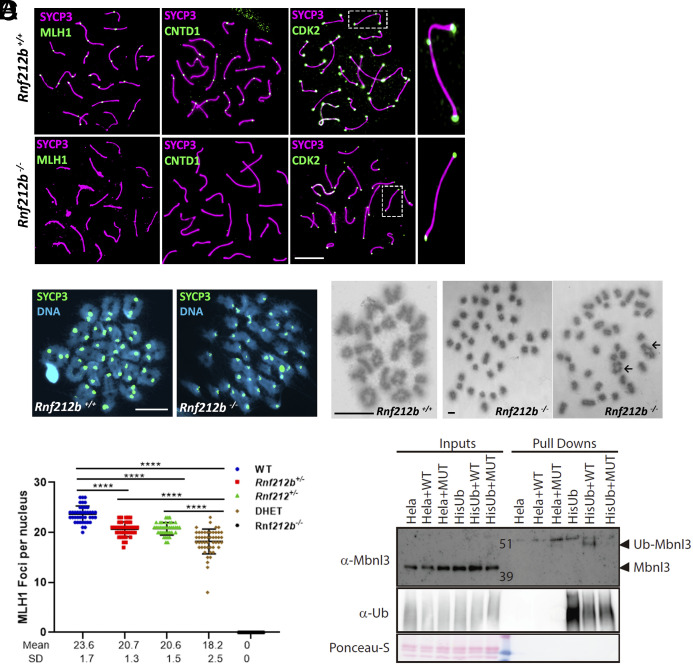
Fig. 6.
Major CO pathway disrupted in the absence of RNF212B, and MBNL3 ubiquitination by RNF212B. (A) Double immunolabeling of SYCP3 with MLH1, CNTD1, and CDK2 in Rnf212b−/−and wild-type mouse spermatocytes (Rnf212b+/+). Right magnified panels show the presence (Up) and absence (Low) of an interstitial CDK2 foci at a CO are shown. (B) Metaphase I cells from chromosome spreads (stained with SYCP3 and DAPI, Left panel) and from Methanol/Acetic chromosome preparations stained with Giemsa (Right) show the presence of univalents (Rnf212b−/−) and a few bivalents (black arrows) in comparison with all bivalents (Rnf212b+/+). (C) Plot quantitation of the number of MLH1 foci in pachynema spermatocytes from Rnf212b+/+(WT), Rnf212b+/−, and Rnf212+/−single mutants, Rnf212+/− Rnf212b+/− (DHet) and Rnf212b−/−. Welch’s t test analysis: ****P < 0.0001. (D) Ubiquitination assay performed on Hela cells to verify MBNL3 ubiquitination by RNF212B activity. Hela cells stably expressing 10xHis-Ub were transfected with the indicated expression vectors. 10xHis-ubiquitin conjugated proteins were purified from cell lysates using Ni-NTA beads. Purified proteins (Pull-Downs) and Lysate samples (Inputs) were analyzed by western blotting using specific Ubiquitin antibody and MBNL3 antibody to detect its ubiquitinated form. We observe a 10 KDa higher band corresponding to the ubiquitinated form of MBNL3 that is only detectable when transfecting HELA-Ub with RNF212B-WT expression vector. No band is detected when transfecting RNF212B dead mutant form (MUT). Bar in all panels, 10 μm. All experiments have been carried out in at least three mice and 12 cells per mouse in (A) and (C), and 5 cells in (B).