Abstract
ASCT2 (alanine serine cysteine transporter 2), a member of the solute carrier 1 family, mediates Na+-dependent exchange of small neutral amino acids across cell membranes. ASCT2 was shown to be highly expressed in tumor cells, making it a promising target for anticancer therapies. In this study, we explored the binding mechanism of the high-affinity competitive inhibitor L-cis hydroxyproline biphenyl ester (Lc-BPE) with ASCT2, using electrophysiological and rapid kinetic methods. Our investigations reveal that Lc-BPE binding requires one or two Na+ ions initially bound to the apo-transporter with high affinity, with Na1 site occupancy being more critical for inhibitor binding. In contrast to the amino acid substrate bound form, the final, third Na+ ion cannot bind, due to distortion of its binding site (Na2), thus preventing the formation of a translocation-competent complex. Based on the rapid kinetic analysis, the application of Lc-BPE generated outward transient currents, indicating that despite its net neutral nature, the binding of Lc-BPE in ASCT2 is weakly electrogenic, most likely because of asymmetric charge distribution within the amino acid moiety of the inhibitor. The preincubation with Lc-BPE also led to a decrease of the turnover rate of substrate exchange and a delay in the activation of substrate-induced anion current, indicating relatively slow Lc-BPE dissociation kinetics. Overall, our results provide new insight into the mechanism of binding of a prototypical competitive inhibitor to the ASCT transporters.
Keywords: neutral amino acid transporter, ASCT2, electrophysiology, kinetics, rapid solution exchange, membrane protein, inhibition mechanism
The solute carrier 1 (SLC1) family is a group of membrane transport proteins including five high-affinity glutamate transporters (excitatory amino acid transporters, EAATs) and two neutral amino acid transporters (alanine serine cysteine transporters, ASCTs) (1, 2, 3). These SLC1 transporters mediate the uptake and efflux of amino acids by cells. ASCT2 (alanine serine cysteine transporter 2) was previously shown to function as a neutral amino acid exchanger (2). ASCT2 plays a crucial role in facilitating the uptake of glutamine, which serves as a vital source of energy and nitrogen for the proliferation of rapidly growing tumor cells (4, 5). In many tumor cell types, ASCT2 is highly upregulated, such as in hepatocellular carcinoma, colorectal cancer, and breast cancer (6, 7, 8). For this reason, ASCT2 is emerging as a promising anticancer drug target. Therefore, a series of competitive and noncompetitive inhibitors targeting ASCT2 have been developed.
SLC1 family members are Na+-dependent transporters, however, the ion coupling stoichiometries differ between the neutral and acidic amino acid transporters in the family. In the EAATs, glutamate is cotransported with three Na+ ions and one proton and one K+ ion is counter-transported (9, 10, 11, 12), while in ASCTs, the uptake of one neutral amino acid is in exchange of countertransported amino acid, modulated by the binding of two or three Na+ ions to the transporter (3, 13, 14). ASCTs and EAATs share a high similarity of the amino acid sequence in the transmembrane domains. The substrate binding mechanism of EAATs has been thoroughly studied. In the substrate-free state, HP2 (hairpin loop 2) is closed; subsequently, two Na+ ions bind to the transporter. This is followed by remodeling of the substrate binding site, facilitating the opening of HP2 for substrate binding. Once the substrate is bound, HP2 closes again. Finally, the binding of the third Na+ occurs (15, 16, 17, 18, 19). The substrate/Na+ binding mechanism for ASCTs, on the other hand, has not been comprehensively characterized.
Currently, several competitive inhibitors have been identified, based on structural similarity with competitive EAAT inhibitors (20). A variety of different amino acid-based scaffolds were explored, for example, benzylproline derivatives, p-nitrophenyl glutamyl derivatives, and serine-based derivatives (21, 22, 23). Among these ASCT2 inhibitors, L-cis hydroxyproline biphenyl ester (Lc-BPE) is one of the most potent, demonstrating an apparent Ki value of 0.74 μM in rat ASCT2 (rASCT2) and 0.86 μM in human ASCT2 (hASCT2) (24). Lc-BPE was developed in our laboratory in 2021, when we combined computational modeling and experimental approaches to identify a distinct subpocket within the substrate binding site. The cryo-EM structure of the hASCT2-Lc-BPE complex captures a pharmacologically relevant outward-open conformation (24). The structure suggests that Lc-BPE is a competitive inhibitor binding to the substrate binding site from the extracellular side. In addition, several other ASCT2 structures were published, including inward- and outward-facing conformations with or without substrate bound (25, 26, 27). One of these structures suggests a one-gate elevator mechanism, in which HP2 functions as the only gate, which controls access to the binding site in both inward- and outward-facing conformations (27).
In this study, we applied electrophysiological techniques to investigate the kinetic properties of the steady-state and presteady-state currents associated with ASCT2 function. Lc-BPE is utilized as a tool to characterize the binding mechanism and electrostatics of sodium, substrate, and competitive inhibitors in ASCT2. The goals were to identify the functional similarities/differences between substrate/competitive inhibitor binding to ASCT2 and their implications for the structural mechanism of Na+ and ligand interaction with the transporter. Our results indicate a transport mechanism wherein one or two Na+ ions initially bind to the unoccupied transporter (likely Na1 and Na3 sites), subsequently followed by the binding of either an amino acid or a competitive inhibitor. For amino acid substrates, an additional Na+ binding results in the formation of a complex that is competent for translocation. However, for competitive inhibitors, this subsequent Na+ binding step cannot take place. This is likely due to competitive inhibitors preventing the closure of HP2. We quantitatively explain the outward charge movement created by inhibitor binding, caused by movement of the zwitterionic amino acid moiety into the binding site, with the negatively charged carboxy group more deeply penetrating the transmembrane electric field. Together, these findings shed additional light on the mechanistic details of the inhibitor/transporter interaction, possibly facilitating the development of more potent competitive inhibitors in the future.
Results
Binding of Lc-BPE is associated with a partially occupied Na+ binding state
Lc-BPE has been previously reported to be a potent competitive, stereo-selective inhibitor of ASCT2. The cryo-EM analysis of the hASCT2 structure in complex with the inhibitor revealed that Lc-BPE is bound to the substrate binding site of ASCT2 (24). The binding site is formed by residues from HP1, TM7, and TM8 and is occluded by the extracellular gate, HP2, as depicted in Figure 1A. The Lc-BPE-bound structure additionally showed a distinctive motion of HP2 within the binding region of the outward-open conformation, relative to the glutamine-bound structure. In contrast to the closed, outward-facing conformation observed in the structure of Asp-bound EAAT1 (28), where the Na2 site is occupied (Fig. 1B), the Na2 site appears to be unoccupied when Lc-BPE binds to ASCT2. The residues that interact with Lc-BPE include S353 in HP1, D464, N471, and T468 in TM8, as well as other residues.
Figure 1.
The [Na+] dependence of Lc-BPE-induced current is compatible with a single-Na+site model.A, Lc-BPE (cyan sticks) binding site of ASCT2 (7BCS, “ligand-down” orientation, HP2 open). HP1 and HP2 are shown in white. Predicted sodium ions in Na1/Na3 sites are shown as purple spheres. B, substrate binding site of EAAT1 (7AWM, HP2 closed). L-Asp is shown as green sticks and sodium ions in Na1/Na3 sites are shown as purple spheres, in Na2 site is shown as a blue sphere. C, typical Lc-BPE-induced ASCT2 anion current (black) at increasing Na+ concentrations. The extracellular solution contained 0.05, 0.1, 1, or 10 mM NaMes (sodium methanesulfonate) and 10 μM Lc-BPE, the internal solution contained 130 mM NaSCN (sodium thiocyanate)/10 mM serine. The current illustrated by the red trace was induced by 2 mM serine in the presence of 140 mM of extracellular NaMes as a control. The application time for Lc-BPE or serine is indicated by the gray bar. D, [Na+] dose-response curve at 10 μM Lc-BPE for ASCT2WT. The red line represents the best nonlinear curve fit to a Michaelis–Menten-like equation. The apparent affinity for Na+ was calculated as Km = 0.04 ± 0.003 mM. The inset shows the structure of Lc-BPE. E, apparent Na+Km values for ASCT2WT, ASCT2D386N, and ASCT2D473N in the presence of 100 μM Lc-BPE. F, [Lc-BPE] dose-response curves for ASCT2WT, ASCT2D386N, and ASCT2D473N in the presence of 140 mM Na+, with apparent Km value of 0.83 ± 0.2 μM for ASCT2WT, 1.6 ± 0.1 μM for ASCT2D386N, 4.4 ± 0.8 μM for ASCT2D473N. G, typical serine-induced ASCT2 anion current at increasing Na+ concentrations. H, [Na+] dose-response curve at 2 mM serine for ASCT2WT. Anion currents were fitted using a two site Michaelis–Menten like equation, . The apparent affinities for Na+ were calculated as Km1 = 0.04 ± 0.02 mM and Km2 = 49 ± 2 mM. The membrane potential was 0 mV in all experiments. ASCT, alanine serine cysteine transporter; EAAT, excitatory amino acid transporter; HP2, hairpin loop 2; Lc-BPE, L-cis hydroxyproline biphenyl ester.
