Abstract
Background
Survival data for Thai patients with 5q spinal muscular atrophy (SMA), the leading cause of infant mortality worldwide, are lacking.
Objective
This study aimed to determine the survival rates and life expectancies of pediatric patients with SMA types 1, 2, and 3.
Methods
We conducted a retrospective cohort analysis of genetically confirmed 5q SMA patients aged 0–18 years who were treated between 1999 and 2021 at the pediatric neuromuscular clinic of Siriraj Hospital, Bangkok, Thailand. Mortality data were sourced from the Civil Registration Office.
Results
The study included 113 patients: 37 with SMA type 1, 53 with type 2, and 23 with type 3. Life expectancy varied significantly by SMA type: 2.2 years for type 1, 11 years for type 2, and 16.5 years for type 3. The median survival times for SMA type 1 and 2 were 1.9 and 19 years, respectively. In SMA type 2, early onset (<1 year) correlated with a shorter median survival than later onset (≥1 year) (log-rank test P = 0.009). Early onset SMA type 2 had a median survival time of 15.9 years, while 75 % of those with later onset SMA type 2 survived until the age of 19 years. Cox proportional hazards analysis revealed that each month's delay in disease onset reduced the annual mortality risk by 17 % for type 1 patients and by 20 % for type 2 patients. Compared with female patients, male patients with type 2 disease had a 12-fold increased mortality risk.
Conclusions
Age at onset is a significant predictor of survival and life expectancy in patients with SMA types 1 and 2. These insights are crucial for genetic counseling and prognostic discussions in clinical settings.
Keywords: Spinal muscular atrophy, Survival analysis, Life expectancy
1. Introduction
Spinal muscular atrophy (SMA) comprises a set of autosomal recessive neuromuscular disorders, notably 5q SMA, which stems from pathogenic variants in the survival motor neuron 1 (SMN1) gene. This subtype is one of the most prevalent autosomal recessive conditions, with an incidence of 1 in 10 000 live births [[1], [2], [3]] and a carrier frequency between 1 in 40 and 1 in 60 [4,5]. Moreover, 5q SMA is the primary cause of infant mortality due to genetic disorders [6,7]. It originates from a homozygous deletion or mutation in the SMN1 gene on chromosome 5q13, leading to motor neuron degeneration [8,9]. SMA is typified by progressive limb and trunk muscular weakness and atrophy. SMA classifications are based on the age at onset of symptoms and the achievement of specific motor milestones [10]. SMA type 1 presents with hypotonia and weakness within 6 months of life, with untreated cases typically leading to death before 2 years of age (range 8.1–24.8 months) [11,12]. Patients with SMA type 2 exhibit gross motor delays, with patients unable to walk independently but able to sit unsupported, and an average life expectancy of 15–25 years [13,14]. Patients with SMA types 3 and 4 face mobility issues but usually have a near-normal lifespan [7,13].
The standard of care for spinal muscular atrophy (SMA) has evolved significantly over time, centering around customized supportive measures, including nutritional and respiratory aids [15,16], which are globally recognized as the foundational treatment approach. The advent of disease-modifying therapies (DMTs) in 2016, however, revolutionized SMA management by fundamentally altering the natural progression of the disease. The first FDA-approved DMT, nusinersen, targets SMN2 mRNA and is administered intrathecally every 4 months [17]. Subsequently, onasemnogene abeparvovec-xioi was introduced. This medication utilizes AAV9 to replace the SMN1 gene through a single intravenous dose [18]. Risdiplam, the third DMT, is an oral option that targets SMN2 mRNA splicing [19]. These DMTs have markedly improved survival rates, reduced the necessity for respiratory interventions, and enhanced motor functions, enabling patients to achieve motor milestones previously deemed unattainable.
In Thailand, DMTs first became available in 2021 (risdiplam and onasemnogene abeparvovec-xioi) through global managed access programs, albeit for a limited number of patients. The absence of reimbursement by the Thai Universal Coverage Scheme has restricted widespread access to these treatments.
