasting blood samples were taken within 24 hours after admission for TG, TC and other indicators. The formula includes TG, TC and BW, TCBI = TG (mg/dL) × TC (mg/dL) × BW (kg)/1000.16
Statistical Analysis
Data conforming to normal distribution were expressed as mean ± standard deviation (SD), two independent sample t-test was used for inter-group comparison, median and quartile [M (P25-P75)] description was used for non-normal distribution data, and non-parametric rank sum test (Mann–Whitney U-test) was used for inter-group comparison. Count data were represented as frequency and percentage (n,%), with the Pearson Chi-square test applied for group comparisons. To assess differences among multiple groups, we conducted either a one-way ANOVA or the Kruskal–Wallis test for parametric and non-parametric data, respectively. Pairwise comparisons were performed on variables that differed between groups, with P-values adjusted using the Bonferroni correction method. Due to the severely skewed distribution of TCBI, Log transformation was used in part of the statistical analysis. According to the sample size of this study and TCBI, the patients were divided into three groups on average (Tertile 1 < 948.33, Tertile 2948.33–1647.15, Tertile 3 > 1647.15). A Binary Logistic regression model was used to examine the relationship between TCBI and SAP. Variables with P less than 0.05 in univariate logistic regression analysis were included in multivariate regression analysis. Adjusted variables included age, coronary heart disease, atrial fibrillation, TOAST classification, NIHSS at admission, dysphagia, endovascular treatment, anticoagulant drug use, hemoglobin and albumin. Restricted cubic spline models were used to provide and explore the shape of the association between TCBI and SAP, fitting a restricted cubic spline function with 4 knots (at the 5th, 35th, 65th, and 95th percentiles). The Receiver operating characteristic (ROC) curve was used to estimate the optimal threshold for biomarkers to predict the occurrence of SAP in AIS patients. Area Under the Receiver Operating Characteristic Curve (AUROC), sensitivity, specificity, positive prediction rate and negative prediction rate were calculated. The optimal threshold was identified using the Youden index. The AUROC efficacy of TCBI was compared with other biomarker values by Z-test.
SPSS 25.0 (IBM Corporation, IL, USA), MedCalc 20.0 (MedCalc Software LTD., Ostend, Belgium), R Programming Language 4.3.2 (Vienna, Austria), and GraphPad Prism 9 (GraphPad Software, USA) were used for all study data analysis and image processing. All P values were two-tailed, and statistical significance was defined by the P value < 0.05.
Results
Baseline Characteristics
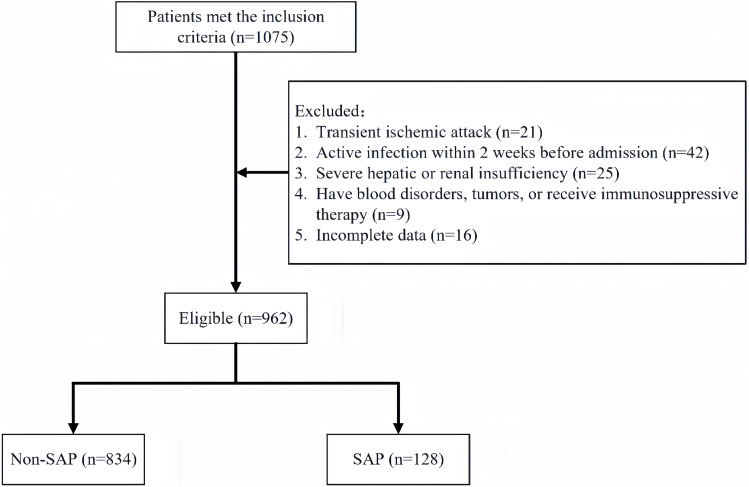
This study screened 1075 AIS patients hospitalized within seven days of symptom onset for analysis. Exclusions were 21 patients with transient ischemic attacks, 42 with fever or active infections pre-admission, 25 with severe hepatic and renal insufficiency, 9 with hematological diseases and tumors, and 16 with incomplete data. Ultimately, 962 patients were enrolled, with 128 developing SAP, corresponding to an incidence rate of 13.3% (Figure 1). Of the total cohort, 369 (38.4%) were females. The median age was 68 (interquartile range, 59–77). The median TCBI was 1259.03 (interquartile range, 812.18 to 1942.30).
Figure 1.
Patient flowchart.
Abbreviations: SAP, Stroke-associated pneumonia.
As shown in Table 1, patients in the SAP group were older, had a lighter body weight, had higher NIHSS at admission, had higher rates of coronary heart disease, atrial fibrillation, cardioembolism and dysphagia, and were more likely to receive endovascular treatment and anticoagulant drug use. Compared with Non-SAP group, SAP group patients had lower levels of hemoglobin, albumin, TC and TG, and higher A2DS2 score. The TCBI in SAP group was significantly lower than that in Non-SAP group (All P < 0.05). In this study, there were no significant differences between the two groups in gender, height, hypertension, diabetes, smoking, drinking, history of stroke, intravenous thrombolysis, antiplatelet drug use, lipid-lowering drug use, LDL-C and HDL-C (All P > 0.05) (Table 1).