It is likely that this predicted difference in Na+ binding site occupancy between Lc-BPE-bound form of ASCT2 and the substrate bound form of EAAT1 manifests itself in the [Na+]-dependence of substrate/inhibitor interaction, which we tested using electrophysiological experiments. It has been previously established that competitive inhibitors, such as Lc-BPE, block the tonic ASCT2 leak anion conductance (9). This block results in inhibition of tonic inward current when permeable thiocyanate SCN– is present inside the cell (Fig. 1C, black currents). Block of the leak anion current by Lc-BPE was Na+ dose dependent, with an apparent Km for Na+ of 0.04 ± 0.003 mM (Fig. 1D), confirming previous reports of high affinity Na+ binding, most likely to the Na1/Na3 site(s) (29). To test the involvement of these two Na+ binding sites, we determined the Km for Na+ of mutant transporters with a mutation to a conserved residue in the Na1 site (D473N) or Na3 site (D386N). While this Km was virtually unchanged for the Na3 site mutant transporter, relative to ASCT2WT, it was significantly increased (lower apparent affinity) for ASCT2D473N (Na1 site mutant, Fig. 1E). Moreover, the Lc-BPE apparent affinity was significantly decreased compared to the WT transporter in both mutants. However, this decrease was significantly more pronounced for ASCT2D473N than for ASCT2D386N (Fig. 1F). When applying serine to ASCT2D473N, no significant anion current was observed, in contrast to ASCT2D386N (Fig. S1A). Together, these results suggest that occupation of the Na1 site, but not the Na3 site is critical for Lc-BPE interaction with ASCT2.
To establish a comparison with the Na+ binding process in the presence of transported substrate, we next determined the [Na+] dependence of serine-induced current. Typical anion currents obtained from ASCT2 at varying concentrations of Na+ are shown in Figure 1G. At low Na+ concentration, serine blocked the leak conductance, resulting in reduced outward current due to the inhibition of SCN− outflow (20). When Na+ concentration was higher than 3 mM, the current reversed to become inwardly directed, which can be attributed to the serine-induced activation of the anion conductance. Therefore, the serine-induced anion current demonstrates a biphasic dependence on the concentration of Na+, with a high apparent affinity observed at low [Na+] (Km1 = 0.04 ± 0.02 mM) and a lower apparent affinity observed at high [Na+] (Km2 = 49 ± 2 mM) (Fig. 1H). This observed biphasic Na+ concentration dependence also indicates that the binding of transported substrate leads to the occupation of at least two sodium-binding sites.
The stability of bound Lc-BPE in MD simulations is affected by the occupation state of the Na1 and Na3 binding sites
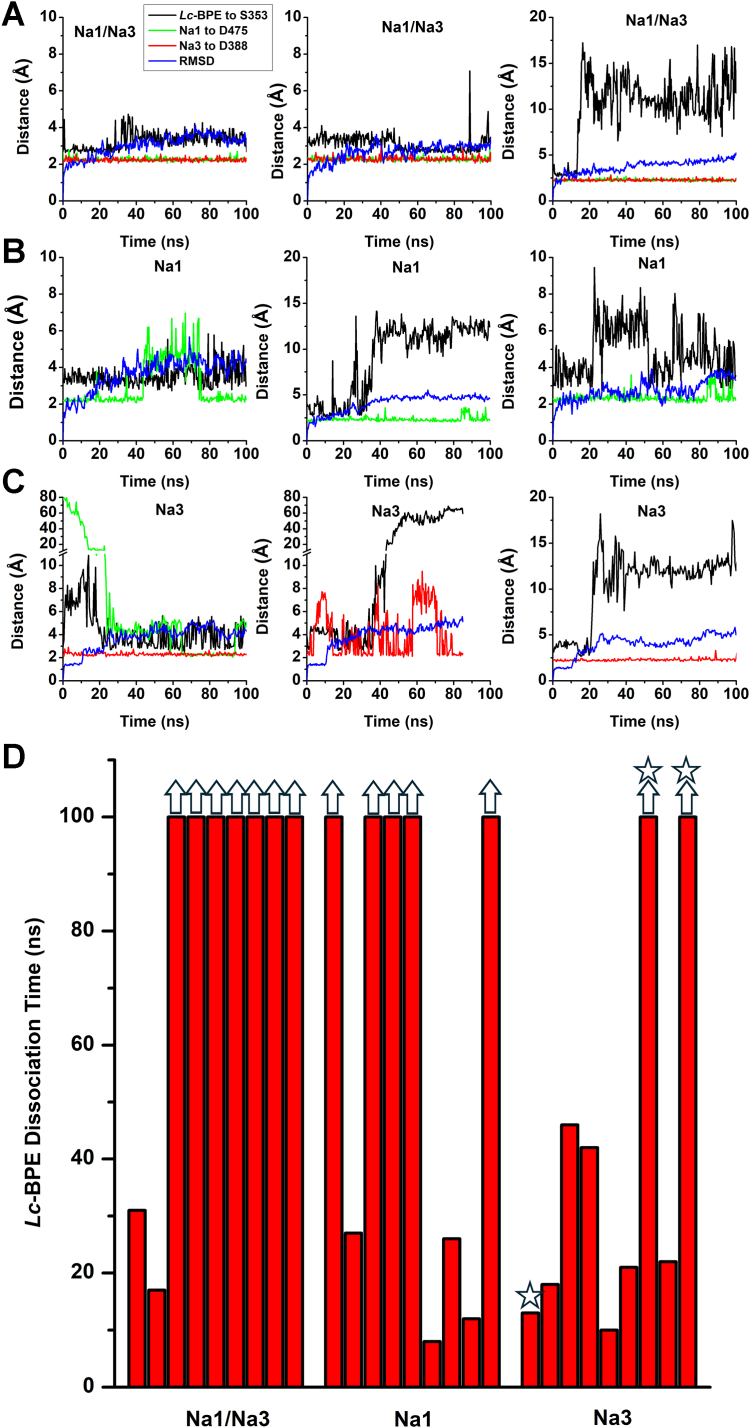
To further examine the occupancy of Na+ binding sites in the Lc-BPE bound state, we performed molecular dynamics (MD) simulations with either one or two Na+ ions bound. We utilized the ASCT2-Lc-BPE complex structure (7BCS) and the EAAT1 structure with three Na ions bound (7AWM) to generate the models of ASCT2. Three models of ASCT2 were generated, each representing different binding components: Lc-BPE bound with Na1, Na3, or Na1/Na3 sites occupied. The Na2 site is distorted in the inhibitor-bound state, and, therefore, was not occupied in MD simulations. Subsequently, each of these models was individually inserted into a lipid-bilayer within a water box. During the 100 ns simulations of Lc-BPE/Na1/Na3, full dissociation of Lc-BPE occurred in two out of the nine simulations, but no dissociation of Na+ ions was observed (Figs. 2, A and D and S2). The distances between Na1, Na3, and selected residues ranged from 2 to 5 Å. During the 100 ns simulations of Lc-BPE/Na1, full dissociation of Lc-BPE occurred in four out of nine simulations, whereas one simulation showed partial dissociation of Na1 (Figs. 2, B and D and S3). During the simulations of Lc-BPE/Na3, full dissociation of Lc-BPE occurred in seven out of the nine simulations, and no dissociation of the Na+ ion at the Na3 site was observed (Figs. 2, C and D and S4). Remarkably, in the Lc-BPE/Na3 model, it has been observed that an additional Na+ ion from the solution spontaneously binds to the Na1 site at 48 ns, 34 ns, and 24 ns, while one of these three Lc-BPE remains in the binding site (Figs. 2C and S4). However, the Lc-BPE/Na1 model did not exhibit any observable binding of additional Na+ ions. For all simulations, there was a marginal initial rise in the RMSD, indicating a relaxation of the structure compared to its initial state. The findings collectively support the conclusion from experiment that occupancy of the Na3 site is less critical for Lc-BPE binding than that of the Na1 site.
