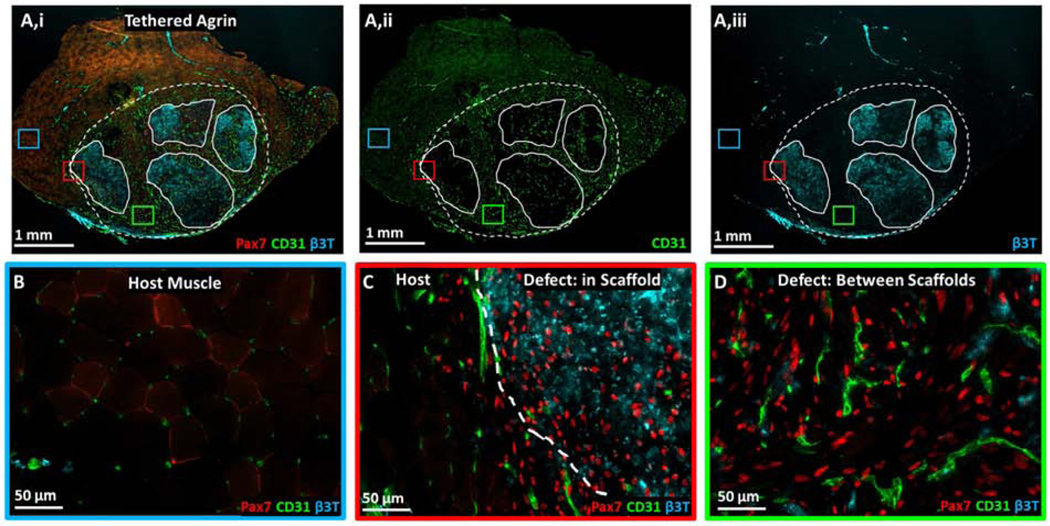
Figure 4. Muscle stem cell, vascular, and neural proteins were upregulated within and surrounding implanted tethered agrin constructs compared to host muscle.
A) Low magnification image of TA cross-section treated with tethered agrin constructs and immunostained for (i) Pax7 (red), CD31 (green), and β3-tubulin (cyan), (ii) CD31 alone, or (iii) β3-tubulin alone. Dashed line: defect region; Solid lines: undegraded scaffold regions. Insets: three regions with corresponding high magnification images demonstrating variations in protein expression in host muscle (blue inset), at the host-defect border (red inset), and within the defect region but between implanted scaffolds (green inset). B) High magnification image of host muscle with low levels of Pax7, CD31, and β3T staining. C) High magnification image of the border between host muscle and an implanted tethered-agrin scaffold within the defessct region. Increases in Pax7, CD31, and β3T expression are visible going into the defect region. D) High magnification image within the defect region but between implanted scaffolds. Pax7 and CD31 remain upregulated between scaffolds but β3T expression is decreased.