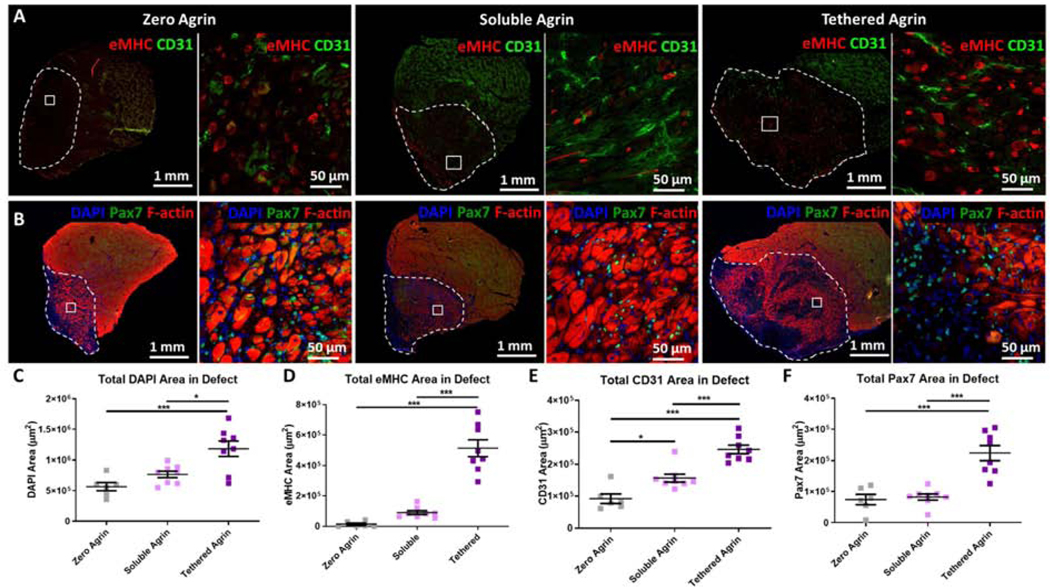
Figure 5. Tethered agrin increases the presence of regenerating myofibers, blood vessels, and satellite cells within VML defects.
A) Differences in expression within the defect site (white dashed line) between groups are visible via immunostaining for embryonic myosin (eMHC; red) and blood vessels (CD31; green). Inset: high magnification image. B) Immunostaining for satellite cells (Pax7; green) and filamentous actin (F-actin; red) demonstrated differences in satellite cell recruitment between treatment groups. C) Defects treated with tethered agrin contained more nuclei than zero or soluble agrin groups. D) Tethered agrin resulted in significantly higher embryonic myosin positive area within the defect region than zero or soluble agrin. E) Significant differences in total blood vessel coverage were seen between all treatment groups with tethered agrin inducing the highest amount of CD31 area within the defect region. F) Tethered agrin constructs resulted in higher Pax7 area within the defect region than zero or soluble agrin. *: p < 0.05; ***: p < 0.001