Figure 2.
The stability of bound Lc-BPE in MD simulations is affected by the occupation state of the Na1 and Na3 binding sites.A, representative trajectories for three independent simulations runs for Lc-BPE/Na1/Na3. B, representative trajectories for three independent simulations run for Lc-BPE/Na1. C, representative trajectories for three independent simulations run for Lc-BPE/Na3. Distances to selected atoms within the binding site for Lc-BPE (black), Na1 (green), Na3 (red) (RMSD is shown in blue). Distances were calculated based on atoms described in Experimental procedures. D, the residency times of Lc-BPE until dissociation. When distance was larger than 10 Å, the Lc-BPE was considered to be dissociated from the binding site. The arrow indicates that dissociation did not occur during the simulation of 100 ns. The star indicates an additional Na+ ion from the solution bound to the Na1 site. Lc-BPE, L-cis hydroxyproline biphenyl ester; MD, molecular dynamics.
Lc-BPE slows recovery of inward transient current induced by substrate application
The kinetics of the reaction steps related to the substrate translocation of ASCT2 and other transporters have been previously investigated by using rapid kinetic methods, i.e. laser photolysis of caged-compounds and piezo-based, rapid solution exchange (30, 31, 32, 33, 34, 35, 36). Here, to investigate the effect of Lc-BPE on the transport kinetics of ASCT2, we applied Lc-BPE rapidly using the piezo-based solution exchange system. This system allows the rapid application, as well as removal of amino acid or inhibitor, providing information on the recovery of current after amino acid and/or inhibitor removal. The kinetics of this recovery most likely reflects the turnover time of the transporter (37). The piezo-based system has a time resolution of 5 to 10 ms, which is sufficient to determine the turnover rate of ASCT2 and how it is influenced by the presence of inhibitor. As depicted in Figure 3A, rapid application of 2 mM serine to the extracellular side of cell membrane resulted in inward transient currents (transport component, no permeating anion present), attributed to the equilibration of the amino acid exchange equilibrium. Consistent with ASCT2’s function as an exchanger, no steady-state current was observed after the initial transient current decay (Fig. 3A). Upon removal of serine, an outward transient current was observed, indicative of restoration of the initial, outward facing transporter conformation. Following this first serine pulse (200 ms duration), a second pulse of serine was applied with varying delay times. In this process, the transporters have to recycle their binding sites to outward facing, completing a full transport cycle, before serine can induce another current response. The currents exhibited a relatively rapid recovery after removal of serine, as illustrated in Figure 3B, with a time constant of τ = 19 ± 2 ms. For ASCT2 mutants, when serine was rapidly applied to ASCT2D386N and ASCT2D473N, the inward transient currents were still detected, but the current amplitude decreased significantly in both mutants (Fig. S1B).
Figure 3.
Lc-BPE binding slows substrate turnover and is associated with outward charge movement.A, transient currents recorded in ASCT2 in response to two consecutive pulses of 2 mM serine, (C) 2 mM serine and 100 μM Lc-BPE or (E) 100 μM Lc-BPE alone in the presence of 140 mM of Na+, with varying interpulse interval (pulse protocol shown at the top, gray bars, homoexchange conditions). The inset shows the time course of the transient current on a magnified time scale. The intracellular solution contained 130 mM NaMes/10 mM serine, the extracellular solution contained 140 mM NaMes. B, recovery of the transient current in the presence of 2 mM Ser, (D) 2 mM serine and 100 μM Lc-BPE or (F) 100 μM Lc-BPE alone for ASCT2WT. The red solid lines represent the best fits to an exponential equation with time constant of 19 ± 2 ms (B, serine), 230 ± 20 ms (D, serine + Lc-BPE) and 320 ± 60 ms (F, Lc-BPE alone). G, similar experiment as in (C) but in the continuous presence of 100 μM Lc-BPE (as shown by the white bar). H, the transient current recorded in ASCT2D386N in response to 100 μM Lc-BPE. I, the transient current recorded in ASCT2D473N in response to 100 μM Lc-BPE. The membrane potential was 0 mV in all experiments. ASCT, alanine serine cysteine transporter; Lc-BPE, L-cis hydroxyproline biphenyl ester.
When serine was applied simultaneously together with a saturating concentration of 100 μM Lc-BPE, the amplitude of the transient current was reduced to 60% of that observed in the absence of Lc-BPE, as depicted in Figure 3C. Furthermore, the rapid decay of the inward transient current was followed by a small, but significant outward overshoot, presumably due to Lc-BPE binding and its subsequent inhibition of the serine response (Fig. 3C, inset). Analysis of the recovery time of the peak current revealed a time constant of 230 ± 20 ms (Fig. 3D), indicating a significant increase (about 10-fold) in recovery time, and, therefore, decrease in the turnover rate compared to the absence of Lc-BPE. In the continuous presence of Lc-BPE, as depicted in Fig. 3G, in contrast to Figure 3C, the transient current was completely abolished, indicating that binding of Lc-BPE to the transporter is slower than that of serine. All together, these results indicate that Lc-BPE exhibits inhibitory effects on both presteady-state current amplitude and turnover of serine exchange. The slow turnover rate in the presence of Lc-BPE can be attributed to the slow dissociation of the inhibitor (see below).
Lc-BPE binding to ASCT2 is associated with outward charge movement
Next, we applied Lc-BPE in the absence of substrate; upon the application of Lc-BPE, outward transient currents were observed (Fig. 3E). This result was unexpected, due to Lc-BPE being a neutral amino acid derivative, which should not carry a net charge. The time constant for transient current recovery was found to be 320 ± 60 ms (Fig. 3F), which is consistent with the time scale of recovery observed for the simultaneous application of serine and Lc-BPE (Fig. 3D). These results indicate that Lc-BPE demonstrates a slow rate of dissociation from the transporter binding site. To initiate the serine-induced inward transient current after Lc-BPE removal, the substrate needs to displace Lc-BPE, which has to dissociate first. Therefore, the simultaneous application of serine and Lc-BPE results in the rate of recovery being determined mostly by inhibitor dissociation rather than substrate-induced turnover. For the Na1 and Na3 site mutant transporters, the outward transient currents were still observed when Lc-BPE was rapidly applied to ASCT2D386N and ASCT2D473N and the current amplitude remained relatively unchanged (Figs. 3, H and I and S1B).
Na+ dependency of Lc-BPE-induced transient current is consistent with the occupation of Na1/Na3 sites, but not the Na2 site
To test if the outward transient current observed upon the application of Lc-BPE is coupled to the binding of Na+ ions, the rapid kinetic experiments were performed using varying concentrations of Na+. Typical currents recorded in the presence of 70 mM, 20 mM, 5 mM, and 1 mM Na+ are depicted in Figs. S5–S7, and Figure 4B, respectively. In the presence of serine, the transient currents exhibited an inward direction at Na+ concentrations of 70 mM, 20 mM, and 5 mM, as depicted in Figs. S5A, S6A and S7A. As expected, the amplitude of serine-induced current was significantly decreased when the concentration of Na+ was reduced from 140 mM to 5 mM (Fig. 4F). However, the recovery time of transient current was unaffected by the Na+ concentration (Fig. 4D). In contrast, at Na+ concentrations of 1 mM and below, the currents reversed sign toward the outward direction, as depicted in Figure 4, A and F, reminiscent of that observed after application of Lc-BPE.
Figure 4.