Table 1.
Comparison of Baseline Data Between SAP Group and Non-SAP Group
| Variables | All Patients n = 962 | Non-SAP n = 834 | SAP n = 128 | P value |
|---|---|---|---|---|
| Demographic information | ||||
| Female, n (%) | 369 (38.4) | 316 (37.9) | 53 (41.4) | 0.446 |
| Age (years), median (IQR) | 68 (59–77) | 67 (58–76) | 73 (64–81) | <0.001 |
| Height (m), mean ± SD | 1.65±0.08 | 1.65±0.08 | 1.65±0.07 | 0.488 |
| Body weight (kg), mean ± SD | 68.08±11.22 | 68.73±11.18 | 63.88±10.61 | <0.001 |
| Hypertension, n (%) | 795 (82.6) | 691 (82.9) | 104 (81.3) | 0.656 |
| Diabetes, n (%) | 331 (34.4) | 285 (34.2) | 46 (35.9) | 0.696 |
| Coronary heart disease, n (%) | 144 (15.0) | 117 (14.0) | 27 (21.1) | 0.037 |
| Atrial fibrillation, n (%) | 99 (10.3) | 70 (8.4) | 29 (22.7) | <0.001 |
| Smoking, n (%) | 233 (24.2) | 199 (23.9) | 34 (26.6) | 0.507 |
| Drinking, n (%) | 177 (18.4) | 157 (18.8) | 20 (15.6) | 0.384 |
| History of stroke, n (%) | 178 (18.5) | 148 (17.7) | 30 (23.4) | 0.123 |
| Clinical features | ||||
| TOAST classification, n (%) | <0.001 | |||
| LAA | 636 (66.1) | 545 (65.3) | 91 (71.1) | |
| CE | 100 (10.4) | 74 (8.9) | 26 (20.3) | |
| SAO | 226 (23.5) | 215 (25.8) | 11 (8.6) | |
| NIHSS at admission (score), median (IQR) | 2 (1–5) | 2 (1–4) | 9 (3–17) | <0.001 |
| Dysphagia, n (%) | 135 (14.0) | 87 (10.4) | 48 (37.5) | <0.001 |
| Treatment, n (%) | ||||
| Endovascular treatment | 55 (5.7) | 24 (2.9) | 31 (24.2) | <0.001 |
| Intravenous thrombolysis | 127 (13.2) | 105 (12.6) | 22 (17.2) | 0.153 |
| Antiplatelet drug use | 884 (91.9) | 771 (92.4) | 113 (88.3) | 0.108 |
| Anticoagulant drug use | 116 (12.1) | 91 (10.9) | 25 (19.5) | 0.005 |
| Lipid-lowering drug use | 946 (98.3) | 821 (98.4) | 125 (97.7) | 0.518 |
| Laboratory data | ||||
| Hemoglobin (g/L), median (IQR) | 141 (129–153) | 142 (130–153) | 137 (122–148) | 0.002 |
| Albumin (g/L), mean ± SD | 39.55±4.28 | 39.78±4.19 | 38.07±4.60 | <0.001 |
| TC (mmol/L), median (IQR) | 4.36 (3.61–5.02) | 4.40 (3.67–5.06) | 4.10 (3.31–4.69) | <0.001 |
| TG (mmol/L), median (IQR) | 1.25 (0.94–1.78) | 1.31 (0.95–1.84) | 1.08 (0.82–1.37) | <0.001 |
| LDL-C (mmol/L), mean ± SD | 2.66±0.89 | 2.68±0.87 | 2.53±1.04 | 0.076 |
| HDL-C (mmol/L), median (IQR) | 1.12 (1.02–1.32) | 1.12 (1.02–1.32) | 1.13 (1.02–1.36) | 0.761 |
| TCBI, median (IQR) | 1259.03 (812.18–1942.30) | 1322.76 (859.93–2046.42) | 898.54 (606.86–1396.16) | <0.001 |
| A2DS2 (score), median (IQR) | 1 (1–3) | 1 (1–2) | 5 (3–7) | <0.001 |
Abbreviations: SD, standard deviation; IQR, interquartile range; SAP, Stroke-associated pneumonia; LAA, Large artery atherosclerosis; CE, Cardioembolism; SAO, Small-artery occlusion; NIHSS, National Institutes of Health Stroke Scale; TC, Total cholesterol; TG, Triglycerides; LDL-C, Low-density lipoprotein cholesterol; HDL-C, High-density lipoprotein cholesterol; TCBI, Triglycerides, total cholesterol, and body weight index.
Association between TCBI and stroke-Associated Pneumonia
According to the quantile of TCBI, all patients were divided into three groups: Tertile 1 < 948.33, Tertile 2948.33–1647.15, and Tertile 3 > 1647.15. In the different TCBI level groups, there was a significant difference in the proportion of SAP(P < 0.001) (Table 2). In addition, Table 2 shows significant differences in age, height, body weight, hypertension, coronary heart disease, atrial fibrillation, TOAST classification, NIHSS at admission, endovascular treatment, intravenous thrombolysis, antiplatelet drug use, anticoagulant drug use, hemoglobin, albumin, TC, TG, LDL-C, HDL-C, and A2DS2 score among the three groups (All P < 0.05) (Table 2).
