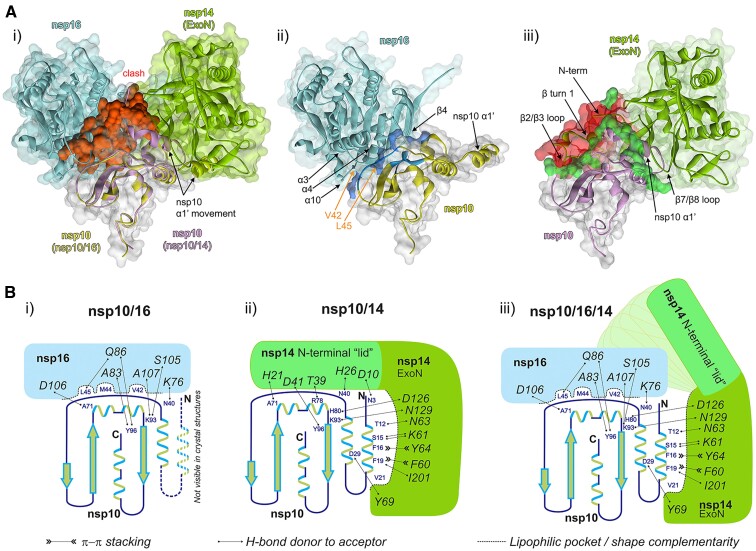
Figure 1.
Nsp10/16/14 heterotrimer complex formation and the lid hypothesis. (Ai) Overlay of the available high-resolution structures of SARS-CoV-2 nsp10/14 and nsp10/16 complexes with nsp10-centered alignment of nsp10/16 (PDB ID: 6WVN) and nsp10/14 (PDB ID: 7DIY). Nsp16 is shown in cyan and the associated nsp10 is shown in yellow. Nsp14 exonuclease domain is shown in green and the associated nsp10 is in magenta. The structural clash between nsp14 and nsp10/16 surfaces is shown in red. (Aii) Interface between nsp10 and nsp16. The strong hydrophobic interaction between nsp10 and nsp16 are shown in blue. The nsp10 residues V42 and L45 are indicated by the orange arrows. (Aiii) The interface between nsp10 and nsp14. The sidechains participating in hydrogen bonds between nsp10 and nsp14 are shown in bright green. The N-terminal region of nsp14, where the β turn 1 and β2/β3 loop are located is where the steric clash (red) is localized. All panels: Variable orientations of nsp10 α1′-helices seen in respective complexes with nsp16 and nsp14 are shown. Arrows indicate major structural features constituting the interface. (B) Schematic representation of interactions guiding the affinities of nsp10, nsp14 and nsp16. (Bi) Nsp16 binds nsp10 at the site overlapping that involved in binding of the lid of nsp14, but nsp10/16 interaction is characterized by a well-developed interface involving deep lipophilic pockets and solvent-shielded hydrogen bonds. The α1 helix of nsp10 is not defined in the crystal structures of the nsp10/16 complexes (PDB IDs: 6W4H, 6YZ1), indicating it is flexible and not involved in binding. (Bii) The α1 helix of nsp10 provides several deeply buried lipophilic and π-stacking interactions with the exonuclease domain of nsp14, and the interaction may be described in terms of shape complementarity. In turn, the interaction of the N-terminal lid region (amino acids 1–50) of nsp14 with nsp10 shows poor shape complementarity and is characterized only by a low number of solvent-exposed hydrogen bonds. (Biii) The formation of the nsp10/16/14 heterotrimer complex is accompanied by the lid displacement and stabilization of the α1 helix.