Outward transient currents in response to Lc-BPE are consistent with occupation of high affinity Na+binding site(s) and show weak voltage dependence.A, transient currents of ASCT2 in response to two pulses of rapid 2 mM serine, or (B) 100 μM Lc-BPE application in the presence of 1 mM Na+, with varying interpulse interval (pulse protocol shown at the top) under homoexchange conditions. The intracellular solution contained 130 mM NaMes/10 mM serine, the extracellular solution contained 140 mM NaMes. The inset shows the time course of the transient current on a magnified time scale. C, recovery of the transient current in the presence of 100 μM Lc-BPE alone at 1 mM Na+. The red solid lines represent the best fits to an exponential equation with time constant of 360 ± 70 ms. The membrane potential was 0 mV in all experiments. D, time constant of transient current recovery of 2 mM serine (black) or 100 μM Lc-BPE (red) application in the presence of 1/5/20/70/140 mM Na+ or 2 mM serine + 100 μM Lc-BPE (blue) in the presence of 140 mM Na+. E, amplitude of transient current induced by Lc-BPE application as a function of [Na+]. F, amplitude of transient current induced by serine application as a function of [Na+]. The red solid lines represent the best nonlinear curve fit to a Michaelis–Menten-like equation. The apparent affinities for Na+ were calculated as Km = 1.0 ± 0.5 mM for (E, Lc-BPE), Km1 = 0.04 ± 0.4 mM, Km2 = 44 ± 20 mM for (F, serine). G, typical serine-induced transient current recordings at varying membrane potentials (from −80 to + 60 mV, [serine] was 2 mM). H, similar experiment as in (G) but for Lc-BPE. I, the amplitude of transient current as function of the membrane potential for application of serine and Lc-BPE. ASCT, alanine serine cysteine transporter; Lc-BPE, L-cis hydroxyproline biphenyl ester.
For the transient current induced by Lc-BPE, in contrast to the results with serine, currents exhibited an outward direction at all Na+ concentrations (Figs. S5C, S6C, S7C and 4B). The amplitude of the currents was virtually independent of [Na+] at concentrations >5 mM (Fig. 4E). However, a notable decrease in amplitude was seen when the concentration was reduced to 1 mM, as depicted in Figure 4, B and E. The recovery time of the transient current was not affected across all Na+ concentrations (Fig. 4D). The apparent Km value for Na+ was determined to be 1.0 ± 0.5 mM for Lc-BPE, as calculated from the transient current (Fig. 4E), consistent with the high affinity observed for the Na+ binding to the apo-form of the transporter (most likely Na1 and Na3 sites), suggesting that the binding of Lc-BPE is solely associated with occupation of the apo transporter binding site(s) with Na+, but does not require Na+ binding to the Lc-BPE-bound form (presumably Na2 site).
Next, we investigated the voltage dependence of the transient currents in the presence of serine or Lc-BPE; the experiments were conducted at varying voltages from −80 to +60 mV (Fig. 4, G and H). Transient currents were then plotted as a function of the membrane potential, as shown in the I-V curves in Figure 4I. Both the serine-induced inward transient current and the Lc-BPE-induced outward transient current were weakly voltage dependent, exhibiting a small but significant increase as the membrane potential became more negative. This observation is in consistent with the hypothesis that binding of Na+ prior to serine or Lc-BPE is voltage dependent, as proposed previously (29), leading to an increase in affinity of serine or Lc-BPE.
Lc-BPE slows the onset of anion currents
To evaluate the inhibition kinetics, we determined the dissociation rate of Lc-BPE using a ligand displacement assay. Here, the ASCT2-expressing cells were preincubated with a certain concentration of Lc-BPE in the presence of intracellular SCN− (recording the anion current component). Following preincubation, Lc-BPE was rapidly replaced with 2 mM serine, which saturated the substrate binding site. After preincubation with Lc-BPE, a significant slowing of serine-induced anion current rise was observed (Fig. 5A). At 0.2 μM, 1 μM, and 5 μM preincubation, the substrate-induced anion current rise was biphasic, with a rapidly rising phase (phase 1) and a slow-rising phase (phase 2), presumably corresponding to the fraction of the transporter without or with Lc-BPE bound before serine application. The biphasic current was then fitted with two exponential components, the apparent time constants in 1 μM Lc-BPE preincubation were 4.9 ± 1.2 ms for phase 1 and 120 ± 14 ms for phase 2 (Fig. 5, A and B). In the absence of Lc-BPE, only the rapidly rising phase was observed. This phase had a time constant of 3.1 ± 1.2 ms, which is in line with phase 1 time constant observed in the presence of Lc-BPE. Subsequently, we further investigated Lc-BPE dissociation at even higher concentrations. At a concentration of 10, 100 μM, only the slow-rising phase was seen, indicating that the binding site for Lc-BPE was fully occupied before serine displacement (Figs. 5, A and B). As expected for inhibitor displacement, which is rate-limited by Lc-BPE dissociation, the time constants for both phases were independent of the Lc-BPE concentration (Fig. 5B).
Figure 5.
Lc-BPE dissociation rate constant is independent of Lc-BPE concentration but dependent on the membrane potential.A, typical traces of whole-cell current recordings of ligand displacement experiments. Cells were preincubated with buffer only (black trace), 1 μM Lc-BPE (red trace) or 100 μM Lc-BPE (blue trace). Serine (2 mM) was rapidly applied through solution exchange to ASCT2. (The application time for Lc-BPE and serine is indicated by the gray bar). The intracellular solution contained 130 mM NaSCN/10 mM serine, the extracellular solution contained 140 mM NaMes. B, Lc-BPE dissociation time constants in the presence of increasing concentrations of Lc-BPE. A two-exponential equation was used to fit the presteady state for 0.2 μM, 1 μM, and 5 μM Lc-BPE and a single-exponential equation for 0 μM, 10 μM, and 100 μM Lc-BPE upon substrate application (see Experimental procedures). C, typical currents at 0 mV, −100 mV or +60 mV membrane potential. D, Lc-BPE (100 μM) ln of the dissociation rate constant as a function of the cell membrane potential (linear fit, slope was −0.0037 ± 0.0002/mV). ASCT, alanine serine cysteine transporter; Lc-BPE, L-cis hydroxyproline biphenyl ester.
The time constant for Lc-BPE dissociation is sensitive to the membrane potential
Next, the time constant for Lc-BPE dissociation was quantified as a function of the transmembrane potential, using the same ligand displacement assay. This time constant decreased weakly with more negative membrane potential, indicating faster dissociation (Fig. 5, C and D). Based on the observed slope of the ln(k) versus voltage relationship in Fig. 5D, the valence of the Lc-BPE binding, zQ, was determined to be −0.19 ± 0.01 based on equation (1):
| (1) |
Here, V is the membrane potential, R is the gas constant, T the temperature, and F the Faraday constant. This result is consistent with an outward movement of negative charge or an inward movement of positive charge upon Lc-BPE dissociation (opposite of the charge movement from binding determined above), which are expected to accelerate at negative membrane potential. This observation suggests that the binding/dissociation of Lc-BPE is influenced by the electrical potential across the cell membrane, thereby further characterizing them as weakly electrogenic processes.
Prediction of electrostatic contributions to substrate/inhibitor binding mechanism
To further determine the physical basis behind the outward charge movement caused by application of neutral, but zwitterionic amino acid to ASCT2, we performed calculations of the electrostatics of the binding reaction. This approach is based on the solving of a linearized Poisson-Boltzmann equation using the Adaptive Poisson-Boltzmann Solver, APBS (https://apbs.readthedocs.io/en/latest/index.html), with the APBSMem protocol that is based on the placement of the protein in an implicit membrane and application of a membrane potential (38, 39). Calculation of the electrostatic energy change when switching between two states of the system (for example apo to amino acid bound) at varying membrane potentials allows the calculation of the valence associated with a potential charge movement. The setup of the system and calculation of the valence are illustrated in Figure 6, B and C. As expected, translocation of the fully loaded transporter is associated with a positive valence (Fig. 6D), due to the inward movement of net positive charge associate with the bound Na+ ions. In contrast, the cation binding sites of the empty transporter are predicted to carry negative charge (Fig. 6D). Interestingly, glutamine binding to the outward open-loop configuration of ASCT2 is predicted to have a valence of −0.08, consistent with transient outward current induced by substrate binding. A similar, negative valence was calculated for Lc-BPE binding. In contrast, HP2 closing is associated with a small positive valence (Figs. 6, C and D). Where could this negative charge movement upon binding originate from? A possible explanation would be that the negative charge of the α-carboxy group of the amino acid is more deeply buried in the membrane compared to the positive charge of the α-amino function. Consistent with this hypothesis, the valence of binding of a glutamine molecule, in which all charges but one negative charge on the carboxy group were neutralized, was −0.26. In contrast, the absolute valence of binding with only one positive charge on the amino group was smaller, z = +0.20 (Fig. 6D). Together, these results suggest that the negative carboxy group moves deeper into the membrane electric field than the amino group, thus generating an overall negative valence for substrate/inhibitor binding. On the other hand, occlusion of the negative charge of the binding site is predicted to be associated with a very small valence (about −0.01). Overall, the results of the electrostatic calculations are consistent with the experimental results, showing outward charge movement induced by both amino acid and Lc-BPE application.