Table 2.
Baseline Characteristics of Patients According to TCBI Tertiles
| Variables | TCBI Tertiles | P value | ||
|---|---|---|---|---|
| T1 (<948.33) n=320 | T2 (948.33–1647.15) n=321 | T3 (>1647.15) n=321 | ||
| Demographic information | ||||
| Female, n (%) | 134 (41.9) | 124 (38.6) | 111 (34.6) | 0.163 |
| Age (years), median (IQR) | 73 (64–81) | 66 (59–76) | 64 (55–72) | <0.001 |
| Height (m), mean ± SD | 1.64±0.08 | 1.65±0.08 | 1.66±0.07 | <0.001 |
| Body weight (kg), mean ± SD | 63.50±9.72 | 68.73±11.02 | 72.01±11.21 | <0.001 |
| Hypertension, n (%) | 251 (78.4) | 267 (83.2) | 277 (86.3) | 0.030 |
| Diabetes, n (%) | 99 (30.9) | 108 (33.6) | 124 (38.6) | 0.115 |
| Coronary heart disease, n (%) | 68 (21.3) | 51 (15.9) | 25 (7.8) | <0.001 |
| Atrial fibrillation, n (%) | 59 (18.4) | 25 (7.8) | 15 (4.7) | <0.001 |
| Smoking, n (%) | 69 (21.6) | 84 (26.2) | 80 (24.9) | 0.371 |
| Drinking, n (%) | 49 (15.3) | 65 (20.2) | 63 (19.6) | 0.214 |
| History of stroke, n (%) | 67 (20.9) | 54 (16.8) | 57 (17.8) | 0.372 |
| Clinical features | ||||
| TOAST classification, n (%) | <0.001 | |||
| LAA | 201 (62.8) | 206 (64.2) | 229 (71.3) | |
| CE | 56 (17.5) | 24 (7.5) | 20 (6.2) | |
| SAO | 63 (19.7) | 91 (28.3) | 72 (22.4) | |
| NIHSS at admission (score), median (IQR) | 3 (1–7) | 2 (1–4) | 2 (1–4) | 0.001 |
| Dysphagia, n (%) | 49 (15.3) | 41 (12.8) | 45 (14.0) | 0.651 |
| Treatment, n (%) | ||||
| Endovascular treatment | 24 (7.5) | 23 (7.2) | 8 (2.5) | 0.009 |
| Intravenous thrombolysis | 49 (15.3) | 28 (8.7) | 50 (15.6) | 0.015 |
| Antiplatelet drug use | 275 (85.9) | 302 (94.1) | 307 (95.6) | <0.001 |
| Anticoagulant drug use | 61 (19.1) | 32 (10.0) | 23 (7.2) | <0.001 |
| Lipid-lowering drug use | 316 (98.8) | 314 (97.8) | 316 (98.4) | 0.644 |
| Laboratory data | ||||
| Hemoglobin (g/L), median (IQR) | 135 (121–147) | 142 (129.5–155) | 145 (135–156) | <0.001 |
| Albumin (g/L), mean ± SD | 38.70±4.46 | 39.81±4.16 | 40.14±4.09 | <0.001 |
| TC (mmol/L), median (IQR) | 3.60 (3.04–4.20) | 4.36 (3.73–4.97) | 5.00 (4.49–5.67) | <0.001 |
| TG (mmol/L), median (IQR) | 0.84 (0.71–1.02) | 1.24 (1.10–1.50) | 2.04 (1.68–2.86) | <0.001 |
| LDL-C (mmol/L), mean ± SD | 2.20 (1.62–2.64) | 2.70 (2.20–3.19) | 3.02 (2.63–3.64) | <0.001 |
| HDL-C (mmol/L), median (IQR) | 1.16 (1.05–1.38) | 1.14 (1.02–1.35) | 1.07 (0.99–1.22) | <0.001 |
| A2DS2 (score), median (IQR) | 2 (1–4) | 1 (1–3) | 1 (1–3) | <0.001 |
| SAP, n (%) | 69 (21.6) | 39 (12.1) | 20 (6.2) | <0.001 |
Abbreviations: SD, standard deviation; IQR, interquartile range; TCBI, Triglycerides, total cholesterol, and body weight index; LAA, Large artery atherosclerosis; CE, Cardioembolism; SAO, Small-artery occlusion; NIHSS, National Institutes of Health Stroke Scale; TC, Total cholesterol; TG, Triglycerides; LDL-C, Low-density lipoprotein cholesterol; HDL-C, High-density lipoprotein cholesterol; SAP, Stroke-associated pneumonia.
