Figure 1. PRMT1 signals methionine availability to regulate mTORC1 activation in a SAM-dependent manner.
A, Control and PRMT1 deficient HEK293 cells were deprived of methionine for 2 hours (h) and restimulated with methionine (100 μM) for 20 min. HEK293 cells were infected with either tet-on-shLuc or tet-on-shPRMT1 lentiviruses and selected with puromycin for 3 days. The stable cell lines were pretreated with or without doxycycline (DOX) for an additional 2 days before methionine starvation and restimulation. Whole-cell lysates (WCL) or r-GTP- immunoprecipitates were analyzed by immunoblotting with the indicated antibodies. pS6K(S): short-term exposure, pS6K(L): long-term exposure. Due to space limitations, we only present the short-term exposure of pS6K in the remaining figures.
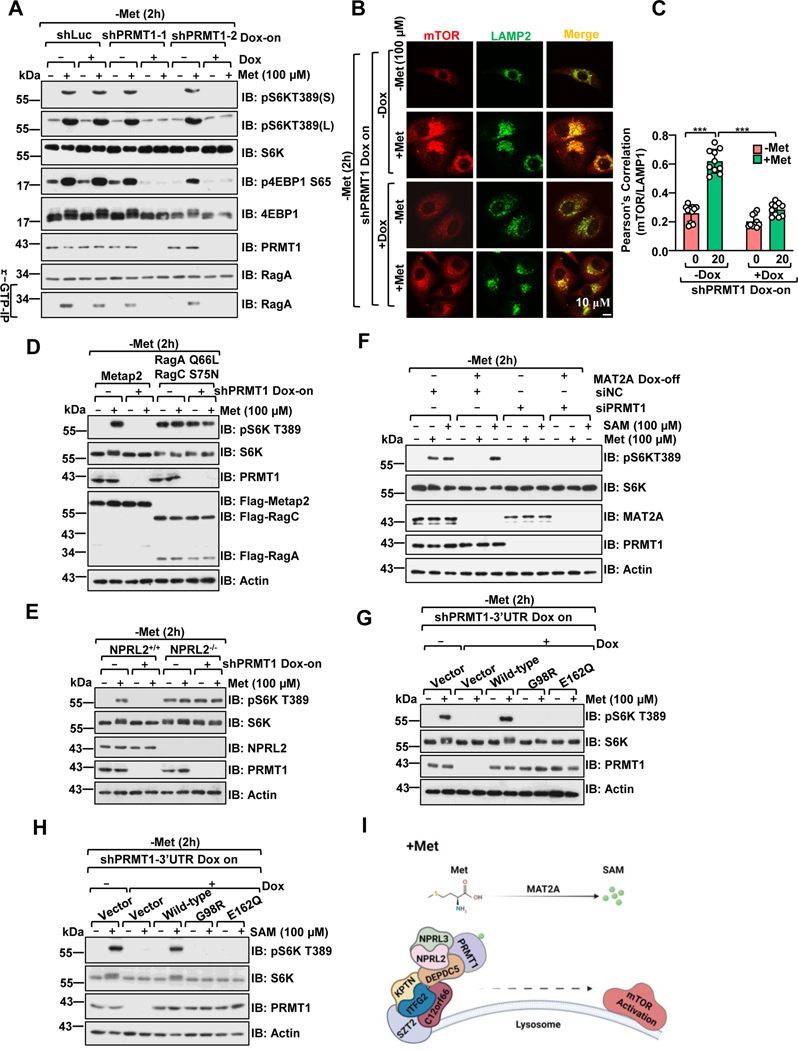
B, HEK 293 cells were treated as in A, and the co-localization of mTORC1 and LAMP2 was analyzed via immunostaining. Scale bar, 10 μm.
C, Pearson’s correlation analysis of mTOR and LAMP2 signals in B. 10 cells were analyzed for each condition, ***p<0.001, unpaired, two-tailed Student’s t-test.
D, Control and PRMT1 deficient HEK293 cells were transfected with constructs to express either Metap2 (control) or RagAQ66L+RagCS75N. Cells were treated as described in A, and cell lysates were analyzed by immunoblotting with the indicated antibodies.
E, Wild-type or NPRL2 knockout HEK293 cells were infected with the indicated lentivirus and treated as in A, and cell lysates were analyzed via immunoblotting with the indicated antibodies.
F, PRMT1 is required to signal methionine sufficiency to mTORC1 via MAT2A-mediated SAM production. The MAT2A Dox-off cell line was generated as described in the previous study25 and transfected with siRNA targeting PRMT1, as indicated. Cells were treated with or without DOX for 48 hours, followed by deprivation of methionine for 2 hours and restimulation with methionine (100 μM) for 20 min or SAM (100 μM) for 6 hours. Cell lysates were analyzed via immunoblotting with the indicated antibodies.
G-H, Wild-type PRMT1, but not the SAM-binding-deficient mutants (G98R and E162Q), restored mTORC1 activation upon methionine (G) or SAM (H) stimulation. HEK293 cells expressing tet-on-shPRMT1 targeting the PRMT1–3’UTR were infected with either PRMT1 wild-type, G98R, or E162Q lentiviruses. The stable cell lines were pre-treated with or without DOX for an additional 2 days, deprived of methionine for 2 hours, and restimulated with methionine (100 μM) for 20 min or SAM (100 μM) for 6 hours. Cell lysates were analyzed via immunoblotting with the indicated antibodies.
I, A schematic illustration showing that PRMT1 signals methionine availability to govern mTORC1 activation through the GATOR1 complex.
See also Figure S1.