The survival outcomes of 5q SMA patients in Thailand remain undocumented. This study aimed to analyze the survival rates of children with SMA types 1 and 2 and to determine the life expectancy of children with SMA types 1, 2, and 3 in Thailand.
2. Methods
2.1. Study design and study population
We conducted a retrospective chart review at Siriraj Hospital. As Thailand's foremost referral center for neuromuscular disorders, the hospital attracts the majority of SMA patient referrals for diagnosis and care. Our cohort included pediatric patients (0–18 years) with genetically confirmed SMA who were assessed and monitored at Siriraj Hospital's pediatric neurology or neuromuscular clinics between 1999 and 2021. For genetic diagnostics to confirm SMA, quantitative polymerase chain reaction, polymerase chain reaction-restriction fragment length polymorphism, or multiplex ligation-dependent probe amplification was used. SMA subtypes were classified by onset age and peak motor milestones. Patients who were lost to follow-up or had incomplete records were excluded. Patients treated with DMTs remained included despite the short interval (under 3 months) from the first DMT administration to the study's conclusion. The data collected comprised citizen identification number, birth date, ages at onset and diagnosis, and treatment specifics. Dates of death were verified through the Civil Registration Office. Ethical approval was granted by the Siriraj Institutional Review Board (COA Si 860/2021)
2.2. Data analysis
We utilized descriptive statistics to analyze demographic data. Survival probabilities were computed through the Kaplan–Meier method, with comparisons made via the log-rank test, and further analyses were conducted using the Cox proportional hazards model. Statistical procedures were performed using Stata 17 software. P values less than 0.05 were considered to indicate statistical significance.
3. Results
3.1. Clinical characteristics
Our study evaluated 113 children with SMA. One patient was excluded due to loss of follow-up and did not receive genetic confirmation of SMA, and they passed away at 2.4 years. The ages of the participants at the time of the study ranged from newborn to 18 years, and males constituted 59 % (67) of the cohort. The clinical characteristics of the patients are detailed in Table 1. Of the cohort, 32.75 % (37) had SMA type 1, 46.90 % (53) had type 2, and 20.35 % [20] had type 3. The initial symptoms of SMA type 1 include hypotonia, a floppy infant appearance, delayed gross motor development, and respiratory failure. For SMA type 2, the early signs are delayed gross motor development and an inability to walk. SMA type 3 initially presents with muscle weakness and difficulty walking. For SMA type 1 patients, the mean ages at onset and at diagnosis were 3.2 and 6.9 months, respectively. SMA type 2 patients had mean onset and diagnosis ages of 11.1 and 29.6 months, respectively, while the corresponding ages for the SMA type 3 patients were 4.3 and 9.3 years, respectively.
Table 1.
Clinical demographics and characteristics of pediatric SMA cohort (N = 113).
| Characteristic | N (%) |
|---|---|
| Male | 67 (59.3) |
| SMA Type | |
| SMA 1 | 37 (32.7) |
| SMA 2 | 53 (46.9) |
| SMA 3 | 23 (20.4) |
| Age of onset (years)a | |
| SMA 1 | 0.3 (0–0.9) |
| SMA 2 | 0.9 (0.3–1.8) |
| SMA 3 | 4.3 (1.2–14) |
| Age at diagnosis (years)a | |
| SMA 1 | 0.5 (0–3) |
| SMA 2 | 2.5 (0.4–12) |
| SMA 3 | 9.3 (2–18) |
| Patients receiving disease-modifying therapies | 20 (17.7) |
| Risdiplam | 13 (65) |
| Onasemnogene abeparvovec | 7 (35) |
| SMN 2 gene analysis (number of patients undergoing) | 35 (30.9) |
| SMN 2 copies number = 2 | 13 (11.50) |
| SMN 2 copies number = 3 | 21 (18.58) |
| SMN 2 copies number = 4 | 1 (0.88) |
Data are presented as mean (range).