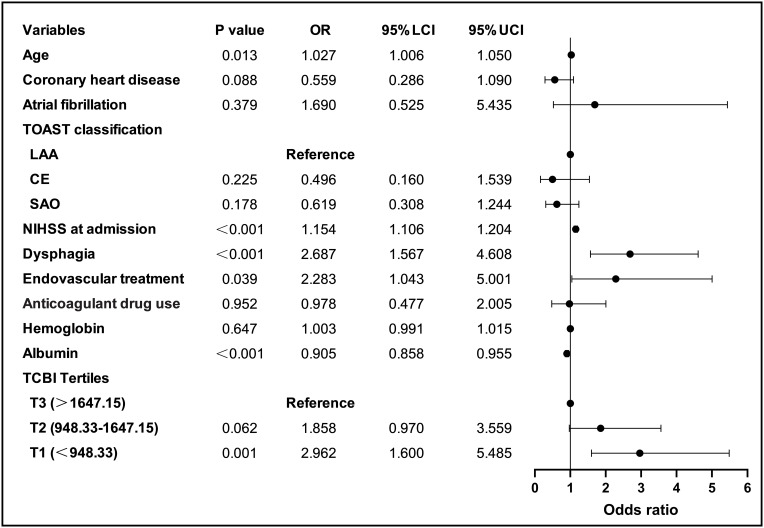
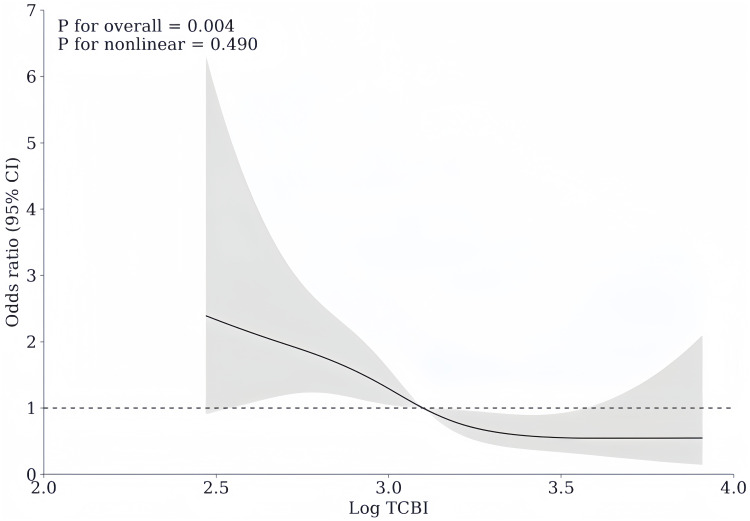
As indicated in the forest plot in Figure 2, with the highest tertile serving as the reference after adjusting for the confounders such as age, coronary heart disease, atrial fibrillation, TOAST classification, NIHSS at admission, dysphagia, endovascular treatment, anticoagulant drug use, hemoglobin and albumin, the lowest tertile of TCBI (<948.33) was substantially related to a greater risk of SAP (odds ratio [OR] = 2.962, 95% confidence interval [CI]: 1.600–5.485, P = 0.001), while the middle tertile of TCBI was not significantly associated with the prevalence of SAP (OR = 1.858, 95% CI = 0.970–3.559, P = 0.062). When taken as a continuous variable, TCBI was still related to SAP in the logistic regression analysis, with the confounders being controlled (unadjusted: OR = 0.127, 95% CI = 0.062–0.260, P < 0.001; adjusted: OR = 0.223, 95% CI = 0.094–0.528, P = 0.001). Figure 2 also showed that age, NIHSS at admission, dysphagia, endovascular treatment, and albumin were also independent predictors of SAP (All P < 0.05) (Figure 2). In addition, the RCS curve revealed a linear relationship between TCBI and SAP, after adjusting for confounding variables (P for nonlinear = 0.490, P for overall = 0.004) (Figure 3).
Figure 2.
Forest plot of odds ratios for SAP.
Abbreviations: LAA, Large artery atherosclerosis; CE, Cardioembolism; SAO, Small-artery occlusion; NIHSS, National Institutes of Health Stroke Scale; TCBI, Triglycerides, total cholesterol, and body weight index.
Figure 3.
Association of TCBI with risk of stroke-associated pneumonia. Odds ratios and 95% confidence intervals derived from restricted cubic spline regression, with knots placed at the 5th, 35th, 65th, and 95th percentiles of the distribution of Log TCBI. The reference point is the median of Log TCBI. Odds ratios were adjusted for the same variables as model in. Figure 2.
