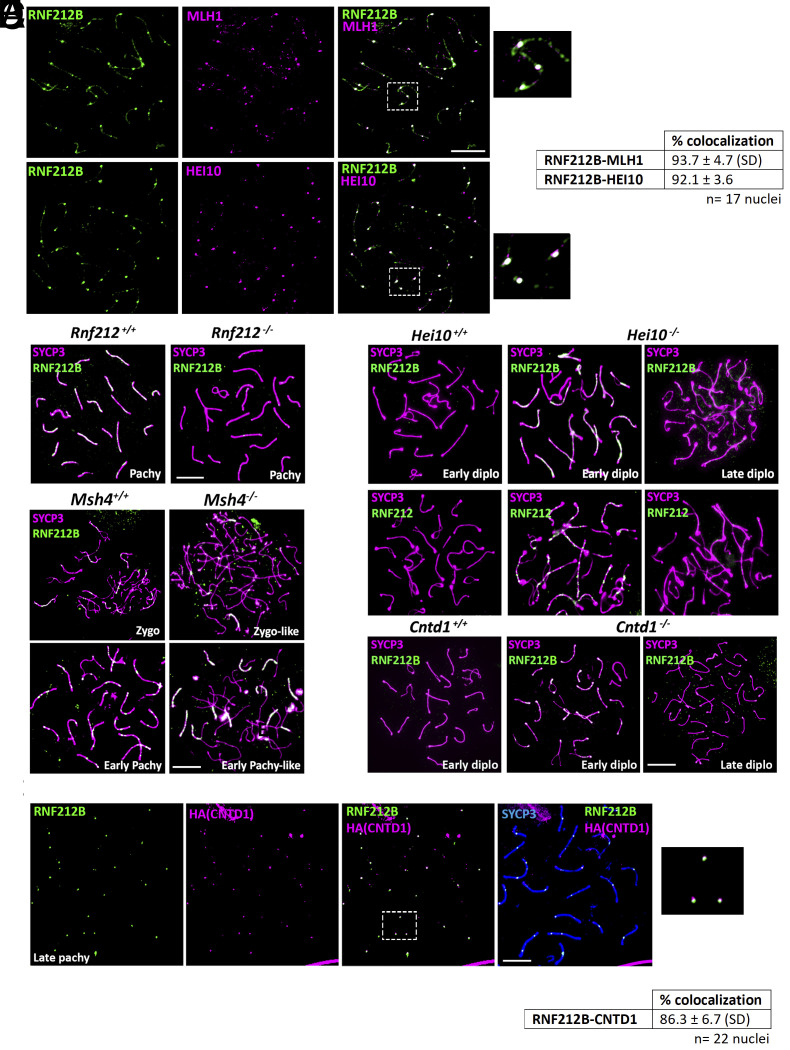
Fig. 2.
RNF212B colocalizes with CO-associated proteins and its loading is dependent on RNF212, and its unloading on HEI10 and CNTD1. (A) Double immunolabeling of RNF212B with MLH1 and HEI10 in pachynema spermatocytes. Right panel show magnification of the indicated region. The quantitation of the colocalization of RNF212B with MLH1 and HEI10 are shown in right table. (B) Double immunolabeling of RNF212B and SYCP3 in Rnf212−/−in pachynema spermatocytes showing absence of foci in comparison with the numerous foci of the wild-type control. (C) Double immunolabeling of RNF212B and SYCP3 in Msh4+/+ and Msh4−/−spermatocytes at zygonema and early pachynema, showing persistence of RNF212B loading in the absence of MSH4. (D) Double immunolabeling of SYCP3 with RNF212B and RNF212 in Hei10−/− early diplonema spermatocytes showing numerous foci at the synapsed LEs in comparison with the wild-type diplonemas which lack foci and in Hei10−/− late diplonemas when foci have completely disappeared. (E) Colabeling of RNF212B, anti-HA (CNTD1), and SYCP3 showing colocalization. The Right panel shows magnification of the indicated region. The quantitation of the colocalization of RNF212B with CNTD1 is shown in the lower table. (F) Double immunolabeling of RNF212B and SYCP3 in Cntd1−/−early diplonema spermatocytes showing numerous foci at the synapsed LEs in comparison with the wild-type diplonema spermatocytes which lack foci and in Cntd1−/− late diplonema when foci have completely disappear. Bar in panels 10 μm. All experiments have been carried out in at least three mice and 15 cells per mouse.