Among the 113 patients, 20 patients (17.7 %) were treated with DMTs. Risdiplam was administered to 13 children: 2 with SMA type 1 at 7 and 45 months, and 11 with SMA type 2 at a median age of 91.3 months (range 28–232). Onasemnogene abeparvovec-xioi was given to 7 children: 5 with SMA type 1 at a median age of 15.4 months (range 6–24) and 2 with SMA type 2 at 18 and 31 months. None of the cases have undergone treatment with nusinersen or similar agents.
3.2. Life expectancy
The analysis of survival outcomes for patients with SMA type 1 showed that 21.62 % (8 of 37) survived, while 78.38 % (29 of 37) had died. The median age of the surviving children was 5.3 years, and all of these patients needed invasive ventilation. The mean life expectancy was 2.2 years with a 95 % confidence interval of 1.4–3.1.
Among SMA type 2 patients, 83.02 % (44 of 53) were alive at the end of the study period, and 16.98 % (9 of 53) had died. The median age of the surviving children was 14 years. The mean life expectancy was 11 years with a 95 % confidence interval of 5–15.
SMA type 3 patients exhibited the highest survival rate, with 91.30 % (21 of 23) alive and 8.70 % (2 of 23) deceased. The median ages of the living patients in this group were 22.2 years. Two individuals diagnosed with SMA type 3 passed away in 2013 and 2014 at the ages of 16.2 and 16.8 years, respectively. The mean life expectancy was 16.5 years with a 95 % confidence interval of 16.2–16.7. Detailed life expectancy data across all SMA types are provided in Table 2. Incidence of endpoints (mortality and/or permanent ventilator dependency) among SMA patients are provided in Table S1.
Table 2.
Comparative mean life expectancies across SMA types 1–3.
| Type | Numbers of patients | Alive n (%) | Deceased n (%) | Mean life expectancy (y) | 95 % confidence interval |
|---|---|---|---|---|---|
| SMA1 | 37 | 8 (21.6) | 29 (78.4) | 2.2 | 1.4 - 3.1 |
| SMA2 | 53 | 44 (83.0) | 9 (17.0) | 11 | 5.0–15.0 |
| SMA3 | 23 | 21 (91.3) | 2 (8.7) | 16.5 | 16.2–16.7 |
3.3. Survival analysis and factors influencing outcomes
We explored potential determinants of survival in SMA patients, focusing on age at onset, the use of genetic-based therapies (DMTs), and sex.
Patients with SMA type 1 were stratified by age at onset into those with an onset age of less than 2 months and those with an onset age of 2 months or more (Fig. 1). The median survival was 1.28 years for the former and 2.28 years for the latter, without a significant difference (log-rank test P = 0.08).
Fig. 1.
Kaplan–Meier survival estimates for SMA type 1 patients stratified by age at onset (n = 17 for 0–2 months; n = 20 for >2 months).
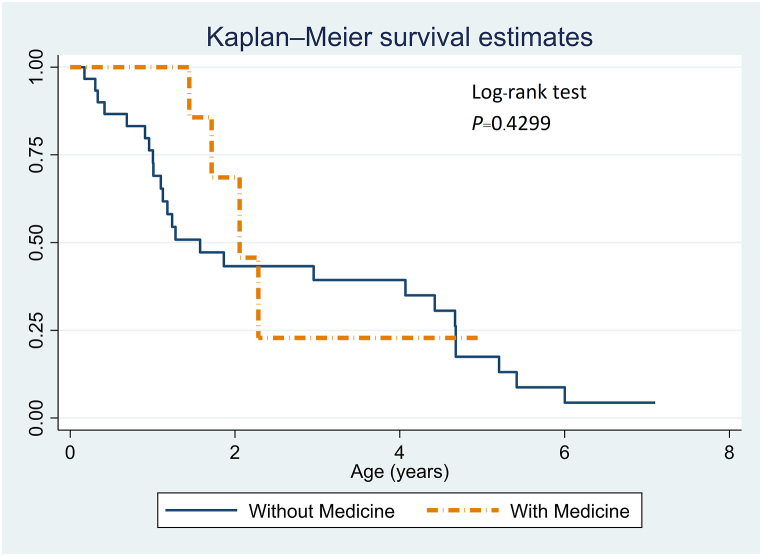
In examining the influence of genetic-based medications on patients with SMA type 1, different outcomes emerged based on patient age (Fig. 2). Patients under 2 years of age who received genetic-based treatments showed improved survival outcomes compared to those who did not receive such treatments. Conversely, for patients older than 2 years, the data suggested that outcomes were somewhat less favorable in the treatment group than in the nontreatment group.