Figure 6.
Proposed simplified mechanism of competitive inhibitor (In) binding to transporter (T).A, the amino acid substrate is abbreviated as S, the inhibitor as I. The bars illustrate the anion-conducting states. B, illustration of the setup of the electrostatic calculations of the valence of the charge movement caused by glutamine (atoms represented as spheres) binding (arrow). The protein backbone is shown in green (PDB code: 6mpb), the accessibility map calculated using APBSMem is shown as the blue mesh, the membrane potential gradient calculated using a noncharged transporter is shown in red. C, relative energy difference between two states of the system for gln binding (unbound and bound state, top) and HP2 closure (in absence of gln, bottom) as a function of the membrane potential. The valence for the transition, (D) is obtained from the slope of the relationships shown in (C). HP2, hairpin loop 2; PDB, Protein Data Bank.
Lc-BPE application has only minor effect on the glutamate transporter
It was reported that Lc-BPE also inhibits excitatory amino acid carrier 1 (EAAC1), the rat analog of human EAAT3, although with lower apparent affinity (24). Glutamate transport by EAAC1 is electrogenic, involving the cotransport of three Na+ ions and one proton, as well as the counter transport of one K+ ion, for each transported glutamate molecule. Consistent with this stoichiometry, large inward transient currents were observed upon rapid application of glutamate, followed by a steady-state current component caused by electrogenic transport (Figs. 7A and S8, A and C). The time constant for transient current recovery was calculated to be 140 ± 8 ms (Fig. 7B). Recovery of the transient current was slowed and peak amplitudes were reduced in the presence of 100 μM DL-TBOA (40), a competitive inhibitor specifically targeting glutamate transporters (Fig. S8, A and C). TBOA application alone (without glutamate) resulted in a small, transient outward current, followed by an inwardly directed overshoot current (Fig. S8E), consistent with previous results with kainate, another glutamate transporter inhibitor (41). These results were as expected for competitive inhibitor behavior with EAAC1. In contrast to TBOA, Lc-BPE had virtually no effect on the recovery time constant of the transient current (110 ± 10 ms, Fig. 7, C and D). Furthermore, in contrast to ASCT2, the application of Lc-BPE alone did not induce significant transient current in EAAC1 (Fig. 7E). Together, these results indicate that while Lc-BPE may be able to inhibit EAAC1 anion current, it has much less effect on stoichiometric charge movement by the transporter.
Figure 7.
Lc-BPE has minor effect on substrate translocation in EAAC1.A, transient currents recorded in EAAC1 in response to two consecutive pulses of 20 μM glutamate, (C) 20 μM glutamate and 100 μM Lc-BPE or (E) 100 μM Lc-BPE alone application in the presence of 140 mM of Na+, with varying interpulse interval (pulse protocol shown at the top, gray bars). The intracellular solution contained 130 mM KMes, the extracellular solution contained 140 mM NaMes. B, recovery of the transient current in the presence of 20 μM glutamate or D, 20 μM glutamate and 100 μM Lc-BPE. The red solid lines represent the best fits to an exponential equation with time constant of 140 ± 8 ms (glutamate) and 110 ± 10 ms (glutamate + Lc-BPE). E, application of 100 μM Lc-BPE alone in the presence of 140 mM of Na+. The membrane potential was 0 mV in all experiments. EAAC1, excitatory amino acid carrier 1; Lc-BPE, L-cis hydroxyproline biphenyl ester.
Discussion
In this report, we detail the involvement of electrostatics in the binding mechanism of competitive inhibitors, substrates as well as Na+ ions in the glutamine transporter ASCT2, including a comprehensive analysis of results from electrophysiological and rapid kinetic experiments. First, our results indicate that Lc-BPE binding is consistent with a model that only requires Na+ binding to the apo-form of the transporter. In this model, at least one or two Na+ ion(s) initially bind(s) to the transporter when it is unoccupied (presumably to the Na1/Na3 sites), resulting in the formation of a high-affinity inhibitor/substrate binding site (Fig. 6A). Results from mutagenesis and MD simulations indicate that the Na1 site, rather than the Na3 site, plays a more critical role in Lc-BPE binding, although occupancy of both Na1 and Na3 sites resulted in the best ligand stability in MD simulations. Second, in contrast to transported substrate, HP2 cannot fully close in the Lc-BPE-bound state; thus, the Na2 site is not formed and cannot be occupied. Third, initial binding of Lc-BPE and substrate are associated with outward charge movement, despite these compounds having a net neutral charge. Fourth, Lc-BPE not only suppresses the substrate-induced anion current, but also slows the kinetics of the serine-induced current and the turnover of serine exchange. Finally, displacement experiment suggests that the rate of activation of anion current induced by substrate is slowed when the cell was preincubated with Lc-BPE, pointing to relatively slow Lc-BPE dissociation kinetics, i.e. long residency time of inhibitor in the binding site.
The sequence of sodium and amino acid binding to the transporters is conserved in the SLC1 family (29, 33, 42, 43). Amino acid transport requires the cotransport of two or three Na+ ions. It was proposed that in the apo-state of the transporters, HP2 is closed, preventing access to the substrate binding site (19). Subsequently, in ASCTs, Na+ binds to Na1/Na3 sites with very high apparent affinity in the submillimolar range, which is consistent with our result in Figure 1. This is one of the major functional differences between the ASCT and EAAT members of the family, where in the EAATs initial Na+ binding is of much lower apparent affinity, in the 100 mM range. The binding to and occupation of the Na1/Na3 sites induces remodeling of the substrate binding site, leading to the opening of HP2 and facilitating substrate binding. This process is accompanied by the closure of HP2, which effectively secures the substrate in the outward-occluded state (16, 17, 18). On the other hand, binding of the competitive inhibitor Lc-BPE prevents loop closure, with the consequence that the third Na+ ion is unable to bind to the Na2 site. The cryo-EM structure of Lc-BPE-hASCT2 showed that HP2 is less resolved, suggesting the HP2 gate exhibits flexibility and has the capacity to move further to accommodate bulkier inhibitors (24), thus disrupting the structure of the Na2 site. The result is that Lc-BPE binding cannot induce the inward charge movement seen upon application of transportable substrates, which is indicative of Na+ binding to the Na2 site and structural changes following this binding. The likely sequence of binding of Na+ and substrate/inhibitor, accounting for the experimental data for WT and Na-site mutant transporters is illustrated in the mechanism shown in Figure 6A.
Results from the rapid kinetic experiments also indicate that the presence of Lc-BPE leads to a complete inhibition of serine-induced inward transient charge movement, as well as a substantial decrease in the turnover rate of the transporter, as expected for a competitive inhibitor. This is evident from the observed >10-fold reduction in the recovery rate of transient current after substrate removal (Fig. 3). Due to the long residency time of Lc-BPE in the binding site (see below) the recovery kinetics should depend primarily on the inhibitor dissociation rate rather than the substrate turnover rate. On the other hand, rapid application of Lc-BPE alone induced outward transient currents. In some experiments, as depicted in Figure 3E, discernible inward overshoots were detected, possibly resulting from the binding of Na+ ions to the unoccupied transporters (44). A transient outward current can generally be induced by the outward movement of positively charged ions or the inward movement of negatively charged ions, until a new state or equilibrium is reached. Due to its largely net neutral nature at physiological pH (the isoelectric point of proline is 6.3), it is, therefore, not immediately evident why Lc-BPE binding would result in charge movement. However, the application of serine at low Na+ concentrations, when the Na2 site cannot be occupied, also induced outward transient currents, hence exhibiting a similar functional effect as a competitive inhibitor. Therefore, it is unlikely that this outward current is an artifact.
What could be the molecular basis of the outward charge movement upon substrate/inhibitor binding, despite the net neutral charge of the zwitterionic species? Our results from calculations of the electrostatic interaction of the amino acid with its binding site point to the following hypothesis: The negatively charged α-carboxy group of the amino acid penetrates the membrane electric field more deeply than the positively charged α-amino group. The predicted overall valence of the charge movement is small (less than 10% of one charge), consistent with the weak voltage dependence of the dissociation time constant. This voltage dependence demonstrates that a more positive membrane potential results in a longer residency time of the ligand in the binding site, as expected, if the residency time is dominated by the membrane potential’s effect on the bound negative charge of the α-carboxy group of the ligand. In contrast, the amplitude of the substrate/inhibitor induced current shows the opposite voltage dependence, increasing with a more negative potential, indicating that this amplitude is not controlled by ligand binding, but rather by some other, voltage dependent step, possibly conformational changes of HP2.