Predictive Value of TCBI for Stroke-Associated Pneumonia
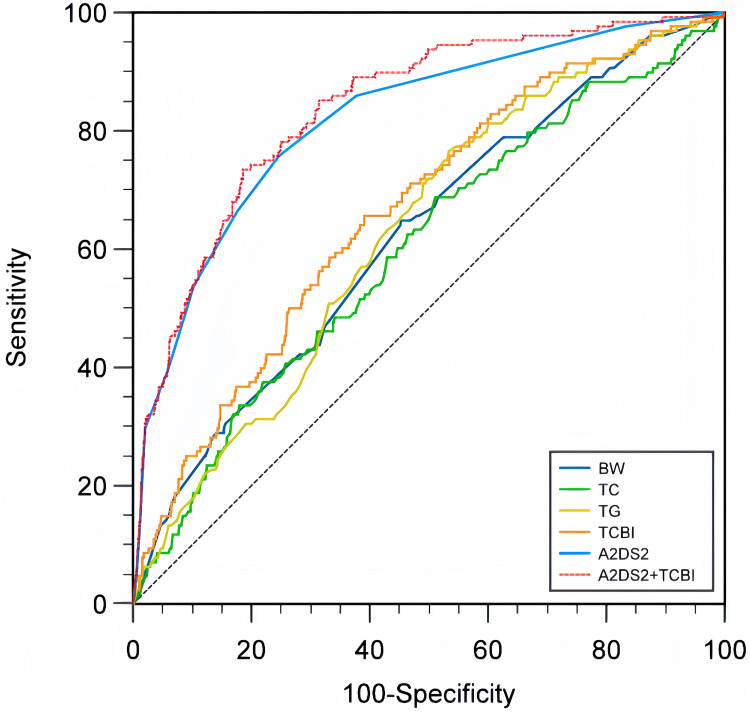
Results from the ROC analysis indicated that the optimal cutoff value of TCBI for predicting SAP was 1112.37, with an AUROC of 0.664 (95% CI = 0.633–0.694). The sensitivity and specificity were 65.62% and 60.91%, respectively. The positive predictive rate and the negative predictive rate were 20.49% and 92.03%, respectively. Compared to other biomarkers, TCBI demonstrated superior predictive capabilities for SAP over TC (AUROC = 0.599, 95% CI = 0.568–0.630; TCBI vs.TC, P = 0.002) and TG (AUROC = 0.628, 95% CI = 0.597–0.659; TCBI vs. TG, P = 0.003). However, there was no significant difference when compared with BW (AUROC = 0.623, 95% CI = 0.591–0.654; TCBI vs. BW, P = 0.206) (Table 3 and Figure 4).
Table 3.
Comparison of Predictive Power of TCBI Vs Other Indicators in the Prediction of SAP
| Variables | AUROC (95% CI) | Cutoff Value | Youden Index | Sensibility (%) | Specificity (%) | Positive Prediction Rate (%) | Negative Prediction Rate (%) | P value |
|---|---|---|---|---|---|---|---|---|
| TCBI | 0.664 (0.633–0.694) | 1112.37 | 0.2654 | 65.62 | 60.91 | 20.49 | 92.03 | Reference |
| BW | 0.623 (0.591–0.654) | 65.30 | 0.1964 | 64.84 | 54.80 | 18.04 | 91.04 | 0.206 |
| TC | 0.599 (0.568–0.630) | 4.42 | 0.1767 | 68.75 | 48.92 | 17.05 | 89.85 | 0.002 |
| TG | 0.628 (0.597–0.659) | 1.37 | 0.2321 | 76.56 | 46.64 | 18.05 | 92.84 | 0.003 |
| A2DS2 | 0.819 (0.794–0.843) | 2 | 0.5108 | 75.78 | 75.30 | 32.01 | 95.30 | <0.001 |
| A2DS2+TCBI | 0.838 (0.813–0.860) | 0.1418 | 0.5485 | 73.44 | 81.41 | 37.75 | 95.23 | <0.001 |
Abbreviations: SAP, stroke-associated pneumonia; AUROC, Area Under the Receiver Operating Characteristic Curve; TCBI, Triglycerides, total cholesterol, and body weight index; BW, Body weight; TC, Total cholesterol; TG, Triglyceride.
Figure 4.
Comparison of AUROC of TCBI with other biomarkers for predicting SAP.
Abbreviations: BW, Body weight; TC, Total cholesterol; TG, Triglycerides; TCBI, Triglycerides, cholesterol, body weight index; SAP, Stroke-associated pneumonia.
The AUROC of A2DS2 model was 0.819 (95% CI = 0.794–0.843). The modified A2DS2 model combining A2DS2 model with TCBI has an AUROC of 0.838 (95% CI = 0.813–0.860). The difference in prediction efficiency between the modified A2DS2 model and the original A2DS2 model was statistically significant (P = 0.004) (Table 3 and Figure 4).
Discussion
To our knowledge, this study is the first to investigate the relationship between TCBI and SAP. Following adjustment for potential confounders, results indicated that lower baseline TCBI levels were associated with an increased risk of SAP occurrence. There was a linear dose–response relationship between TCBI and SAP. Additionally, our results again confirmed the predictive efficacy of the A2DS2 model for SAP. Moreover, incorporating TCBI with the conventional A2DS2 model significantly enhanced its accuracy, suggesting TCBI’s potential as a valuable biomarker for SAP in clinical settings.
Prior research indicates a high prevalence of malnutrition among stroke patients. Individuals with AIS presenting normal nutritional levels can still exhibit malnutrition due to factors such as advanced age, dysphagia, immunosuppression, cognitive impairments, and restricted mobility. Additionally, acute-phase malnutrition is believed to correlate with a diminished functional prognosis and an elevated mortality rate from all causes.22 New evidence shows that initiating enteral nutrition early and integrating probiotic therapy enhances nutritional status, diminishes systemic inflammation, bolsters immune function, and decreases the incidence of infectious complications among stroke patients.23 Hence, assessing nutritional status suitably is crucial for effective risk stratification and treatment planning. Several nutritional assessment instruments, such as the Nutritional Risk Screening 2002 and the Malnutrition Universal Screening Tool, have been employed to identify malnutrition in AIS patients.24 These indicators consist of subjective parameters, for instance, recent weight changes, which are arbitrary. In addition, certain AIS patients manifesting symptoms like decreased consciousness and aphasia are unable to participate in scale assessments, rendering these instruments unsuitable for universal application in the AIS population. Consequently, there is a necessity for more objective and broadly applicable methodologies to evaluate patients’ nutritional status.
