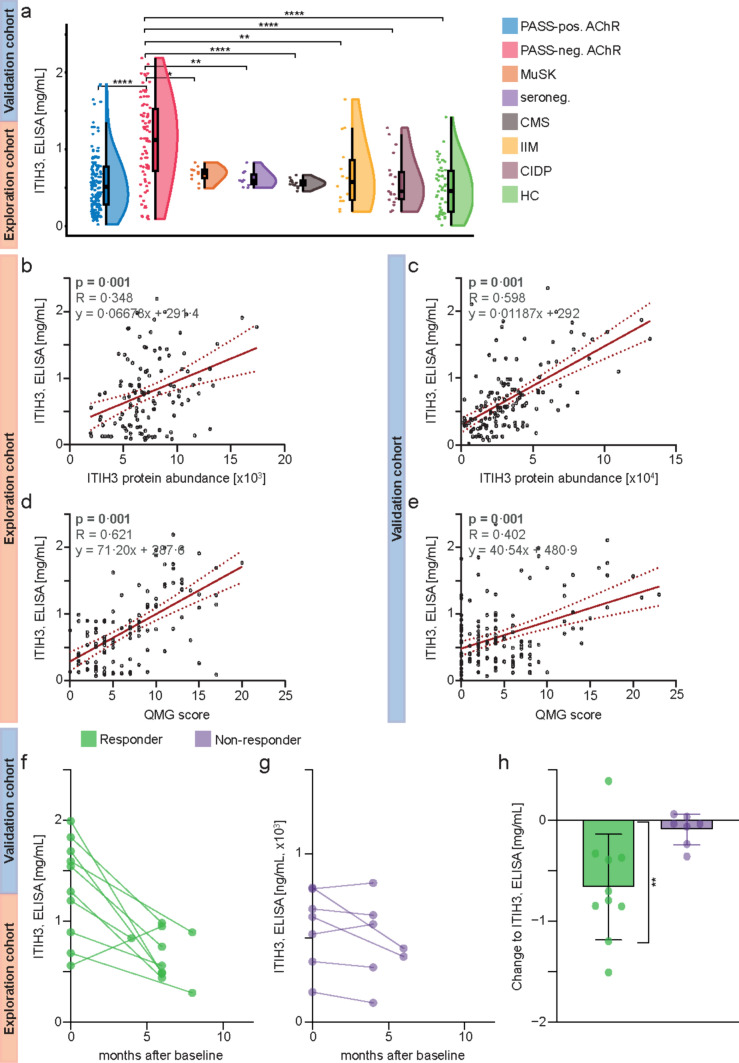
Fig. 4.
Validation of ITIH3 as a biomarker for disease activity and treatment response a Raincloud plot comparing ITIH3 protein abundance levels between PASS-positive and PASS-negative anti-AChR-Ab-positive MG patients measured by ELISA. Here, data sets from the exploration and validation cohorts were combined. Likewise, ITIH3 levels are indicated for anti-MuSK-Ab-positive (n = 10) and seronegative (n = 10) MG patients as well as for patients with CMS (n = 14), IIM (n = 12 with IBM and n = 2 with IMNM), and CIDP (n = 19). HCs indicate baseline levels (n = 53). A p value > 0.05 was classified as not significant, p < 0.05 (*) as significant, p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****) as highly significant. b + c Linear regression analysis of ITIH3 protein abundance measured by ELISA and by mass spectrometry in each cohort as indicated. d + e Linear regression analysis of ITIH3 protein levels measured by ELISA and individual QMG scores in each cohort as indicated. In the upper left hand of the plot, p-values are indicated next to the R statistic and the linear function equation describing the regression. f Scatter plot displaying the change to serum ITIH3 as measured by ELISA for treatment responders between baseline and follow-up. Each line indicates the change for an individual patient. g Scatter plot displaying the change to serum ITIH3 as measured by ELISA for treatment non-responders between baseline and follow-up. Each line indicates the change for an individual patient. Treatment response was defined as an improvement of ≥ 3 points on the MG-ADL scale. h Bar graph indicating the change to ITIH3 between follow-up and baseline for responders and non-responders. A one sample t test with zero as hypothetical mean was used for statistical analysis of each group. A p value > 0.05 was classified as not significant, p < 0.05 (*) as significant, p < 0.01 (**), p < 0.001 (***), and p < 0.0001 (****) as highly significant. Anti-AChR-Ab anti-acetylcholine receptor antibody, CIDP chronic inflammatory demyelinating polyradiculoneuropathy, CMS congenital myasthenic syndrome, HC healthy control, IBM inclusion body myositis, IIM idiopathic inflammatory myopathy, IMNM immune-mediated necrotizing myopathy, ITIH3 inter-alpha-trypsin inhibitor heavy chain H3, MG myasthenia gravis, MuSK muscle-specific kinase, PASS patient-acceptable symptom state, seroneg. seronegative, QMG quantitative MG