Overall, these results open intriguing possibilities for the electrophysiological detection of ligand binding to transmembrane proteins, and/or conformational changes associated with those binding events. Even for net neutral ligands, charge movement may be detected electrophysiologically, if there is an asymmetrical charge distribution when the molecule is bound in the transmembrane electric field. While electrogenic partial reactions have been observed in the electroneutral Na/H exchanger (45) and serotonin transporter (46), both involving charged ions/substrates, to our knowledge, electrophysiological analysis of charge movements induced by neutral molecules has not been reported in the literature. Ultimately, this may be the main significance of this work, extending electrophysiological methods to membrane proteins that have not been thought to be amenable to a current recording approach. Therefore, this may have implications for the study of other important electroneutral transporters. For example, Na+-independent amino acid transporters, such as the large amino acid transporter, LAT1, could be reconsidered to test with an electrophysiological approach.
Are there alternative explanations for the outwardly directed charge movement upon inhibitor/substrate application? One possibility could be that less than 100% of the substrate binding sites are outward facing before Lc-BPE application. In other words, the translocation equilibrium is not fully pushed to the outward-facing state. Due to the zero-trans conditions for amino acid substrate used in these experiments (saturating [serine] inside the cell, zero [serine] on the outside), this is not likely. In addition, lowering external [Na+] would be expected to further push the equilibrium to outward facing, but the outward current is maintained to very low external [Na+]. However, if this was the case, Lc-BPE binding would be expected to increase the population of the outward facing state. Thus, the outward current would be caused by outward movement of the positively charged transport domain, which is loaded with amino acid and Na+. In a recent publication, Burtscher et al. report similar rapid transient currents induced by binding of the inhibitor cocaine to the serotonin transporter, serotonin transporter (46). Cocaine is positively charged. The binding current was attributed to the displacement of Gouy-Chapman surface charge. In contrast, the amino acid derivatives used in our report are net neutral. Therefore, the Gouy-Chapman hypothesis is less likely. Other limitations of our study are related to the reliance on functional measurements, albeit with high time resolution and sensitivity. Ultimately, the determination of subtle conformational changes that determine inward or outward charge movement will have to come from structural studies.
Our results also provide further insight into the residency time of the competitive inhibitor, Lc-BPE, in the binding site. The dissociation kinetics of Lc-BPE in displacement experiments are characterized by a slow dissociation rate. The activation rate by serine of the anion current is slowed when the cell is preincubated with the Lc-BPE inhibitor, reflecting the dissociation of the inhibitor. The displacement of the bound inhibitor by the substrate takes place within a time scale of 120 ms. Such a slow apparent dissociation time constant agrees with the high binding affinity exhibited by Lc-BPE, in the sub-μM range. In contrast, residency time for serine is much shorter, with the higher dissociation rate being a necessity for rapid transporter turnover during amino acid exchange.
To summarize, in the present study, the binding mechanism of a competitive inhibitor in ASCT2 was elucidated through the analysis of presteady-state kinetic data. Our results suggest that the binding of a competitive inhibitor is coupled to the binding of Na+ to the Na1/Na3 site, while the Na2 site remains unoccupied. In contrast, the binding of substrate, subsequently leading to transport, necessitates the occupancy of three Na sites, including the Na2 site. At low concentrations of sodium, the Na2 site is not occupied, even in the substrate bound form, resulting in electrophysiological behavior that is indistinguishable from that of the inhibitor, Lc-BPE. With respect to the actual electrostatic contribution to the substrate/inhibitor binding process, it was determined that movement of the negatively charged α-carboxy group into the transmembrane electric field dominates the voltage dependence of the process, even though the amino acid has a net neutral charge. The presence of Lc-BPE also resulted in a slowing of substrate exchange turnover and a decrease in the rate of activation of substrate-induced anion current, due to its long residency time in the ASCT2 binding site. Given the increasing significance of ASCT2 as a potential target for anticancer drugs, our findings have the potential to contribute to the mechanism-based development and optimization of ASCT2 inhibitors.
Experimental procedures
Cell culture and transfection
Human embryonic kidney 293T cells (HEK293T, American Type Culture Collection No. CRL 1573) were cultured as described previously (47, 48). rASCT2 or EAAC1 complementary DNAs constructs inserted in the pBK-CMV vector were transiently transfected into cells using jet-PRIME transfection reagent. Cells were tested for mycoplasma and authenticated by short tandem repeat profiling (ATCC). Transfections were performed according to the protocol supplied by Polyplus-Transfection. The cells used for electrophysiological analysis were cultured for 20 to 30 h after transfection. Mutagenesis was performed using the QuikChange protocol according to the directions of the supplier (Agilent). Mutations were confirmed using sequencing.
Electrophysiology
Currents associated with rASCT2 and EAAC1 were measured in the whole-cell current recording configuration with an EPC7 patch-clamp Amplifier (ALA Scientific). Whole-cell currents were recorded under voltage-clamp conditions. The resistance of the recording electrode was 3 to 6 MΩ. Series resistance was not compensated because of the small whole-cell currents carried by EAAC1 and ASCT2. For ASCT2, the composition of the solutions for measuring amino acid exchange currents in the transport mode was as follows: 140 mM sodium methanesulfonate (NaMes), 2 mM Mg gluconate 2, 2 mM CaMes2, 10 mM Hepes, with additional amino acid substrates or/and inhibitor, pH 7.3 (extracellular), and 130 mM NaMes (for the measurement of anion current, intracellular NaMes was replaced with sodium thiocyanate (NaSCN)), 2 mM Mg gluconate 2, 5 mM EGTA, 10 mM Hepes, pH 7.3 (intracellular), as published previously (47, 49, 50). For EAAC1, the composition of the solutions for measuring amino acid exchange currents in the transport mode, the intracellular NaMes was replaced by KMes. For experiments on Na+ dependence, the concentration of extracellular NaMes was adjusted and compensated for by NMGMes.
Rapid solution exchange
Fast solution exchanges were performed using the SF-77B (Warner Instruments LLC) piezo-based solution exchange instrument, allowing a time resolution in the 5 to 10 ms range. Amino acid substrate was applied through a theta capillary glass tubing (TG200-4, outer diameter = 2.00 mm, inner diameter = 1.40 mm; Warner Instruments), with the tip of the theta tubing pulled to a diameter of 350 μm and positioned at 0.5 mm to the cell (37). For paired-pulse experiments, currents were recorded with 10/20/40 ms interval time after removal of amino acid.
Voltage-jump experiments
Voltage jumps (−100 to +60 mV) were applied to perturb the translocation equilibrium and to determine the voltage dependence of the transient current and anion conductance. To determine ASCT2-specific currents, external solution contained 140 mM NaMes in the presence of amino acid substrate and/or competitive inhibitor (as control). The internal solution contained 130 mM NaMes in the presence of 10 mM amino acid substrate (for the displacement experiment, intracellular NaMes was replaced with NaSCN). Capacitive transient compensation and series resistance compensation of up to 80% was used using the EPC-7 amplifier.
Electrostatic calculations of Gln and Lc-BPE binding valence
We utilized the APBSmem 2.1.0 (38) (https://apbsmem.sourceforge.io/), to compute electrostatic energies based on the adaptive Poisson-Boltzmann solver, APBS (39). These calculations involved examining the binding of the inhibitor Lc-BPE to ASCT2 within an implicit membrane environment. Our analysis considered substrate Gln binding, along with the HP2 open and closure states. In the presence of an internal membrane potential, V, the following modified version of the linearized Poisson-Boltzmann equation is used, according to the procedure first introduced by Roux (51):
| (2) |
Here, ε represents the spatially dependent dielectric constant, Φ is the electrostatic potential, and κ stands for the Debye–Hückel screening constant and kb the Boltzmann constant. The function fI as a Heaviside step function, specifically set at 1 in the intracellular solution and 0 in the membrane, protein, and extracellular solution. Details can be found in reference (38).
The total electrostatic energy, E, is then computed by summing up over the product of the local charge and the potential (52):
| (3) |
The valence is computed by applying various internal membrane potentials (V) and calculating the difference in total electrostatic energy ΔE between protein configurations with bound and free substrate. The valence is determined from the slope of the ΔE versus membrane potential plot (53). Further details are available in previous publications (41, 54).