The TCBI is a novel and simple nutritional tool that reflects the intrinsic metabolism of fat, cholesterol, carbohydrates, and sugars, primarily by simple multiplication of body weight and variables that reflect lipid metabolism.17 The TCBI was initially developed to evaluate the nutritional status of individuals afflicted with atherosclerotic cardiovascular disease. It has been observed that populations suffering from coronary artery disease with lower TCBI scores experience increased rates of all-cause mortality, cardiovascular mortality, and cancer mortality.16 Recent studies indicate that TCBI could serve as a significant prognostic nutrition-related marker in critically ill cardiac patients needing mechanical circulatory support, experiencing acute decompensated heart failure, suffering from acute myocardial infarction, or undergoing transcatheter aortic valve replacement.17,18,25,26
In a Chinese cohort with H-type hypertension, a higher stroke incidence was observed among individuals with low TCBI levels. Multivariable-adjusted analyses indicated a negative association between TCBI and stroke prevalence.19 This may be attributed to the fact that malnutrition can induce inflammation and oxidative stress.27,28 An investigation involving 9708 participants indicates that TCBI levels correlate with both short-term and long-term functional disability, as well as all-cause mortality, following a stroke.20 Despite the growing interest, research on the association between TCBI and stroke remains sparse. Consequently, we initiated a prospective study to assess the relationship between TCBI and SAP. The findings supported our preliminary hypothesis: patients exhibiting lower TCBI levels exhibited a higher propensity for developing SAP, a relationship that persisted as significant even after adjusting for potential confounders. Numerous investigations have demonstrated a correlation between early malnutrition and an elevated risk of SAP. For the prompt identification of patients at high risk, objective nutritional assessment tools, including the Geriatric Nutrition Risk Index (GNRI), the Controlling Nutritional Status (CONUT), and the Prognostic Nutritional Index (PNI), are recommended.15,29 However, these three scoring indicators are not widely used in clinical practice due to the complexity of calculation methods and parameters. The advantages of TCBI are easy to obtain, simple to calculate, objective and unrestricted in AIS population. Furthermore, TCBI showed significant predictive value for SAP. Our findings support the use of TCBI as a simple nutritional marker for the surveillance of SAP occurrences.
The underlying mechanism between lower TCBI and increased SAP risk is not fully understood. Weight loss is regarded as a risk factor for SAP.6 Body weight and body mass index (BMI) commonly quantify muscle and fat mass, and high BMI (overweight or obese) has been well-acknowledged as a risk factor for AIS.30 Nevertheless, research has demonstrated a linear correlation between elevated BMI and improved survival rates in AIS patients,31,32 a phenomenon termed the “obesity paradox”. Therefore, relying solely on body weight or BMI to forecast adverse stroke outcomes is contentious. Circulating lipid levels are indicators of nutritional status and inflammation, TG and TC can reflect the nutritional status of patients to a certain extent.33,34 Prior research indicates an association between dual X-ray absorptiometry (the gold standard for assessing nutritional status) and GNRI.35,36 Furthermore, multiple evidence suggests a moderately positive correlation between TCBI and GNRI,16–18 indicating that TCBI may serve as an indirect marker of nutritional status. Hence, interpreting the relationship between TCBI and SAP incidence through the lens of malnutrition is justifiable. The association between malnutrition and immune function is well-documented. Malnutrition may lead to reduced lymphocyte production, thereby impairing immune function,37,38 and immune dysfunction significantly contributes to the pathology of SAP events. Malnutrition may also contribute to both the development and worsening of SAP by inducing inflammation and oxidative stress.27,28 Additionally, based on the correlation between inflammatory indicators and nutritional scores, malnutrition is thought to reflect a high inflammatory and metabolic state of the body, which also contributes to the development of SAP.15
However, our study has several limitations. Firstly, considering the results from a single-center cohort, a larger sample size from prospective registries across multiple regions and countries is necessary to generalize the findings. Secondly, TCBI was only measured at admission, preventing the assessment of its dynamic changes and their correlation with SAP throughout the hospitalization period. Thirdly, the study did not include an evaluation of other nutritional indices like CONUT, GNRI, or PNI, nor did it explore the comparative relevance and applicability of TCBI against these tools. Lastly, the absence of data on functional outcomes and mortality precluded analysis of the relationship between TCBI, SAP, and stroke outcomes, which needs to be clarified in further studies.
Conclusion
In Conclusion, our study demonstrates that low TCBI was associated with a higher risk of SAP. T1 layer (TCBI < 948.33) had the highest risk for SAP prevalence. Moreover, integrating TCBI into traditional A2DS2 model significantly improves its predictive performance. TCBI measured for the first time after admission can be used for early identification of patients at high risk of SAP. Timely attention and effective intervention may improve the prognosis of patients.