Fig. 2.
Kaplan–Meier survival analysis for SMA type 1 patients: medication impact (n = 7 with medication; n = 30 without).
Regarding the sex of SMA type 1 patients, the analysis did not reveal a significant difference in median survival times (males = 1.72 years, females = 2.06 years; log-rank test P = 0.96; Fig. 3). However, the Cox proportional hazards analysis revealed a significant relationship between age at onset and survival time (hazard ratio = 0.83; P = 0.04). This result indicates that with each additional month of age at onset, the yearly probability of death decreases by 17 %.
Fig. 3.
Sex-based Kaplan-Meier survival distribution in SMA type 1 (n = 20 males; n = 17 females).
SMA type 2 patients were divided based on onset age into two groups: those with onset before 1 year and those with onset at 1 year or later (Fig. 4). Patients with onset before 1 year had a median survival of 15.86 years, while 75 % of those with onset at 1 year or later survived until the age of 19 years, with a significant difference (log-rank test P = 0.0092).
Fig. 4.
Kaplan–Meier survival curves for SMA type 2 patients categorized by age at onset (n = 53).
The effect of genetic-based medication usage on survival (Fig. 5) showed that patients with SMA type 2 who were receiving medication had improved outcomes, although the difference was not significant (log-rank test P = 0.149). The nonmedicated SMA type 2 patients had a median survival of 19 years, whereas all medicated SMA type 2 patients were still alive at the conclusion of the study. Kaplan–Meier survival functions demonstrate the impact of age at onset on mortality and ventilator dependency, as shown in Figure S1-S6. Sex-based analysis (Fig. 6) revealed no significant difference in survival among SMA type 2 patients. The median survival for males was 19.01 years; however, the female median survival could not be estimated, as less than 50 % of the females died (log-rank test P = 0.092).
Fig. 5.
Kaplan–Meier survival projections for SMA type 2 patients: medication effect (n = 13 with medication; n = 40 without).
Fig. 6.
Kaplan–Meier survival comparisons between male and female patients in SMA type 2 (n = 53).
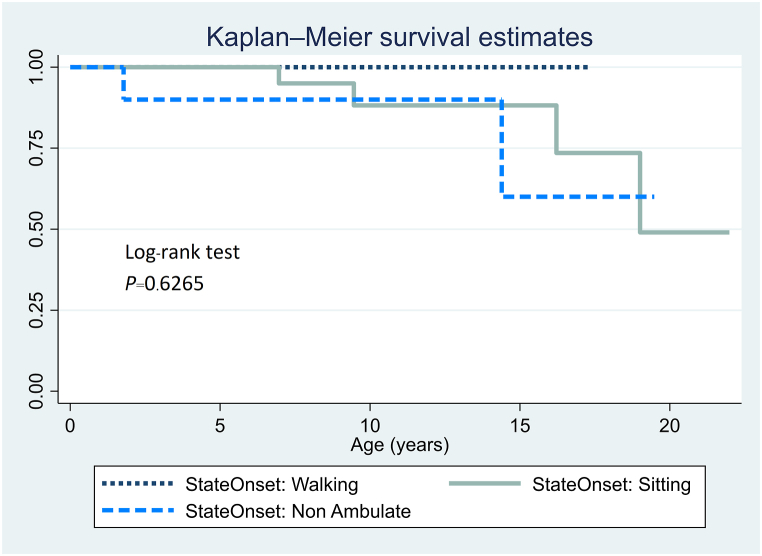
Cox proportional hazards analysis (Table 3) revealed significant associations for SMA type 2 patients. Age at onset correlated with survival (HR = 0.80; P = 0.021), and sex had a notable impact on survival (HR = 12.29; P = 0.036). Sex-based analysis (Fig. 6) and state at onset (Fig. 7) revealed no significant difference in survival among SMA type 2 patients.