Data analysis
The data analysis was performed in Microsoft Excel (https://www.office.com/) and Microcal Origin (https://www.originlab.com/) software. Error bars are shown as mean ± SD, collected from recordings of 6 to 10 cells, for statistical analysis.
To determine the recovery rate of transient currents, nonlinear curve fitting was used with the following exponential function:
| (4) |
Here, I is the current amplitude, Imax is the current for the first pulse, τ the time constant, and t the time.
Transient signals of piezo-based solution-exchange results were analyzed in Clampfit software (Axon Instruments, https://www.moleculardevices.com/products/axon-patch-clamp-system/acquisition-and-analysis-software/pclamp-software-suite) by fitting with a sum of two exponential components:
| (5) |
To determine Na+ apparent Km values, nonlinear curve fitting was used with a Michaelis–Menten like equation:
| (6) |
Here, Imax is the current at saturating substrate concentration, [Na+] is the concentration of Na+.
MD simulations
The model system for MD simulations was generated with VMD software (55) (https://www.ks.uiuc.edu/Research/vmd/), using an ASCT2 structure (7BCS) (24). The Lc-BPE parameter, generated via the multipurpose atom-typer for CHARMM (MATCH) approach (56), using SwissParam (57). The final model was inserted in a preequilibrated POPC lipid bilayer with the dimensions of 130 × 130 × 90 Å. TIP3P water was added to generate a box measuring 100 Å in the z-direction. NaCl was added at a total concentration of 0.15 M, and the system was neutralized. The total number of atoms in the system was 136,293. Simulations were run using the CHARMM36 force field. NAMD (58) simulations were performed using 2000 steps of minimization, followed by 10 ns equilibration runs under constant pressure conditions (NPT), and then for 100 ns (59). The RMSD (calculated from the peptide backbone) increased from 1 Å soon after simulation starts to ∼3.5 Å after 20 ns of equilibration, after which it was at steady state. The cutoff for local electrostatic interactions was set to 12 Å. For long-range electrostatic interactions, we used the particle-mesh Ewald method implemented in NAMD. Bonds to hydrogen atoms and TIP3P water were kept rigid using SHAKE. The time steps of the simulations were 2 fs. The time evolution of distances and distribution function analysis were calculated by Tcl/Tk programs built in the VMD software. For distance calculations, we selected S353 (N) from the transporter and (O08) from the Lc-BPE as reference atoms to evaluate the distance changes of Lc-BPE; D475 (OD1) from the transporter and SOD 1 as reference atoms to evaluate the distance changes of Na1; D388 (OD1) from the transporter and SOD 3 as reference atoms to evaluate the distance changes of Na3.
Data availability
All the data needed to support the conclusions of this study are presented in the article and/or supplementary materials. Raw data are available upon reasonable request.
Supporting information
This article contains supporting information.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
We acknowledge Frank Marcoline for his assistance on the troubleshooting in APBSmem.
Author contributions
Y. D., J. W., and C. G. conceptualization; Y. D., J. W., and C. G. methodology; Y. D., J. W., and C. G. formal analysis; Y. D., J. W., and C. G. writing–review and editing; Y. D., J. W., and C. G. validation; Y. D., J. W., and C. G. investigation; Y. D., J. W., and C. G. visualization; Y. D. and J. W. data curation; Y. D. and J. W. writing–original draft; Y. D. and J. W. software; C. G. supervision; C. G. project administration; C. G. funding acquisition; C. G. resources; C. G. supervision.
Funding and additional information
This study was supported by grants from the National Institutes of Health (R01CA277794) (Schlessinger, PI); and R15 GM135843 awarded to C. G. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Karen Fleming
Supporting information
References
- 1.Arriza J.L., Kavanaugh M.P., Fairman W.A., Wu Y.N., Murdoch G.H., North R.A., et al. Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J. Biol. Chem. 1993;268:15329–15332. [PubMed] [Google Scholar]
- 2.Utsunomiya-Tate N., Endou H., Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J. Biol. Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- 3.Zerangue N., Kavanaugh M.P. ASCT-1 is a neutral amino acid exchanger with chloride channel activity. J. Biol. Chem. 1996;271:27991–27994. doi: 10.1074/jbc.271.45.27991. [DOI] [PubMed] [Google Scholar]
- 4.Jain M., Nilsson R., Sharma S., Madhusudhan N., Kitami T., Souza A.L., et al. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science. 2012;336:1040–1044. doi: 10.1126/science.1218595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alberghina L., Gaglio D. Redox control of glutamine utilization in cancer. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2014.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun H.W., Yu X.J., Wu W.C., Chen J., Shi M., Zheng L., et al. GLUT1 and ASCT2 as predictors for prognosis of hepatocellular carcinoma. PLoS One. 2016;11 doi: 10.1371/journal.pone.0168907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S., Kim D.H., Jung W.H., Koo J.S. Expression of glutamine metabolism-related proteins according to molecular subtype of breast cancer. Endocr. Relat. Cancer. 2013;20:339–348. doi: 10.1530/ERC-12-0398. [DOI] [PubMed] [Google Scholar]
- 8.Toda K., Nishikawa G., Iwamoto M., Itatani Y., Takahashi R., Sakai Y., et al. Clinical role of ASCT2 (SLC1A5) in KRAS-mutated colorectal cancer. Int. J. Mol. Sci. 2017;18:1632. doi: 10.3390/ijms18081632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy L.M., Warr O., Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. J. Neurosci. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerangue N., Kavanaugh M.P. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]
- 11.Kanner B.I., Sharon I. Active transport of L-glutamate by membrane vesicles isolated from rat brain. Biochemistry. 1978;17:3949–3953. doi: 10.1021/bi00612a011. [DOI] [PubMed] [Google Scholar]
- 12.Wadiche J.I., Amara S.G., Kavanaugh M.P. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- 13.Broer A., Wagner C., Lang F., Broer S. Neutral amino acid transporter ASCT2 displays substrate-induced Na+ exchange and a substrate-gated anion conductance. Biochem. J. 2000;346 Pt 3:705–710. [PMC free article] [PubMed] [Google Scholar]
- 14.Scopelliti A.J., Heinzelmann G., Kuyucak S., Ryan R.M., Vandenberg R.J. Na+ interactions with the neutral amino acid transporter ASCT1. J. Biol. Chem. 2014;289:17468–17479. doi: 10.1074/jbc.M114.565242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reyes N., Ginter C., Boudker O. Transport mechanism of a bacterial homologue of glutamate transporters. Nature. 2009;462:880–885. doi: 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastug T., Heinzelmann G., Kuyucak S., Salim M., Vandenberg R.J., Ryan R.M. Position of the third Na+ site in the aspartate transporter GltPh and the human glutamate transporter, EAAT1. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verdon G., Oh S., Serio R.N., Boudker O. Coupled ion binding and structural transitions along the transport cycle of glutamate transporters. Elife. 2014;3 doi: 10.7554/eLife.02283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guskov A., Jensen S., Faustino I., Marrink S.J., Slotboom D.J. Coupled binding mechanism of three sodium ions and aspartate in the glutamate transporter homologue Glt(Tk) Nat. Commun. 2016;7 doi: 10.1038/ncomms13420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen S., Guskov A., Rempel S., Hanelt I., Slotboom D.J. Crystal structure of a substrate-free aspartate transporter. Nat. Struct. Mol. Biol. 2013;20:1224–1226. doi: 10.1038/nsmb.2663. [DOI] [PubMed] [Google Scholar]