Acknowledgments
The authors thank the study participants and their relatives as well as the clinical staff for their support and contributions to this study.
Funding Statement
Huai’an municipal Science and Technology Program Project (HAB202322).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kishore AK, Vail A, Chamorro A, et al. How is pneumonia diagnosed in clinical stroke research? A systematic review and meta-analysis. Stroke. 2015;46(5):1202–1209. doi: 10.1161/STROKEAHA.114.007843 [DOI] [PubMed] [Google Scholar]
- 2.Badve MS, Zhou Z, van de Beek D, et al. Frequency of post-stroke pneumonia: systematic review and meta-analysis of observational studies. Int J Stroke. 2019;14(2):125–136. doi: 10.1177/1747493018806196 [DOI] [PubMed] [Google Scholar]
- 3.de Montmollin E, Ruckly S, Schwebel C, et al. Pneumonia in acute ischemic stroke patients requiring invasive ventilation: impact on short and long-term outcomes. J Infect. 2019;79(3):220–227. doi: 10.1016/j.jinf.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zhang P, Wu S, et al. Stroke-related complications in large hemisphere infarction: incidence and influence on unfavorable outcome. Ther Adv Neurol Disord. 2019;12:1756286419873264. doi: 10.1177/1756286419873264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eltringham SA, Kilner K, Gee M, et al. Impact of dysphagia assessment and management on risk of stroke-associated pneumonia: a systematic review. Cerebrovasc Dis. 2018;46(3–4):99–107. doi: 10.1159/000492730 [DOI] [PubMed] [Google Scholar]
- 6.Patel UK, Kodumuri N, Dave M, et al. Stroke-associated pneumonia: a retrospective study of risk factors and outcomes. Neurologist. 2020;25(3):39–48. doi: 10.1097/NRL.0000000000000269 [DOI] [PubMed] [Google Scholar]
- 7.Gong S, Zhou Z, Zhou M, et al. Validation of risk scoring models for predicting stroke-associated pneumonia in patients with ischaemic stroke. Stroke Vasc Neurol. 2016;1(3):122–126. doi: 10.1136/svn-2016-000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishore AK, Vail A, Bray BD, et al. Clinical risk scores for predicting stroke-associated pneumonia: a systematic review. Eur Stroke J. 2016;1(2):76–84. doi: 10.1177/2396987316651759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hotter B, Hoffmann S, Ulm L, et al. External validation of five scores to predict stroke-associated pneumonia and the role of selected blood biomarkers. Stroke. 2021;52(1):325–330. doi: 10.1161/STROKEAHA.120.031884 [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann S, Malzahn U, Harms H, et al. Development of a clinical score (A2DS2) to predict pneumonia in acute ischemic stroke. Stroke. 2012;43(10):2617–2623. doi: 10.1161/STROKEAHA.112.653055 [DOI] [PubMed] [Google Scholar]
- 11.Smith CJ, Bray BD, Hoffman A, et al. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. J Am Heart Assoc. 2015;4(1):e001307. doi: 10.1161/JAHA.114.001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song X, He Y, Bai J, et al. A nomogram based on nutritional status and A(2)DS(2) score for predicting stroke-associated pneumonia in acute ischemic stroke patients with type 2 diabetes mellitus: a retrospective study. Front Nutr. 2022;9:1009041. doi: 10.3389/fnut.2022.1009041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hao R, Qi X, Xia X, et al. Malnutrition on admission increases the in-hospital mortality and length of stay in elder adults with acute ischemic stroke. J Clin Lab Anal. 2022;36(1):e24132. doi: 10.1002/jcla.24132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta A, De Paola L, Pana TA, et al. The relationship between nutritional status at the time of stroke on adverse outcomes: a systematic review and meta-analysis of prospective cohort studies. Nutr Rev. 2022;80(12):2275–2287. doi: 10.1093/nutrit/nuac034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li D, Liu Y, Jia Y, et al. Association between malnutrition and stroke-associated pneumonia in patients with ischemic stroke. BMC Neurol. 2023;23(1):290. doi: 10.1186/s12883-023-03340-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi S, Iwata H, Wada H, et al. A novel and simply calculated nutritional index serves as a useful prognostic indicator in patients with coronary artery disease. Int J Cardiol. 2018;262:92–98. doi: 10.1016/j.ijcard.2018.02.039 [DOI] [PubMed] [Google Scholar]
- 17.Minami-Takano A, Iwata H, Miyosawa K, et al. A novel nutritional index serves as a useful prognostic indicator in cardiac critical patients requiring mechanical circulatory support. Nutrients. 2019;11(6):1420. doi: 10.3390/nu11061420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishiwata S, Yatsu S, Kasai T, et al. Prognostic effect of a novel simply calculated nutritional index in acute decompensated heart failure. Nutrients. 2020;12(11):3311. doi: 10.3390/nu12113311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Wang X, Yu C, et al. Association of a novel nutritional index with stroke in Chinese population with hypertension: insight from the China H-type hypertension registry study. Front Nutr. 2023;10:997180. doi: 10.3389/fnut.2023.997180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang G, Pan Y, Zhang R, et al. A novel nutritional index and adverse outcomes in ischemic stroke: results from the third China National Stroke Registry. Nutr, Metab Cardiovasc Dis. 2022;32(6):1477–1484. doi: 10.1016/j.numecd.2022.02.015 [DOI] [PubMed] [Google Scholar]