Table 3.
Cox proportional hazards analysis: influence of onset age (months) and sex on survival in SMA types 1 and 2.
| Type | Factor | Hazard ratio | Standard error | z | P > z | 95 % confidence interval |
|---|---|---|---|---|---|---|
| SMA1 | Age at onset (mo) | 0.830 | 0.076 | −2.02 | 0.043 | 0.693–0.994 |
| SMA2 | Age at onset (mo) | 0.804 | 0.075 | −2.31 | 0.021 | 0.669–0.967 |
| Sex | 12.294 | 14.732 | 2.09 | 0.036 | 1.174–128.733 |
Fig. 7.
Kaplan–Meier survival analysis according to state at onset for SMA type 2 patients (n = 53).
The cohort of 23 patients with SMA type 3 constituted 20.3 % of the study population. However, the dataset was too small for analysis through the Kaplan–Meier survival method, log-rank test, and Cox proportional hazard modeling.
4. Discussion
This retrospective cohort analysis utilized patient records covering a duration of 24 years, with follow-up extending to 16 years. The objective of this study was to elucidate the life expectancy and survival rates of Thai pediatric patients diagnosed with SMA in the era preceding the introduction of therapeutic interventions. The study also aimed to identify factors associated with prolonged life.
In this investigation, the distributions of SMA types 1, 2, and 3 within the Thai cohort were 33 %, 47 %, and 20 %, respectively. This contrasts with the distribution observed in nations lacking newborn screening programs, such as Japan, where SMA types 1, 2, and 3 account for 42.1 %, 32.1 %, and 20.8 % of patients, respectively [21]. Our Thai distributions also diverge from those reported in countries with newborn SMA screenings, characterized by proportions of 40%–60 % for SMA type 1, 20%–40 % for SMA type 2, and 15%–25 % for SMA type 3 [21]. The lower proportion of SMA type 1 patients in Thailand may be attributed to underdiagnosis and the referral of only intubated patients to our institute. Additionally, genetic confirmation of SMA is confined to three academic hospitals within the Thai public health system, potentially influencing diagnostic accessibility.
Our study revealed mean life expectancies of 2.2 years for SMA type 1, 11 years for SMA type 2, and 16.5 years for SMA type 3, which is consistent with findings from Korea [11] and the United States [12]. These earlier studies reported median survival times for SMA type 1 patients of 20.8–24.8 months and 8.1–22.0 months, respectively. Farrar et al. [14] reported a median survival of 15.6 years (range 5.6–40.9 years) for SMA type 2 patients, consistent with our observations. For SMA type 3 patients, the literature suggests that these patients may enjoy nearly normal life expectancies [7,13]. However, our study did not include a survival analysis for this group due to the need for extended follow-up and the focus on children under 18 years of age, limiting the inclusion of type 3 patients.
In our study, the median age for death or the requirement of permanent ventilation support among SMA type 1 patients without DMTs was 19 months (Figure S2). In comparison, the previous natural history study by the Paediatric Neuromuscular Clinical Research (PNCR) group reported a median age of 13.5 months for death or the need for permanent ventilation support
In addition, to study the impact of the improved standard of care on the survival of SMA patients without DMTs, we analyzed survival by comparing those born on or before 2011 with those born after 2011. The median survival time for SMA type 1 patients born on or before 2011 was 0.48 years, while for those born after 2011, it was 1.25 years (log-rank test, p = 0.023; Figure S7). This difference may result from improvements in general medical care and the standard of care for SMA type 1 patients. For SMA type 2 patients, we could not analyze the difference in median survival time due to the small sample size and insufficient follow-up period, Figure S8.)