- 20.Grewer C., Grabsch E. New inhibitors for the neutral amino acid transporter ASCT2 reveal its Na+-dependent anion leak. J. Physiol. 2004;557:747–759. doi: 10.1113/jphysiol.2004.062521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh K., Tanui R., Gameiro A., Eisenberg G., Colas C., Schlessinger A., et al. Structure activity relationships of benzylproline-derived inhibitors of the glutamine transporter ASCT2. Bioorg. Med. Chem. Lett. 2017;27:398–402. doi: 10.1016/j.bmcl.2016.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Esslinger C.S., Cybulski K.A., Rhoderick J.F. Ngamma-aryl glutamine analogues as probes of the ASCT2 neutral amino acid transporter binding site. Bioorg. Med. Chem. 2005;13:1111–1118. doi: 10.1016/j.bmc.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Albers T., Marsiglia W., Thomas T., Gameiro A., Grewer C. Defining substrate and blocker activity of alanine-serine-cysteine transporter 2 (ASCT2) Ligands with Novel Serine Analogs. Mol. Pharmacol. 2012;81:356–365. doi: 10.1124/mol.111.075648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garibsingh R.A., Ndaru E., Garaeva A.A., Shi Y., Zielewicz L., Zakrepine P., et al. Rational design of ASCT2 inhibitors using an integrated experimental-computational approach. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2104093118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X., Plotnikova O., Bonin P.D., Subashi T.A., McLellan T.J., Dumlao D., et al. Cryo-EM structures of the human glutamine transporter SLC1A5 (ASCT2) in the outward-facing conformation. Elife. 2019;8 doi: 10.7554/eLife.48120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garaeva A.A., Oostergetel G.T., Gati C., Guskov A., Paulino C., Slotboom D.J. Cryo-EM structure of the human neutral amino acid transporter ASCT2. Nat. Struct. Mol. Biol. 2018;25:515–521. doi: 10.1038/s41594-018-0076-y. [DOI] [PubMed] [Google Scholar]
- 27.Garaeva A.A., Guskov A., Slotboom D.J., Paulino C. A one-gate elevator mechanism for the human neutral amino acid transporter ASCT2. Nat. Commun. 2019;10:3427. doi: 10.1038/s41467-019-11363-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Canul-Tec J.C., Kumar A., Dhenin J., Assal R., Legrand P., Rey M., et al. The ion-coupling mechanism of human excitatory amino acid transporters. EMBO J. 2022;41 doi: 10.15252/embj.2021108341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zander C.B., Albers T., Grewer C. Voltage-dependent processes in the electroneutral amino acid exchanger ASCT2. J. Gen. Physiol. 2013;141:659–672. doi: 10.1085/jgp.201210948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z., Papageorgiou G., Corrie J.E., Grewer C. Pre-steady-state currents in neutral amino acid transporters induced by photolysis of a new caged alanine derivative. Biochemistry. 2007;46:3872–3880. doi: 10.1021/bi0620860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong Y., Wang J., Garibsingh R.A., Hutchinson K., Shi Y., Eisenberg G., et al. Conserved allosteric inhibition mechanism in SLC1 transporters. Elife. 2023;12 doi: 10.7554/eLife.83464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Corrie J.E., Munasinghe V.R., Trentham D.R., Barth A. Studies of decarboxylation in photolysis of alpha-carboxy-2-nitrobenzyl (CNB) caged compounds. Photochem. Photobiol. Sci. 2008;7:84–97. doi: 10.1039/b711398f. [DOI] [PubMed] [Google Scholar]
- 33.Watzke N., Bamberg E., Grewer C. Early intermediates in the transport cycle of the neuronal excitatory amino acid carrier EAAC1. J. Gen. Physiol. 2001;117:547–562. doi: 10.1085/jgp.117.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi Y., Wang J., Ndaru E., Grewer C. Pre-steady-state kinetic analysis of amino acid transporter SLC6A14 reveals rapid turnover rate and substrate translocation. Front Physiol. 2021;12 doi: 10.3389/fphys.2021.777050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grewer C., Madani Mobarekeh S.A., Watzke N., Rauen T., Schaper K. Substrate translocation kinetics of excitatory amino acid carrier 1 probed with laser-pulse photolysis of a new photolabile precursor of D-aspartic acid. Biochemistry. 2001;40:232–240. doi: 10.1021/bi0015919. [DOI] [PubMed] [Google Scholar]
- 36.Wang J., Zielewicz L., Dong Y., Grewer C. Pre-steady-state kinetics and reverse transport in rat glutamate transporter EAAC1 with an immobilized transport domain. Neurochem. Res. 2022;47:148–162. doi: 10.1007/s11064-021-03247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J., Dong Y., Grewer C. Functional and kinetic comparison of alanine cysteine serine transporters ASCT1 and ASCT2. Biomolecules. 2022;12:113. doi: 10.3390/biom12010113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Callenberg K.M., Choudhary O.P., de Forest G.L., Gohara D.W., Baker N.A., Grabe M. APBSmem: a graphical interface for electrostatic calculations at the membrane. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baker N.A., Sept D., Joseph S., Holst M.J., McCammon J.A. Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U. S. A. 2001;98:10037–10041. doi: 10.1073/pnas.181342398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shimamoto K., Lebrun B., Yasuda-Kamatani Y., Sakaitani M., Shigeri Y., Yumoto N., et al. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol. Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 41.Zielewicz L., Wang J., Ndaru E., Grewer C.T. Transient kinetics reveal mechanism and voltage dependence of inhibitor and substrate binding to glutamate transporters. ACS Chem. Biol. 2019;14:1002–1010. doi: 10.1021/acschembio.9b00194. [DOI] [PubMed] [Google Scholar]
- 42.Wadiche J.I., Kavanaugh M.P. Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. J. Neurosci. 1998;18:7650–7661. doi: 10.1523/JNEUROSCI.18-19-07650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bergles D.E., Tzingounis A.V., Jahr C.E. Comparison of coupled and uncoupled currents during glutamate uptake by GLT-1 transporters. J. Neurosci. 2002;22:10153–10162. doi: 10.1523/JNEUROSCI.22-23-10153.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanui R., Tao Z., Silverstein N., Kanner B., Grewer C. Electrogenic steps associated with substrate binding to the neuronal glutamate transporter EAAC1. J. Biol. Chem. 2016;291:11852–11864. doi: 10.1074/jbc.M116.722470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calinescu O., Linder M., Wohlert D., Yildiz O., Kuhlbrandt W., Fendler K. Electrogenic cation binding in the electroneutral Na+/H+ antiporter of pyrococcus abyssi. J. Biol. Chem. 2016;291:26786–26793. doi: 10.1074/jbc.M116.761080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burtscher V., Hotka M., Li Y., Freissmuth M., Sandtner W. A label-free approach to detect ligand binding to cell surface proteins in real time. Elife. 2018;7 doi: 10.7554/eLife.34944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grewer C., Gameiro A., Rauen T. SLC1 glutamate transporters. Pflugers Arch. 2014;466:3–24. doi: 10.1007/s00424-013-1397-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanai Y., Hediger M.A. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- 49.Wang J., Zielewicz L., Grewer C. A K(+)/Na(+) co-binding state: simultaneous versus competitive binding of K(+) and Na(+) to glutamate transporters. J. Biol. Chem. 2019;294:12180–12190. doi: 10.1074/jbc.RA119.009421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang J., Zhang K., Goyal P., Grewer C. Mechanism and potential sites of potassium interaction with glutamate transporters. J. Gen. Physiol. 2020;152 doi: 10.1085/jgp.202012577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roux B. Influence of the membrane potential on the free energy of an intrinsic protein. Biophys. J. 1997;73:2980–2989. doi: 10.1016/S0006-3495(97)78327-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva J.R., Pan H., Wu D., Nekouzadeh A., Decker K.F., Cui J., et al. A multiscale model linking ion-channel molecular dynamics and electrostatics to the cardiac action potential. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11102–11106. doi: 10.1073/pnas.0904505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choudhary O.P., Ujwal R., Kowallis W., Coalson R., Abramson J., Grabe M. The electrostatics of VDAC: implications for selectivity and gating. J. Mol. Biol. 2010;396:580–592. doi: 10.1016/j.jmb.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J., Albers T., Grewer C. Energy landscape of the substrate translocation equilibrium of plasma-membrane glutamate transporters. J. Phys. Chem. B. 2018;122:28–39. doi: 10.1021/acs.jpcb.7b09059. [DOI] [PubMed] [Google Scholar]
- 55.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14 doi: 10.1016/0263-7855(96)00018-5. 33-38, 27-38. [DOI] [PubMed] [Google Scholar]
- 56.Zoete V., Cuendet M.A., Grosdidier A., Michielin O. SwissParam: a fast force field generation tool for small organic molecules. J. Comput. Chem. 2011;32:2359–2368. doi: 10.1002/jcc.21816. [DOI] [PubMed] [Google Scholar]
- 57.Yesselman J.D., Price D.J., Knight J.L., Brooks C.L., 3rd MATCH: an atom-typing toolset for molecular mechanics force fields. J. Comput. Chem. 2012;33:189–202. doi: 10.1002/jcc.21963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Phillips J.C., Braun R., Wang W., Gumbart J., Tajkhorshid E., Villa E., et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J., Li P., Yu X., Grewer C. Observing spontaneous, accelerated substrate binding in molecular dynamics simulations of glutamate transporters. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data needed to support the conclusions of this study are presented in the article and/or supplementary materials. Raw data are available upon reasonable request.