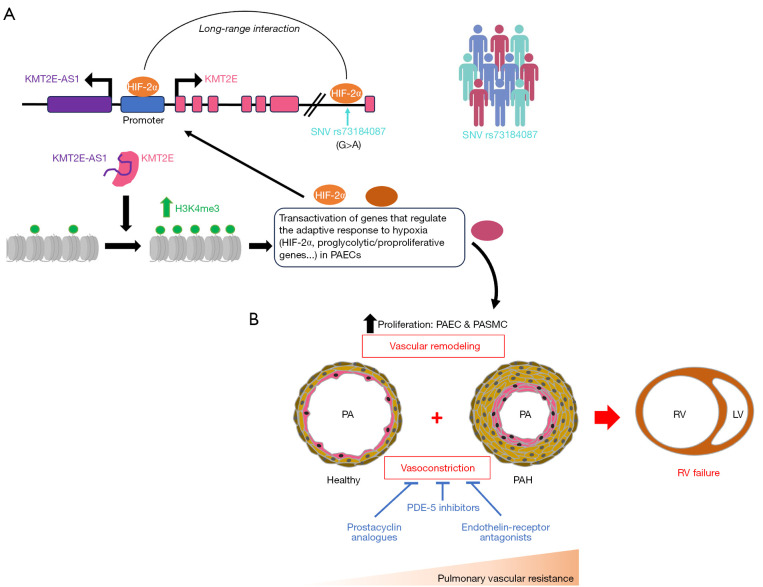
Figure 1.
Potential implications of the results presented in the study against background PAH therapy. (A) Graphic summary of results presented in the study. Under hypoxia or pseudohypoxia, HIF-2α activation in PAECs increases expression of the lncRNA KMT2E-AS1 and neighboring gene KMT2E. KMT2E-AS1 binds and stabilizes KMT2E leading to increase H3K4me3 deposition and transactivation of genes favoring metabolic adaptation and proliferation, including HIF-2α. This creates a feed-forward loop sustaining pathogenic endothelial activity and pulmonary vascular remodeling. A SNV (rs73184087) within a KMT2E intron was found to be associated with the risk of developing PH. SNV rs73184087 displays allele-specific binding to HIF-2α and long-range interaction with the shared lncRNA-KMT2E promoter. (B) Pathogenesis of PAH. PAH is characterized by vasoconstriction and vascular remodeling of small PAs leading to progressive increase in pulmonary vascular resistance, and consequently RV failure. Currently available treatments focus primarily on vasodilation rather than on vascular remodeling and offer only limited beneficial effects on survival. HIF-2α, hypoxia-inducible factor-2α; SNV, single nucleotide variant; PAEC, pulmonary artery endothelial cell; PASMC, pulmonary artery smooth muscle cell; PA, pulmonary artery; PAH, pulmonary arterial hypertension; RV, right ventricle; LV, left ventricle; PDE-5, phosphodiesterase-5; lncRNA, long non-coding RNA; PH, pulmonary hypertension.