Age at onset critically impacts survival outcomes in patients with SMA. Our findings indicated onset ages of 3.2 months for SMA type 1, 11.1 months for type 2, and 4.3 years for type 3, consistent with prior studies on SMA types 1 and 2 [12,[20], [22], [23][24]]. However, our results for SMA type 3 diverged, potentially due to our smaller cohort. We stratified SMA type 1 patients using a 2-month onset age threshold to form two subgroups. This stratification did not reveal a significant difference in survival between patients with an onset between birth and 2 months and those with an onset beyond 2 months. In contrast, Ge et al. reported a significantly superior survival probability in patients with an onset of ≥2 months compared to those with an onset of <2 months [22]. Similarly, for SMA type 2, a 1-year onset age threshold showed that patients with a later onset had significantly improved median survival, contrary to our findings. These variances could be attributed to differences in the sample sizes, the reliability of caregiver-reported onset data at initial hospital evaluations, and the age cutoffs used.
In this analysis, sex was identified as a critical determinant, revealing a marked difference in median survival times between male and female SMA type 2 patients. Additionally, the Cox proportional hazards analysis for SMA type 2 demonstrated a significant association between sex and survival, with male mortality rates being twelvefold greater than those of their female counterparts. This disparity may be linked to the differential rates of medication administration between the sexes. Within our sample, medication was provided to 40 % of the female patients (20 in total, 8 medicated) compared to only 15 % of the male patients (33 in total, 5 medicated). Therefore, the lower medication usage among males may be a contributing factor to their increased mortality rates.Our study examined medication as an interesting factor because various treatment options are now available for SMA patients. However, our analysis revealed no significant difference in the median survival times of SMA type 1 and type 2 patients who were treated with medication and those who were not.
In our cohort of 30 nonmedicated SMA type 1 patients, six needed ventilatory support, with a median onset age of 3.2 months (range: 0–14 months). Among the seven patients receiving medication, five were ventilator dependent, exhibiting an average onset age of 3.12 months (range: 1–5 months) and initiating treatment at an average age of 1.03 years. Fig. 2 shows that medicated patients aged 0–2 years demonstrated superior outcomes compared to their nonmedicated counterparts, a distinction that diminished beyond the age of 2. Despite similar median onset ages in the medicated and nonmedicated SMA type 1 subgroups, they differed in age-range breadth and the proportion of ventilator-dependent patients, indicating potentially enhanced surveillance in the nonmedicated cohort. Specifically, the 30 patients in the nonmedicated group presented a wider onset age spectrum (0–14 months) and included six (20 %) who were ventilator dependent and may have received better surveillance. In contrast, the seven patients in the medicated group had a narrower age range (1–5 months) and included five (71 %) who were ventilator dependent. A follow-up study, possibly employing a matched case ≥ control design, is warranted to further elucidate the interplay between medication use, ventilator dependency, and onset age in SMA type 1 patients.
Cox proportional hazards analysis for SMA type 1 revealed a significant correlation between onset age and survival period. The analysis suggested that with each additional month of age at onset, the annual risk of mortality decreased by 17 %. This finding is consistent with the patterns observed in SMA type 2. Specifically, for each additional month of age at onset, the yearly probability of death decreased by 20 %.
5. Conclusion
This study established a median life expectancy of 2.2 years for SMA type 1 patients. Additionally, the study highlighted that each additional month of age at onset significantly impacts survival, correlating with a 17 % decrease in the annual death risk. For SMA type 2 patients, the median life expectancy was 11 years, with survival significantly influenced by both onset age and female sex. These findings, derived from detailed survival analyses, offer critical insights for genetic and prognostic counseling across SMA types.