- 21.Smith CJ, Kishore AK, Vail A, et al. Diagnosis of stroke-associated pneumonia: recommendations from the pneumonia in stroke consensus group. Stroke. 2015;46(8):2335–2340. doi: 10.1161/STROKEAHA.115.009617 [DOI] [PubMed] [Google Scholar]
- 22.Sabbouh T, Torbey MT. Malnutrition in stroke patients: risk factors, assessment, and management. Neurocrit Care. 2018;29(3):374–384. doi: 10.1007/s12028-017-0436-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Hu Y, Yuan X, et al. Effect of early enteral nutrition combined with probiotics in patients with stroke: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2022;76(4):592–603. doi: 10.1038/s41430-021-00986-3 [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Ye S, Huang X, et al. Comparing the prognostic significance of nutritional screening tools and ESPEN-DCM on 3-month and 12-month outcomes in stroke patients. Clin Nutr. 2021;40(5):3346–3353. doi: 10.1016/j.clnu.2020.11.001 [DOI] [PubMed] [Google Scholar]
- 25.Kim HR, Kang MG, Kim K, et al. Comparative analysis of three nutrition scores in predicting mortality after acute myocardial infarction. Nutrition. 2021;90:111243. doi: 10.1016/j.nut.2021.111243 [DOI] [PubMed] [Google Scholar]
- 26.Sudo M, Shamekhi J, Aksoy A, et al. A simply calculated nutritional index provides clinical implications in patients undergoing transcatheter aortic valve replacement. Clin Res Cardiol. 2023. doi: 10.1007/s00392-023-02220-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikizler TA, Cano NJ, Franch H, et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the international society of renal nutrition and metabolism. Kidney Int. 2013;84(6):1096–1107. doi: 10.1038/ki.2013.147 [DOI] [PubMed] [Google Scholar]
- 28.Ciancarelli I, Morone G, Iosa M, et al. Influence of oxidative stress and inflammation on nutritional status and neural plasticity: new perspectives on post-stroke neurorehabilitative outcome. Nutrients. 2022;15(1):108. doi: 10.3390/nu15010108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai C, Yan D, Xu M, et al. Geriatric Nutritional Risk Index is related to the risk of stroke-associated pneumonia. Brain Behav. 2022;12(8):e2718. doi: 10.1002/brb3.2718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell AB, Cole JW, McArdle PF, et al. Obesity increases risk of ischemic stroke in young adults. Stroke. 2015;46(6):1690–1692. doi: 10.1161/STROKEAHA.115.008940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim BJ, Lee SH, Jung KH, et al. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology. 2012;79(9):856–863. doi: 10.1212/WNL.0b013e318266fad1 [DOI] [PubMed] [Google Scholar]
- 32.Liu Z, Sanossian N, Starkman S, et al. Adiposity and outcome after ischemic stroke: obesity paradox for mortality and obesity parabola for favorable functional outcomes. Stroke. 2021;52(1):144–151. doi: 10.1161/STROKEAHA.119.027900 [DOI] [PubMed] [Google Scholar]
- 33.Reuben DB, Ix JH, Greendale GA, et al. The predictive value of combined hypoalbuminemia and hypocholesterolemia in high functioning community-dwelling older persons: macArthur studies of successful aging. J Am Geriatr Soc. 1999;47(4):402–406. doi: 10.1111/j.1532-5415.1999.tb07230.x [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Pereira SL, Luo M, et al. Evaluation of blood biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients. 2017;9(8):829. doi: 10.3390/nu9080829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen SC, Chung WS, Wu PY, et al. Associations among geriatric nutrition risk index, bone mineral density, body composition and handgrip strength in patients receiving hemodialysis. Nutrition. 2019;65:6–12. doi: 10.1016/j.nut.2019.02.013 [DOI] [PubMed] [Google Scholar]
- 36.Mizuiri S, Nishizawa Y, Doi T, et al. Association and predictive value of geriatric nutritional risk index, body composition, or bone mineral density in haemodialysis patients. Nephrology (Carlton). 2021;26(4):341–349. doi: 10.1111/nep.13826 [DOI] [PubMed] [Google Scholar]
- 37.Ignacio de Ulíbarri J, González-Madroño A, de Villar NG, et al. CONUT: a tool for controlling nutritional status. First validation in a hospital population. Nutr Hosp. 2005;20(1):38–45. [PubMed] [Google Scholar]
- 38.Gu A, Malahias MA, Strigelli V, et al. Preoperative malnutrition negatively correlates with postoperative wound complications and infection after total joint arthroplasty: a systematic review and meta-analysis. J Arthroplasty. 2019;34(5):1013–1024. doi: 10.1016/j.arth.2019.01.005 [DOI] [PubMed] [Google Scholar]