Consent statement
Patient consent was not required for this study.
Funding
This study was supported by the Siriraj Research Developmental Fund, Faculty of Medicine Siriraj Hospital, Mahidol University (R016437001).
Data availability statement
Data associated with the study has not been deposited into a publicly available repository. Data will be made available on request.
CRediT authorship contribution statement
Piyanart Sittiyuno: Writing – original draft, Investigation, Formal analysis. Pimchanok Kulsirichawaroj: Writing – review & editing, Validation, Methodology, Formal analysis. Pattara Leelahavarong: Formal analysis, Conceptualization. Oranee Sanmaneechai: Writing – review & editing, Supervision, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors express their gratitude to Doctor Chulaluk Komoltri for her valuable assistance with the statistical analyses and to Ms. Tanaporn Netsuwan for her help with data collection. We also extend our thanks to Associate Professor Varalak Srinonprasert and the National Health Security Office for their support in obtaining the data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e32732.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Verhaart I.E.C., Robertson A., Leary R., McMacken G., König K., Kirschner J., et al. A multi-source approach to determine SMA incidence and research ready population. J. Neurol. 2017;264(7):1465–1473. doi: 10.1007/s00415-017-8549-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jedrzejowska M., Milewski M., Zimowski J., Zagozdzon P., Kostera-Pruszczyk A., Borkowska J., et al. Incidence of spinal muscular atrophy in Poland--more frequent than predicted? Neuroepidemiology. 2010;34(3):152–157. doi: 10.1159/000275492. [DOI] [PubMed] [Google Scholar]
- 3.Arkblad E., Tulinius M., Kroksmark A.K., Henricsson M., Darin N. A population-based study of genotypic and phenotypic variability in children with spinal muscular atrophy. Acta Paediatr. 2009;98(5):865–872. doi: 10.1111/j.1651-2227.2008.01201.x. [DOI] [PubMed] [Google Scholar]
- 4.Prior T.W., Snyder P.J., Rink B.D., Pearl D.K., Pyatt R.E., Mihal D.C., et al. Newborn and carrier screening for spinal muscular atrophy. Am. J. Med. Genet. 2010;152a(7):1608–1616. doi: 10.1002/ajmg.a.33474. [DOI] [PubMed] [Google Scholar]
- 5.Ogino S., Leonard D.G., Rennert H., Ewens W.J., Wilson R.B. Genetic risk assessment in carrier testing for spinal muscular atrophy. Am. J. Med. Genet. 2002;110(4):301–307. doi: 10.1002/ajmg.10425. [DOI] [PubMed] [Google Scholar]
- 6.Lunn M.R., Wang C.H. Spinal muscular atrophy. Lancet. 2008;371(9630):2120–2133. doi: 10.1016/S0140-6736(08)60921-6. [DOI] [PubMed] [Google Scholar]
- 7.Kolb S.J., Kissel J.T. Spinal muscular atrophy. Neurol. Clin. 2015;33(4):831–846. doi: 10.1016/j.ncl.2015.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorson C.L., Hahnen E., Androphy E.J., Wirth B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl. Acad. Sci. U.S.A. 1999;96(11):6307–6311. doi: 10.1073/pnas.96.11.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Markowitz J.A., Singh P., Darras B.T. Spinal muscular atrophy: a clinical and research update. Pediatr. Neurol. 2012;46(1):1–12. doi: 10.1016/j.pediatrneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 10.Zerres K., Rudnik-Schöneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch. Neurol. 1995;52(5):518–523. doi: 10.1001/archneur.1995.00540290108025. [DOI] [PubMed] [Google Scholar]
- 11.Park H.B., Lee S.M., Lee J.S., Park M.S., Park K.I., Namgung R., et al. Survival analysis of spinal muscular atrophy type I. Korean J Pediatr. 2010;53(11):965–970. doi: 10.3345/kjp.2010.53.11.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkel R.S., McDermott M.P., Kaufmann P., Darras B.T., Chung W.K., Sproule D.M., et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–817. doi: 10.1212/WNL.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zerres K., Rudnik-Schöneborn S., Forrest E., Lusakowska A., Borkowska J., Hausmanowa-Petrusewicz I. A collaborative study on the natural history of childhood and juvenile onset proximal spinal muscular atrophy (type II and III SMA): 569 patients. J. Neurol. Sci. 1997;146(1):67–72. doi: 10.1016/s0022-510x(96)00284-5. [DOI] [PubMed] [Google Scholar]
- 14.Farrar M.A., Vucic S., Johnston H.M., du Sart D., Kiernan M.C. Pathophysiological insights derived by natural history and motor function of spinal muscular atrophy. J. Pediatr. 2013;162(1):155–159. doi: 10.1016/j.jpeds.2012.05.067. [DOI] [PubMed] [Google Scholar]
- 15.Finkel R.S., Mercuri E., Meyer O.H., Simonds A.K., Schroth M.K., Graham R.J., et al. Diagnosis and management of spinal muscular atrophy: Part 2: Pulmonary and acute care; medications, supplements and immunizations; other organ systems; and ethics. Neuromuscul. Disord. 2018;28(3):197–207. doi: 10.1016/j.nmd.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Mercuri E., Finkel R.S., Muntoni F., Wirth B., Montes J., Main M., et al. Diagnosis and management of spinal muscular atrophy: Part 1: recommendations for diagnosis, rehabilitation, orthopedic and nutritional care. Neuromuscul. Disord. 2018;28(2):103–115. doi: 10.1016/j.nmd.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Finkel R.S., Chiriboga C.A., Vajsar J., Day J.W., Montes J., De Vivo D.C., et al. Treatment of infantile-onset spinal muscular atrophy with nusinersen: a phase 2, open-label, dose-escalation study. Lancet. 2016;388(10063):3017–3026. doi: 10.1016/S0140-6736(16)31408-8. [DOI] [PubMed] [Google Scholar]
- 18.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377(18):1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 19.Baranello G., Darras B.T., Day J.W., Deconinck N., Klein A., Masson R., et al. Risdiplam in type 1 spinal muscular atrophy. N. Engl. J. Med. 2021;384(10):915–923. doi: 10.1056/NEJMoa2009965. [DOI] [PubMed] [Google Scholar]
- 20.Ou S.F., Ho C.S., Lee W.T., Lin K.L., Jones C.C., Jong Y.J. Natural history in spinal muscular atrophy Type I in Taiwanese population: a longitudinal study. Brain Dev. 2021;43(1):127–134. doi: 10.1016/j.braindev.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 21.Kimizu T., Ida S., Okamoto K., Awano H., Niba E.T.E., Wijaya Y.O.S., et al. Spinal muscular atrophy: diagnosis, incidence, and newborn screening in Japan. Int J Neonatal Screen. 2021;7(3) doi: 10.3390/ijns7030045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ge X., Bai J., Lu Y., Qu Y., Song F. The natural history of infant spinal muscular atrophy in China: a study of 237 patients. J. Child Neurol. 2012;27(4):471–477. doi: 10.1177/0883073811420152. [DOI] [PubMed] [Google Scholar]
- 23.Aguerre V., De Castro F., Mozzoni J., Gravina L.P., Araoz H.V., Monges S. Natural history of type 1 spinal muscular atrophy in a series of argentinian children. J. Neuromuscul. Dis. 2020;7(4):453–458. doi: 10.3233/JND-200508. [DOI] [PubMed] [Google Scholar]
- 24.Finkel R.S., McDermott M.P., Kaufmann P., Darras B.T., Chung W.K., Sproule D.M., et al. Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology. 2014;83(9):810–817. doi: 10.1212/WNL.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with the study has not been deposited into a publicly available repository. Data will be made available on request.